Recovery of Rare Earth Elements through Spent NdFeB Magnet Oxidation (First Part)
Abstract
:1. Introduction
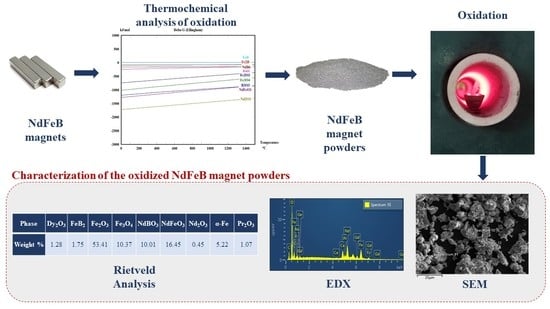
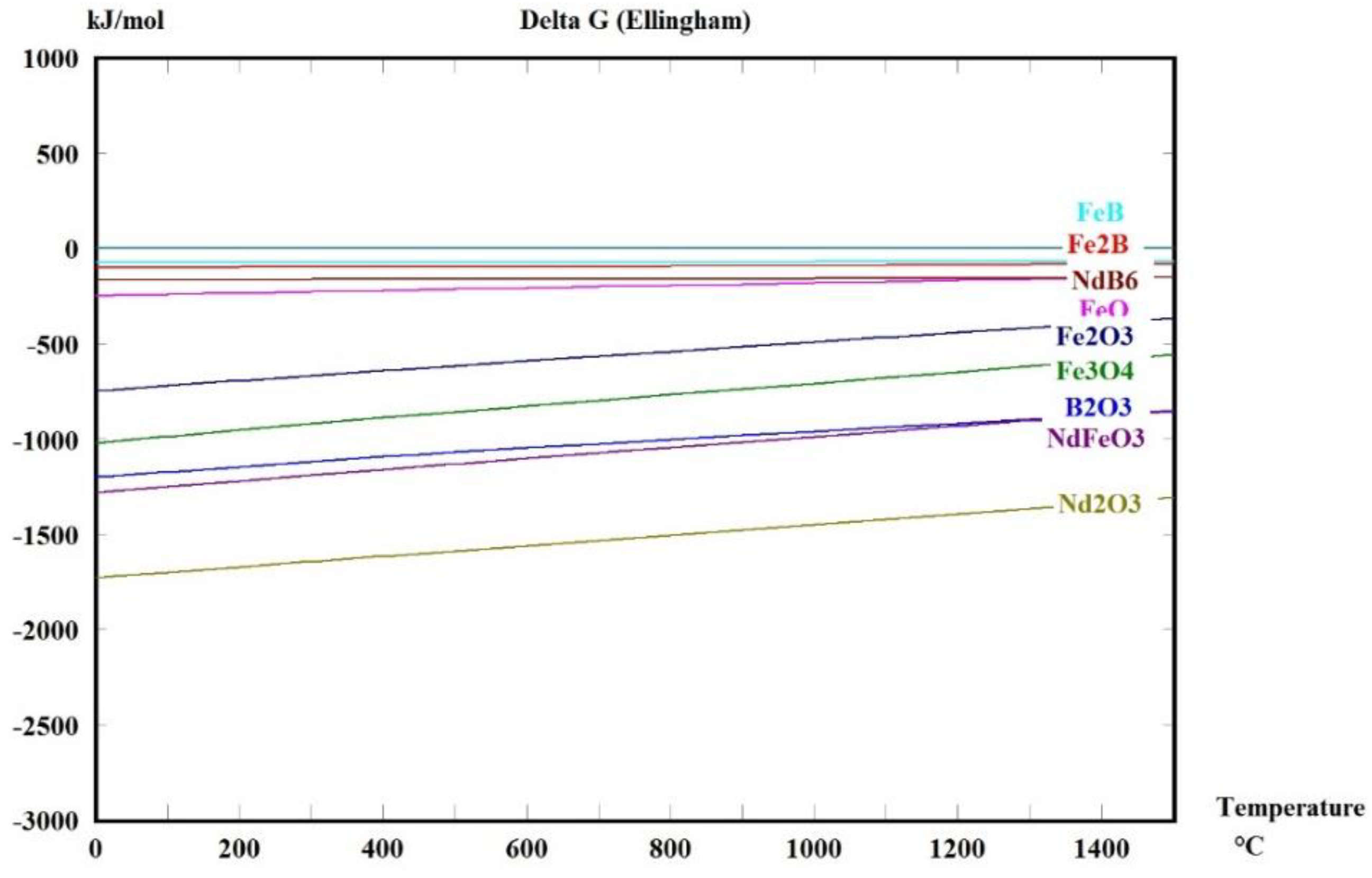
2. Thermochemical Analysis of the Oxidation
M: Fe, Nd, Dy, B
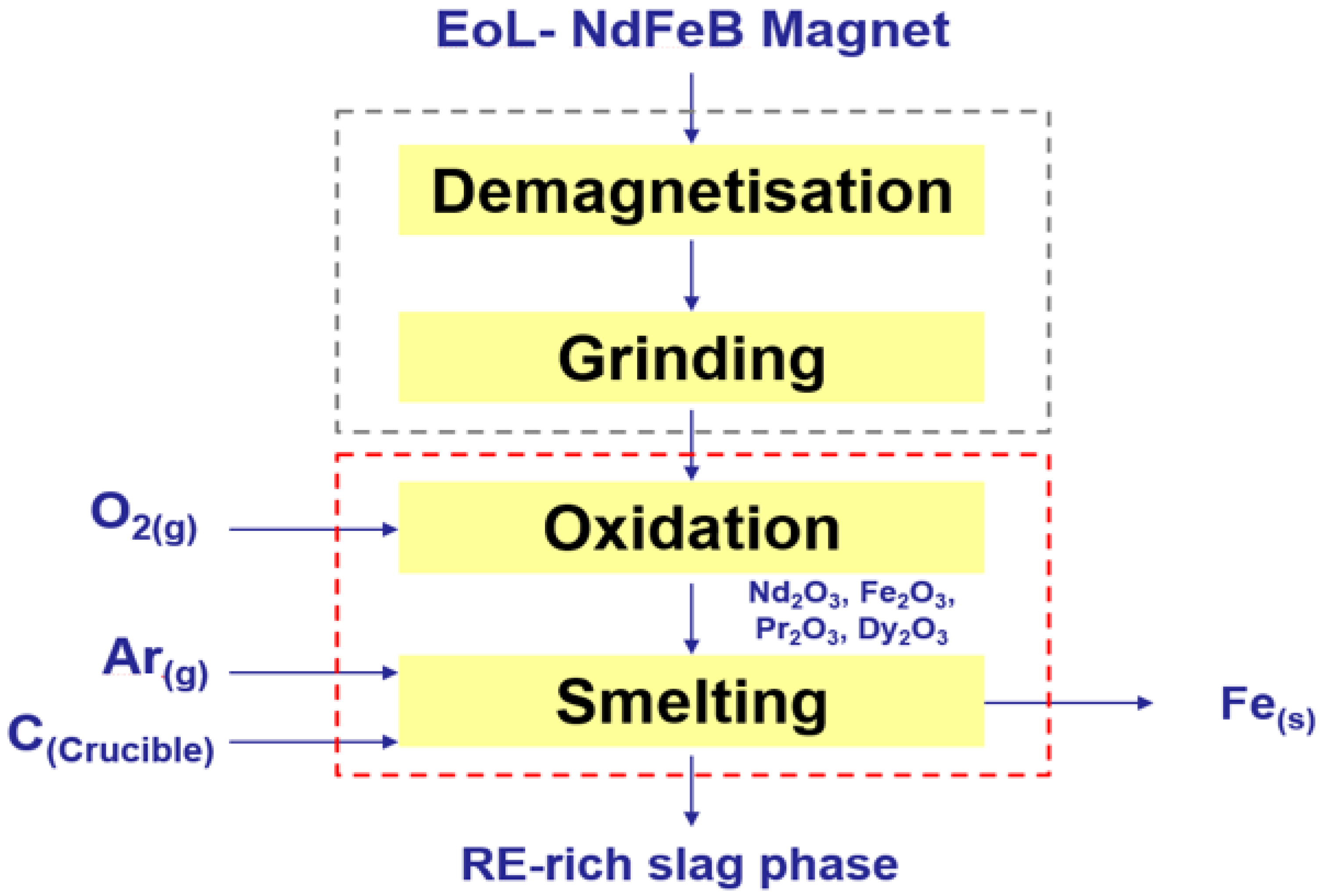
3. Experimental Procedure
3.1. Materials and Methods
3.2. Results and Discussion
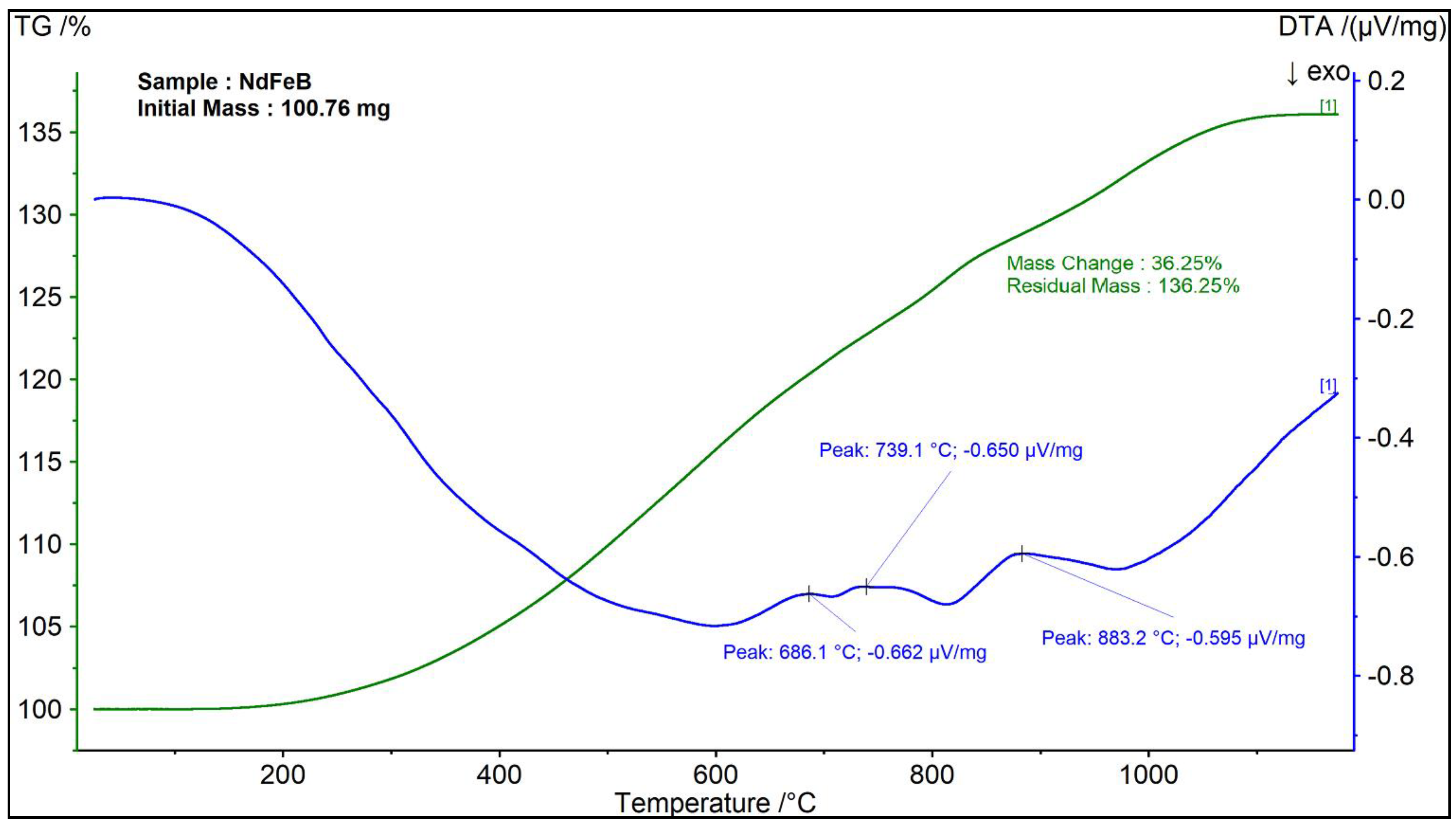
3.2.1. Differential Thermal (DTA) and Thermalgravimetric Analysis (TGA)
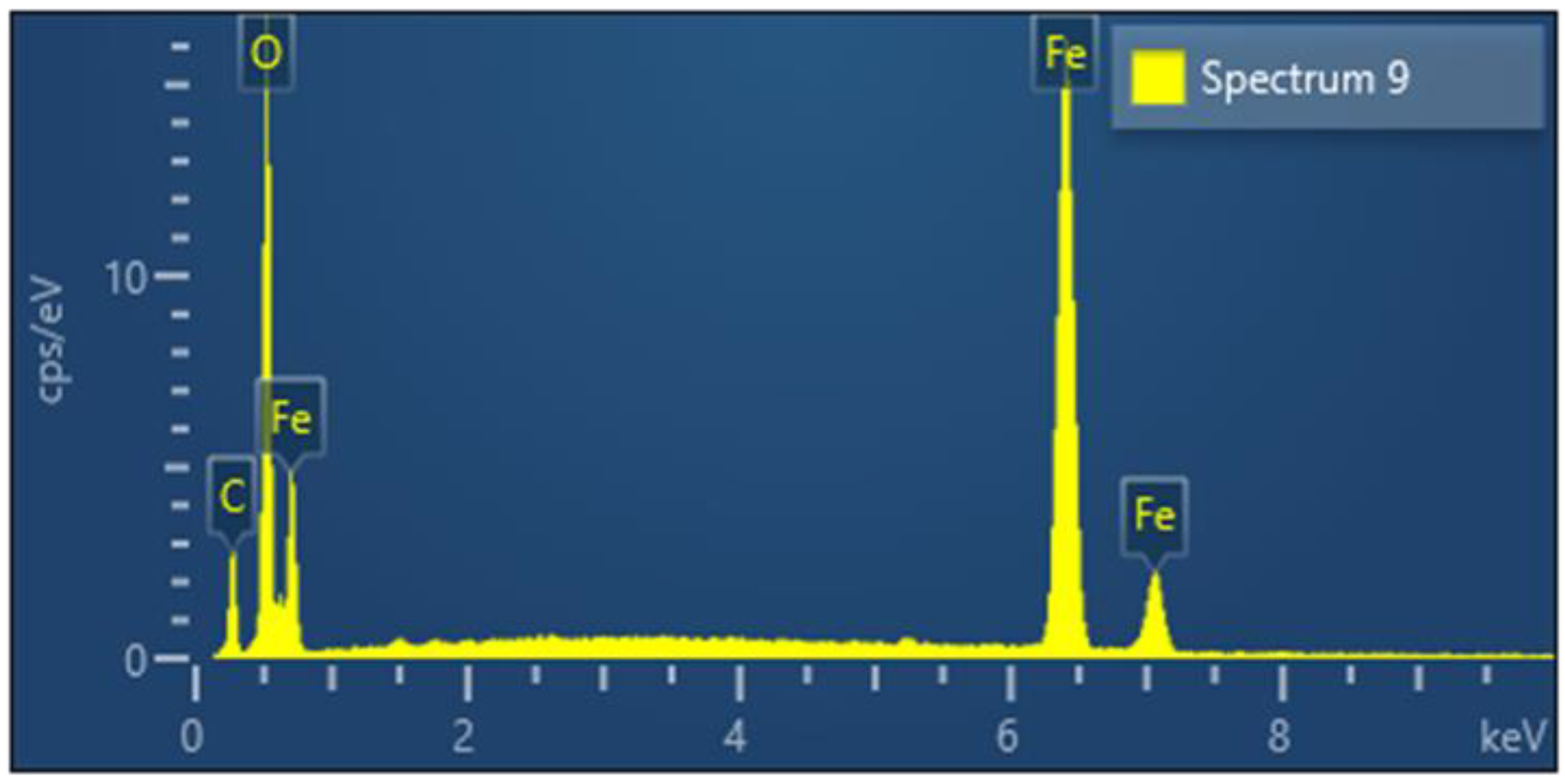
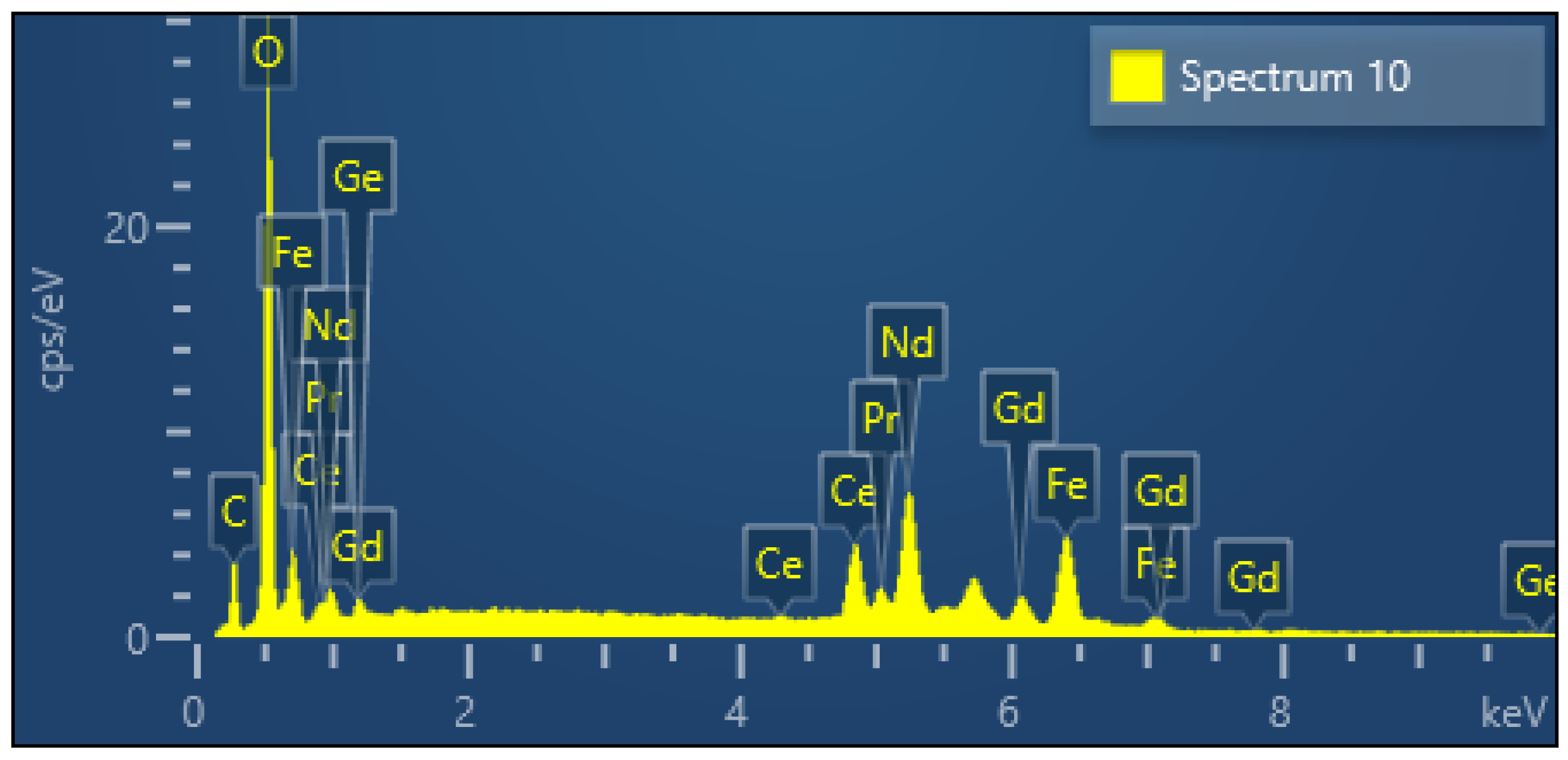
3.2.2. Oxidation of Particles in the Furnace
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, P.J.; Carpenter, D.; Boutin, C.; Allison, J.E. Rare earth elements (REEs): Effects on germination and growth of selected crop and native plant species. Chemosphere 2013, 96, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.; Ciminelli, V. Process development for the recovery of high-grade lanthanum by solvent extraction. Hydrometallurgy 2004, 73, 237–244. [Google Scholar] [CrossRef]
