Metallic Glassy Hollow Microfibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bulk MG Tubes
2.2. Electrodeposition of Cu
2.3. Morphology and Microstructure Characterization
3. Results and Discussion
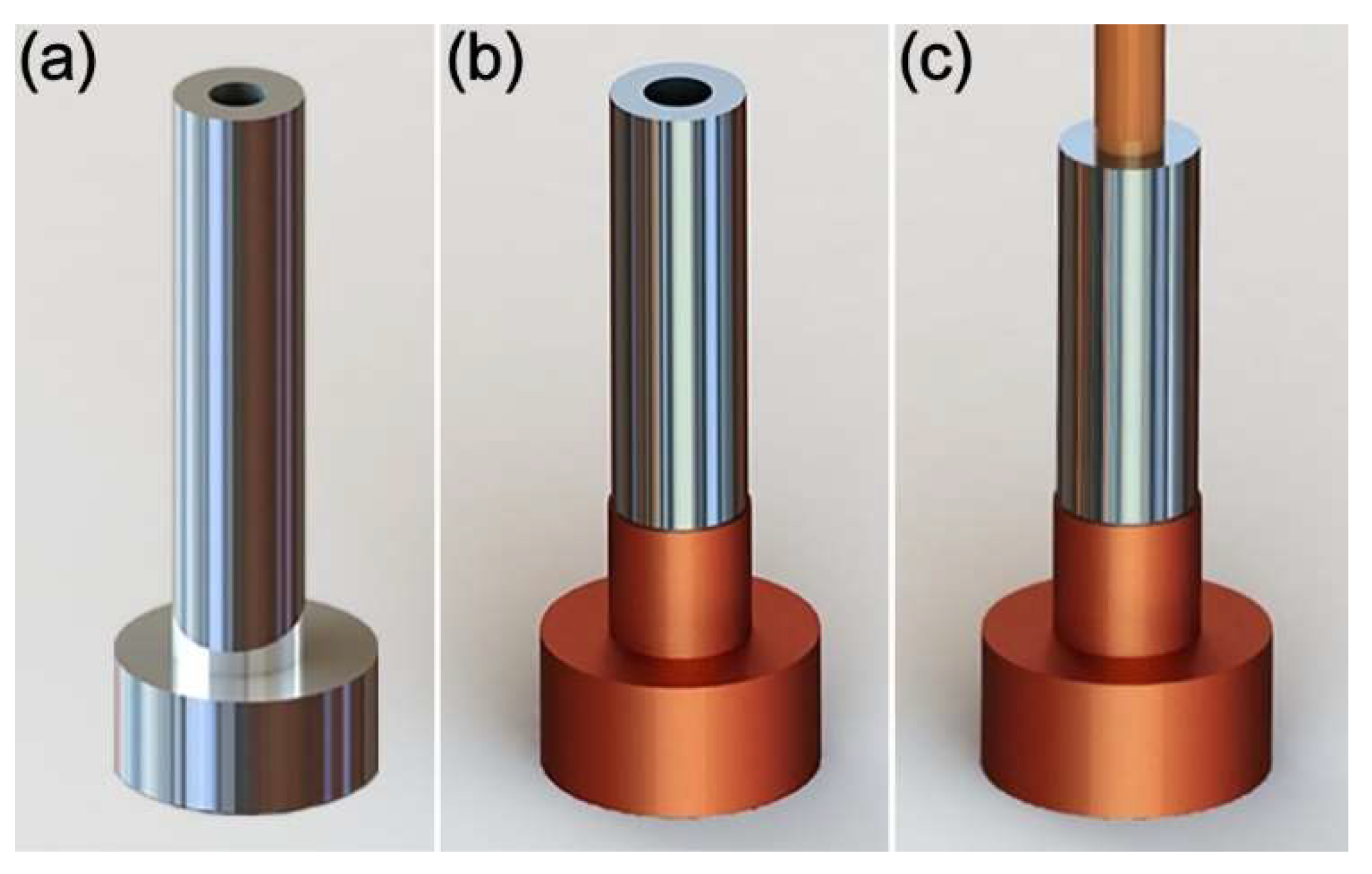
3.1. Preparing Preform for MG HMs
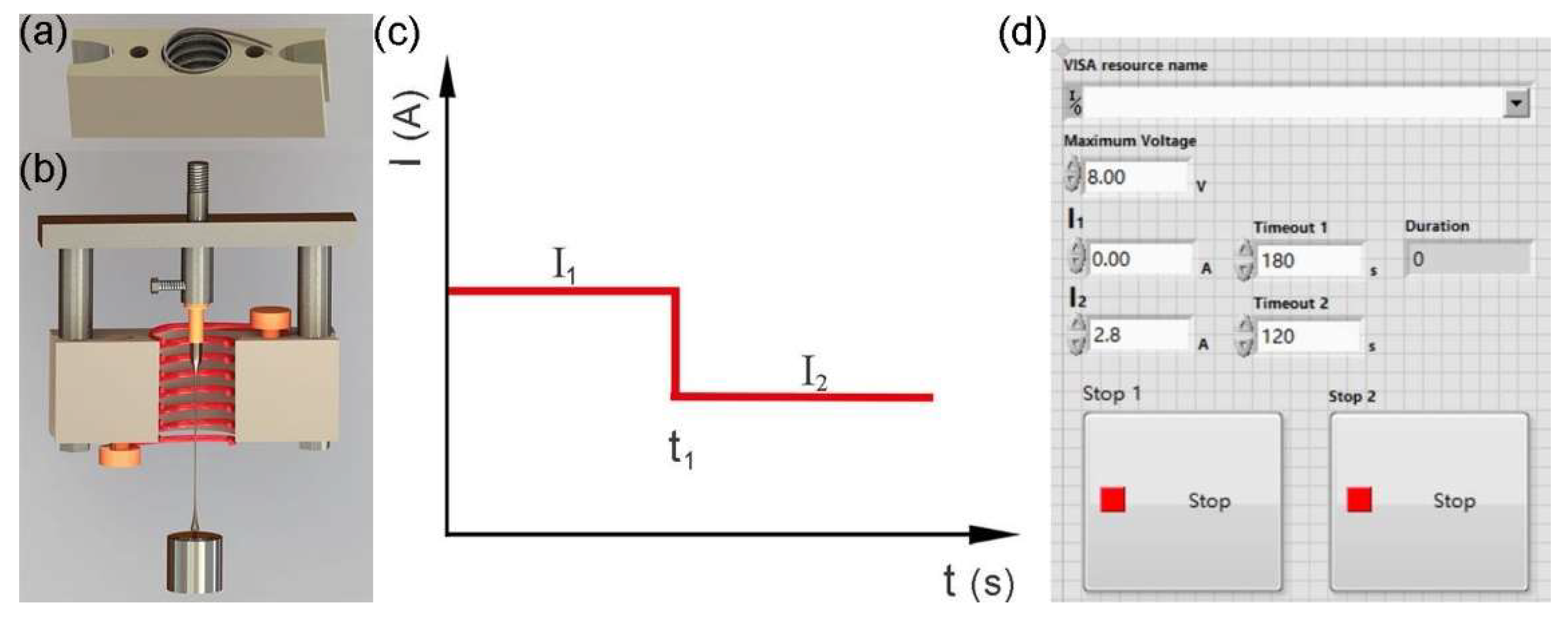
3.2. The Instrumentation for Fabricating MG HMs
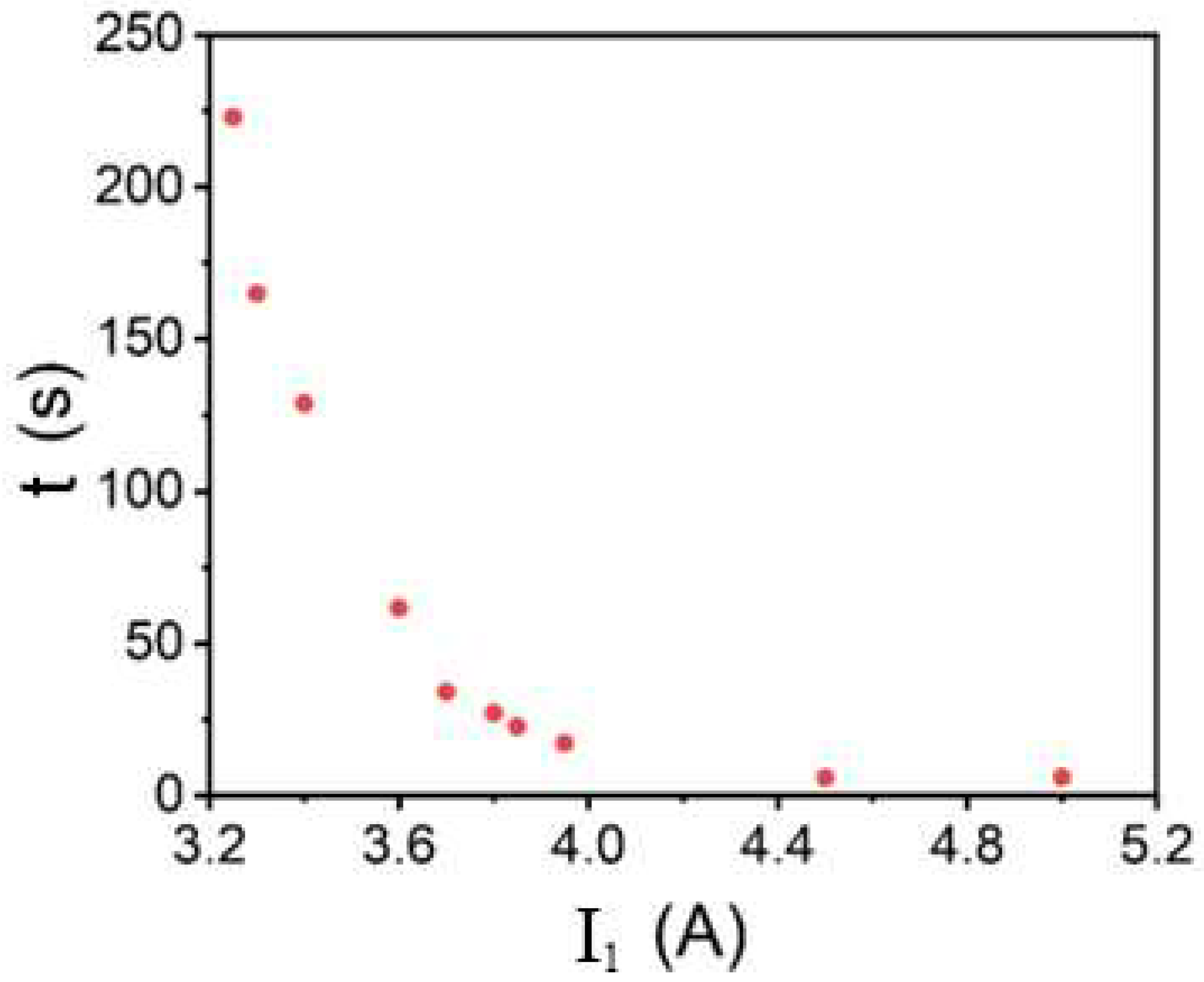
3.3. Fabrication of MG HMs
3.4. Morphology of the MG HMs
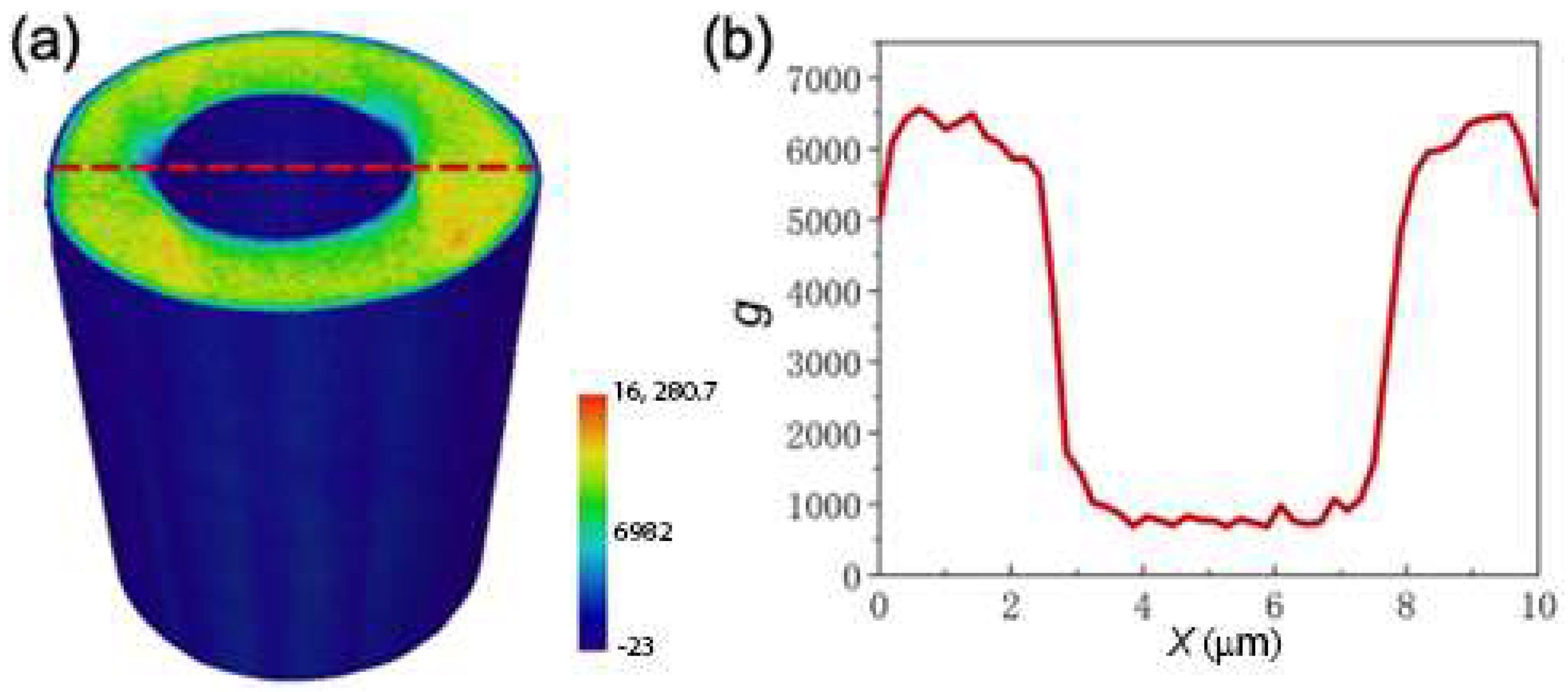
3.5. Heterogeneous Microstructure of the MG HMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baughman, R.H. Carbon nanotube actuators. Science 1999, 284, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Solovev, A.A.; Mei, Y.; Bermudez Urena, E.; Huang, G.; Schmidt, O.G. Catalytic microtubular jet engines self-propelled by accumulated gas bubbles. Small 2009, 5, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhuang, Z.; Zhao, H.; Lin, M.; Zhao, D.; Mai, L. Intricate hollow structures: Controlled synthesis and applications in energy storage and conversion. Adv. Mater. 2017, 29, 1602914. [Google Scholar] [CrossRef]
- Xu, B. Tubular micro/nanomachines: From the basics to recent advances. Adv. Funct. 2017, 28, 1705872. [Google Scholar] [CrossRef]