- Maestro, P.; Huguenin, D. Industrial applications of rare earths: Which way for the end of the century? J. Alloys Compd. 1995, 225, 520–528. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Gupta, C.K. The Rare Earths, Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–84. [Google Scholar]
- Hoshino, M.; Sanematsu, K.; Watanabe, Y. REE mineralogy and resources. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: New York, NY, USA, 2016; Volume 49, pp. 129–291. [Google Scholar]
- Davris, P.; Stopic, S.; Balomenos, E.; Panias, D.; Paspaliaris, I.; Friedrich, B. Leaching of rare earth elements from Eudialyte concentrate by supressing silicon dissolution. Miner. Eng. 2017, 108, 115–122. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Gronen, L.; Milivojevic, M.; Obradovic, S.; Friedrich, B. Neural Network Modeling for the Extraction of Rare Earth Elements from Eudialyte Concentrate by Dry Digestion and Leaching. Metals 2018, 8, 267. [Google Scholar] [CrossRef]
- Ma, Y.; Stopić, S.; Friedrich, B. Hydrometallurgical Treatment of an Eudialyte Concentrate for Preparation of Rare Earth Carbonate. Johns. Matthey Technol. Rev. 2019, 63, 2–13. [Google Scholar] [CrossRef]
- Yun, Y.; Stopic, S.; Friedrich, B. Valorization of Rare Earth Elements from a Steenstrupine Concentrate Via a Combined Hydrometallurgical and Pyrometallurgical Method. Minerals 2020, 10, 248. [Google Scholar] [CrossRef]
- Kilicarslan, A.; Voßenkaul, D.; Stoltz, S.; Stopic, S.; Nezihi, M.; Friedrich, B. Selectivity Potential of Ionic Liquids for Metal Extraction from Rare Earth containing Slags—A QEMSCAN assisted approach. Hydrometallurgy 2017, 169, 59–67. [Google Scholar]
- Buchert, M.; Manhart, A.; Sutter, J. Untersuchung zu Seltenen Erden: Permanentmagnete im Industriellen Einsatz in Baden-Württemberg. Studie im Auftrag des Ministeriums für Umwelt, Klima und Energiewirtschaft Baden-Württemberg. Forum 6: Energieeffiziente Industrielle Antriebe und deren Abhängigkeit von Selten Erden; p. 46. 2014. Available online: http://www.oeko.de/oekodoc/2053/2014-630-de.pdf (accessed on 22 January 2014).
- Stopic, S.; Friedrich, B. Advances in Understanding of the Application of Unit Operations in metallurgy of rare earth elements. Metals 2021, 11, 237–256. [Google Scholar] [CrossRef]