- Miranda, C.; Sampath Kumar, S.; Muthuswamy, J.; Smith, B.S. Photoacoustic micropipette. Appl. Phys. Lett. 2018, 113, 264103. [Google Scholar] [CrossRef]
- Singhal, R.; Bhattacharyya, S.; Orynbayeva, Z.; Vitol, E.; Friedman, G.; Gogotsi, Y. Small diameter carbon nanopipettes. Nanotechnology 2010, 21, 015304. [Google Scholar] [CrossRef]
- Zhang, S.; Mielke, S.L.; Khare, R.; Troya, D.; Ruoff, R.S.; Schatz, G.C.; Belytschko, T. Mechanics of defects in carbon nanotubes: Atomistic and multiscale simulations. Phys. Rev. B 2005, 71, 115403. [Google Scholar] [CrossRef]
- Wang, G.; Li, X. Predicting young’s modulus of nanowires from first-principles calculations on their surface and bulk materials. J. Appl. Phy. 2008, 104, 113517. [Google Scholar] [CrossRef]
- Tamizhanban, R.; Sreejith, K.R.; Jayanth, G.R. An automated pipette puller for fabrication of glass micropipettes. Rev. Sci. Instrum. 2014, 85, 055105. [Google Scholar] [CrossRef]
- Klony Lieberman, A.L. Multifunctional, micropipette based force cantilevers for scanned probe microscopy. Rev. Sci. Instrum. 2014, 85, 055105. [Google Scholar] [CrossRef]
- Jun, W.K.; Duwez, P.O.L. Non-crystalline structure in solidified gold–silicon alloys. Nature 1960, 187, 869–870. [Google Scholar]
- Greer, A.L. Metallic glasses... On the threshold. Mater. Today 2009, 12, 14–22. [Google Scholar] [CrossRef]