- Umweltbundesamt. Seltene Erden in Permanentmagneten. In Für Mensch und Umwelt-Factsheet; Umweltbundesamt: Dessau-Roßlau, Germany, 2019; pp. 1–10. [Google Scholar]
- Sprecher, B.; Xiao, Y.; Walton, A.; Speight, J.; Harris, R.; Kleijn, R.; Visser, G.; Kramer, G.J. Life cycle inventory of the production of rare earths and the subsequent production of NdFeB rare earth permanent magnets. Environ. Sci. Technol. 2014, 48, 3951–3958. [Google Scholar] [CrossRef]
- KMurase, K.; Machida, K.-I.; Adachi, G.-Y. Recovery of rare metals from scrap of rare earth intermetallic material by chemical vapour transport. J. Alloy Compd. 1995, 217, 218–225. [Google Scholar] [CrossRef]
- Takeda, O.; Nakano, K.; Sato, Y. Recycling of rare earth magnet waste by removing rare earth oxide with molten fluoride. Mater. Trans. 2014, 55, 334–341. [Google Scholar] [CrossRef]
- Kaya, E.; Kaya, O.; Stopic, S.; Gürmen, S.; Friedrich, B. NdFeB magnets recycling process: An alternative method to produce mixed rare earth oxide from Scrap NdFeB magnets. Metals 2021, 11, 716. [Google Scholar] [CrossRef]
- Shirayama, S.; Okabe, T.H. Selective Extraction and Recovery of Nd and Dy from Nd-Fe-B Magnet Scrap by Utilizing Molten MgCl2. Metall. Mater. Trans. B 2018, 49, 1067–1077. [Google Scholar] [CrossRef]