- Demetriou, M.D.; Launey, M.E.; Garrett, G.; Schramm, J.P.; Hofmann, D.C.; Johnson, W.L.; Ritchie, R.O. A damage-tolerant glass. Nat. Mater. 2011, 10, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.; Hufnagel, T.; Ramamurty, U. Mechanical behavior of amorphous alloys. Acta Mater. 2007, 55, 4067–4109. [Google Scholar] [CrossRef]
- Wang, W.H. Bulk metallic glasses with functional physical properties. Adv. Mater. 2009, 21, 4524–4544. [Google Scholar] [CrossRef]
- Hu, Y.C.; Wang, Y.Z.; Su, R.; Cao, C.R.; Li, F.; Sun, C.W.; Yang, Y.; Guan, P.F.; Ding, D.W.; Wang, Z.L.; et al. A highly efficient and self-stabilizing metallic-glass catalyst for electrochemical hydrogen generation. Adv. Mater. 2016, 28, 10293–10297. [Google Scholar] [CrossRef]
- Wang, Y.T.; Bai, H.Y.; Pan, M.X.; Zhao, D.Q.; Wang, W.H. Multiple spin-glass-like behaviors in a Pr-based bulk metallic glass. Phys. Rev. B 2006, 74, 064422. [Google Scholar] [CrossRef]
- Chen, H.S.; Turnbull, D. Evidence of a glass–liquid transition in a gold–germanium–silicon alloy. J. Chem. Phys. 1968, 48, 2560–2571. [Google Scholar] [CrossRef]
- Fan, G.J.; Li, J.J.Z.; Rhim, W.-K.; Qiao, D.C.; Choo, H.; Liaw, P.K.; Johnson, W.L. Thermophysical properties of a Cu46Zr42Al7Y5 bulk metallic glass-forming liquid. Appl. Phys. Lett. 2006, 88, 221909. [Google Scholar] [CrossRef]
- Nieh, T.G.; Wadsworth, J. Homogeneous deformation of bulk metallic glasses. Scripta Mater. 2006, 54, 387–392. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, D.Q.; Pan, M.X.; Wang, W.H.; Greer, A.L. Amorphous metallic plastic. Phys. Rev. Lett. 2005, 94, 205502. [Google Scholar] [CrossRef] [Green Version]
- Schroers, J. Processing of bulk metallic glass. Adv. Mater. 2010, 22, 1566–1597. [Google Scholar] [CrossRef] [PubMed]
- Schroers, J.; Hodges, T.M.; Kumar, G.; Raman, H.; Barnes, A.J.; Pham, Q.; Waniuk, T.A. Thermoplastic blow molding of metals. Mater. Today 2011, 14, 14–19. [Google Scholar] [CrossRef]
- Cowell, E.W.; Alimardani, N.; Knutson, C.C.; Conley, J.F.; Keszler, D.A.; Gibbons, B.J.; Wager, J.F. Advancing mim electronics: Amorphous metal electrodes. Adv. Mater. 2011, 23, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Xia, X.X.; Zhao, D.Q.; Pan, M.X.; Bai, H.Y.; Wang, W.H. Micro-and nanoscale metallic glassy fibers. Adv. Eng. Mater. 2010, 12, 1117–1122. [Google Scholar] [CrossRef]
- Inoue, A.; Hagiwara, M.; Masumoto, T. Production of Fe-P-C amorphous wires by in-rotating-water spinning method and mechanical properties of the wires. J. Mater. Sci. 1982, 17, 580–588. [Google Scholar] [CrossRef]
- Kumar, G.; Desai, A.; Schroers, J. Bulk metallic glass: The smaller the better. Adv. Mater. 2011, 23, 461–476. [Google Scholar] [CrossRef]