- Lyman, J.; Palmer, G. Recycling of Rare Earths and Iron from NdFeB Magnet Scrap. High Temp. Mater. Process. 1993, 11, 175–188. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kubo, K.; Katayama, Y.; Tanaka, T.; Yamamoto, T. Extraction of rare earth elements as oxides from a neodymium magnetic sludge. Metall. Mater. Trans. B 2012, 43, 468–476. [Google Scholar] [CrossRef]
- Kruse, S.; Raulf, K.; Pretz, T.; Friedrich, B. Influencing Factors on the Melting Characteristics of NdFeB-Based Production Wastes for the Recovery of Rare Earth Compounds. J. Sustain. Met. 2016, 3, 168–178. [Google Scholar] [CrossRef]
- Kruse, S.; Raulf, K.; Trentmann, A.; Pretz, T.; Friedrich, B. Processing of Grinding Slurries Arising from NdFeB Magnet Production. Chem. Ing. Tech. 2015, 87, 1589–1598. [Google Scholar] [CrossRef]
- Saito, T.; Sato, H.; Ozawa, S.; Yu, J.; Motegi, T. The extraction of Nd from waste Nd–Fe–B alloys by the glass slag method. J. Alloy Compd. 2003, 353, 189–193. [Google Scholar] [CrossRef]