- Yi, J.; Huo, L.S.; Zhao, D.Q.; Pan, M.X.; Bai, H.Y.; Wang, W.H. Toward an ideal electrical resistance strain gauge using a bare and single straight strand metallic glassy fiber. Sci. China-Phys. Mech. Astron. 2012, 55, 609–613. [Google Scholar] [CrossRef]
- Sekol, R.C.; Kumar, G.; Carmo, M.; Gittleson, F.; Hardesty-Dyck, N.; Mukherjee, S.; Schroers, J.; Taylor, A.D. Bulk metallic glass micro fuel cell. Small 2013, 9, 2081–2085. [Google Scholar] [CrossRef]
- Hasan, M.; Kumar, G. High-throughput drawing and testing of metallic glass nanostructures. Nanoscale 2017, 9, 3261–3268. [Google Scholar] [CrossRef]
- Hussain, I.; Jiang, Y.Y.; Jia, Y.D.; Wang, G.; Zhai, Q.J.; Chan, K.C.; Yi, J. Tensile behavior of Cu-coated Pd40Cu30Ni10P20 metallic glassy wire. Sci. Rep. 2018, 8, 5659. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ge, T.P.; Liu, G.L.; Luan, J.H.; He, Q.F.; Yuan, Q.X.; Huang, W.X.; Zhang, K.; Bai, H.Y.; Shek, C.H.; et al. Density fluctuations with fractal order in metallic glasses detected by synchrotron X-ray nano-computed tomography. Acta Mater. 2018, 155, 69–79. [Google Scholar] [CrossRef]
- Geng, C.; Huang, B.; Zhang, N.; Yi, J.; Wang, Q.; Jia, Y.; Li, F.; Luan, J.; Hou, X.; Huang, W.; et al. Evolution of local densities during shear banding in zr-based metallic glass micropillars. Acta Mater. 2022, 235, 118068. [Google Scholar] [CrossRef]