- Bian, Y.; Guo, S.; Jiang, L.; Tang, K.; Ding, W. Extraction of Rare Earth Elements from Permanent Magnet Scraps by FeO–B2O3 Flux Treatment. J. Sustain. Met. 2015, 1, 151–160. [Google Scholar] [CrossRef]
- Tanvar, H.; Dhawan, N. Microwave-Assisted Carbothermic Reduction of Discarded Rare Earth Magnets for Recovery of Neodymium and Iron Values. JOM 2020, 73, 54–62. [Google Scholar] [CrossRef]
- Sharma, R.A. Neodymium Production Processes. JOM 1987, 39, 33–37. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Oxide reduction processes in the preparation of rare-earth metals. Min. Met. Explor. 2013, 30, 38–44. [Google Scholar] [CrossRef]
- Ernst, S. Chemische Technik (Winnacker-Küchler). Band 3: Anorganische Grundstoffe, Zwischenprodukte. Herausgegeben von Roland Dittmeyer, Wilhelm Keim, Gerhard Kreysa und Alfred Oberholz. Angew. Chem. 2006, 118, 29–30. [Google Scholar] [CrossRef]
- Mendiara, T.; Jensen, A.; Glarborg, P. Chemical Looping Reforming of Generator Gas; CHEC Report No. R1001; Technical University of Denmark: Lyngby, Denmark, 2010. [Google Scholar]
- Frohberg, M.G. Thermodynamik für Werkstoffingenieure und Metallurgen; Wiley-VCH: Weinheim, Germany, 1994; ISBN 978-3-527-30922-1. [Google Scholar]