- Busch, R.; Bakke, E.; Johnson, W.L. Viscosity of the supercooled liquid and relaxation at the glass transition of the Zr46.75Ti8.25Cu7.5Ni10Be27.5 bulk metallic glass forming alloy. Acta Mater. 1998, 46, 4725–4732. [Google Scholar] [CrossRef]
- Nakayama, K.S.; Yokoyama, Y.; Ono, T.; Chen, M.W.; Akiyama, K.; Sakurai, T.; Inoue, A. Controlled formation and mechanical characterization of metallic glassy nanowires. Adv. Mater. 2010, 22, 872–875. [Google Scholar] [CrossRef]
- Spaepen, F. Homogeneous flow of metallic glasses: A free volume perspective. Scr. Mater. 2006, 54, 363–367. [Google Scholar] [CrossRef]
- Kruzic, J.J. Bulk metallic glasses as structural materials: A review. Adv. Eng. Mater. 2016, 18, 1308–1331. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yi, J.; Huang, B.; Wang, G. Metallic Glassy Hollow Microfibers. Metals 2022, 12, 1463. https://doi.org/10.3390/met12091463
Zhao J, Yi J, Huang B, Wang G. Metallic Glassy Hollow Microfibers. Metals. 2022; 12(9):1463. https://doi.org/10.3390/met12091463
Chicago/Turabian StyleZhao, Jing, Jun Yi, Bo Huang, and Gang Wang. 2022. "Metallic Glassy Hollow Microfibers" Metals 12, no. 9: 1463. https://doi.org/10.3390/met12091463