- Xie, W.H.; Huang, R.; Zhang, J.Z.; Li, W.; Yang, Y. Study on the interaction of rare earth element neodymium, iron and arsenic at 1173 K. Gongneng Cailiao/J. Funct. Mater. 2018, 49, 1134–1138. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.-M.; Van Gerven, T.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Met. 2017, 3, 122–149. [Google Scholar] [CrossRef]


| Sample NdFeB (%) | Nd | Ni | Fe | B | Pr | Dy |
|---|---|---|---|---|---|---|
| Weight (%) | 15.9 | 0.0006 | 73.8 | 0.662 | 5.6 | 0.201 |
| Phase | DyFe2 | DyFe5 | FeB5 | Nd2FeB14 | Pr2FeB14 |
|---|---|---|---|---|---|
| Weight % | 2.46 | 3.04 | 4.26 | 87.19 | 3.04 |
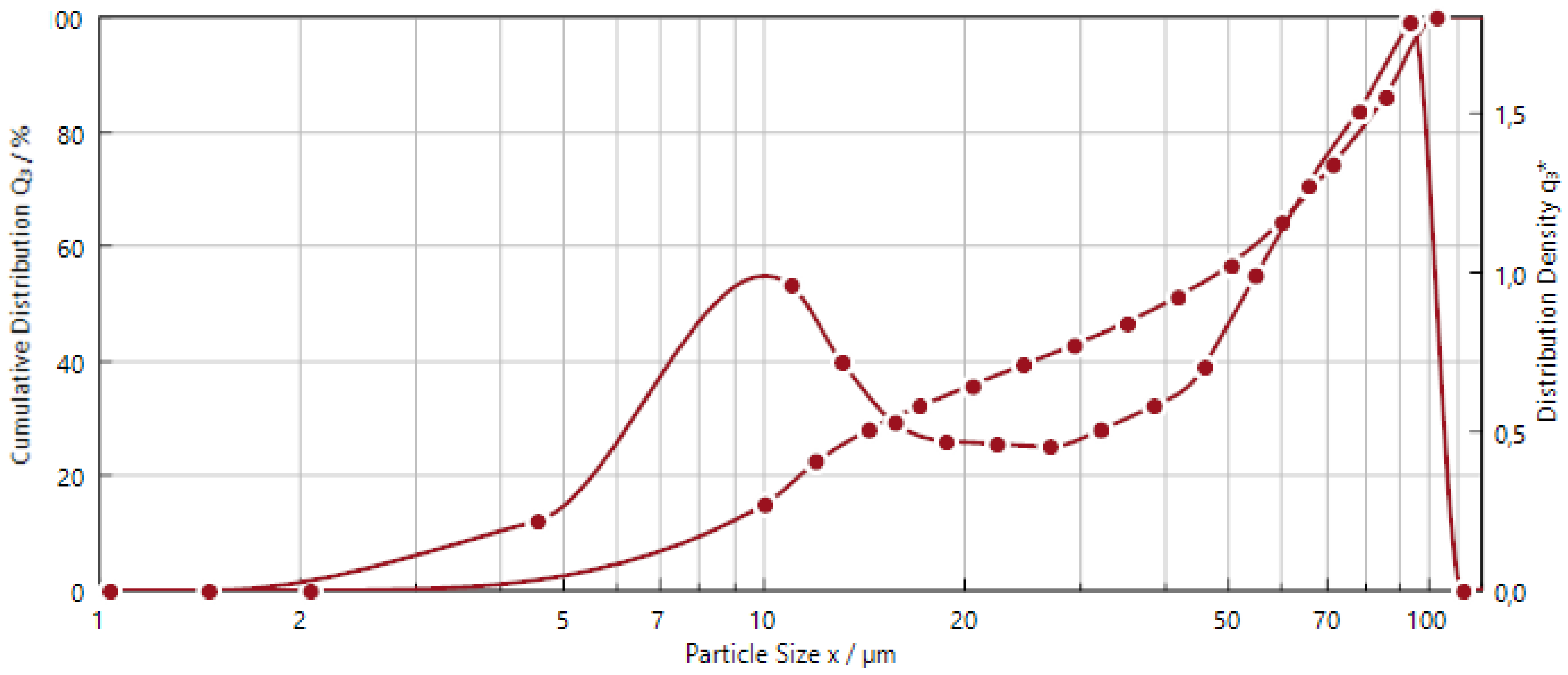
| Samples (µm) | X30 | X50 | X90 |
|---|---|---|---|
| NdFeB | 7.35 | 39.85 | 90.40 |
| Time (min) | 500 °C | 600 °C | 700 °C | 800 °C | 900 °C | 1000 °C | 1100 °C |
|---|---|---|---|---|---|---|---|
| 15 | 0.118 | 0.05 | 0.214 | 0.244 | 0.238 | 0.264 | 0.274 |
| 30 | 0.134 | 0.192 | 0.242 | 0.294 | 0.251 | 0.293 | 0.287 |
| 45 | 0.161 | 0.221 | 0.248 | 0.299 | 0.283 | 0.336 | 0.295 |
| 60 | 0.178 | 0.206 | 0.276 | 0.299 | 0.327 | 0.333 | 0.337 |
| 75 | 0.17 | 0.22 | 0.268 | 0.312 | 0.336 | 0.325 | 0.318 |
| 90 | 0.186 | 0.249 | 0.295 | 0.327 | 0.329 | 0.342 | 0.341 |
| Phase | Dy2O3 | FeB2 | Fe2O3 | Fe3O4 | NdBO3 | NdFeO3 | Nd2O3 | α-Fe | Pr2O3 |
|---|---|---|---|---|---|---|---|---|---|
| Weight % | 1.28 | 1.75 | 53.41 | 10.37 | 10.01 | 16.45 | 0.45 | 5.22 | 1.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stopic, S.; Polat, B.; Chung, H.; Emil-Kaya, E.; Smiljanić, S.; Gürmen, S.; Friedrich, B. Recovery of Rare Earth Elements through Spent NdFeB Magnet Oxidation (First Part). Metals 2022, 12, 1464. https://doi.org/10.3390/met12091464
Stopic S, Polat B, Chung H, Emil-Kaya E, Smiljanić S, Gürmen S, Friedrich B. Recovery of Rare Earth Elements through Spent NdFeB Magnet Oxidation (First Part). Metals. 2022; 12(9):1464. https://doi.org/10.3390/met12091464
Chicago/Turabian StyleStopic, Srecko, Buse Polat, Hanwen Chung, Elif Emil-Kaya, Slavko Smiljanić, Sebahattin Gürmen, and Bernd Friedrich. 2022. "Recovery of Rare Earth Elements through Spent NdFeB Magnet Oxidation (First Part)" Metals 12, no. 9: 1464. https://doi.org/10.3390/met12091464