Microwave Drying Kinetics of Chromium-Rich Electroplating Sludge
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Procedure
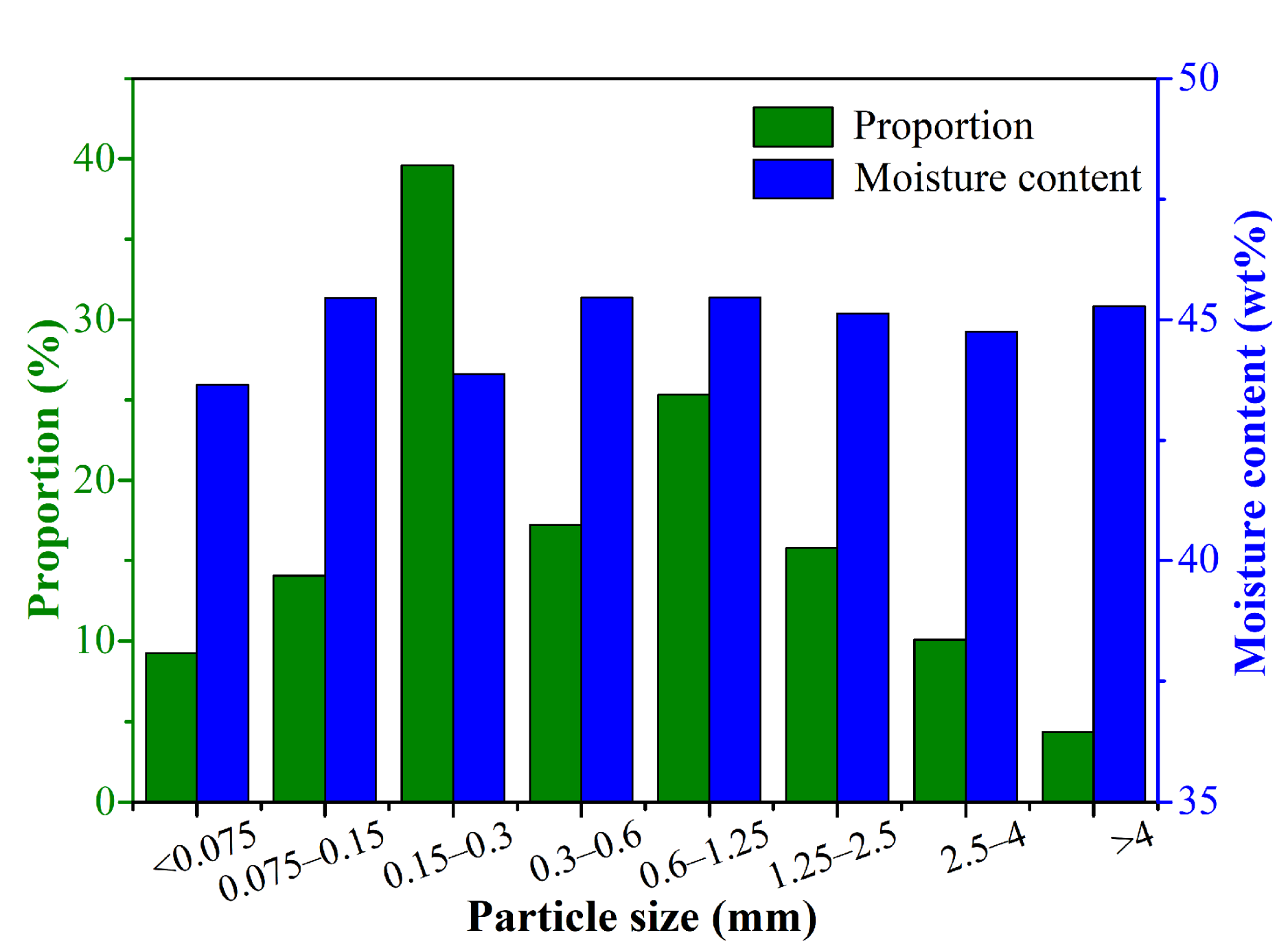
2.2.1. Particle Size Distribution and Corresponding Moisture Content Distribution
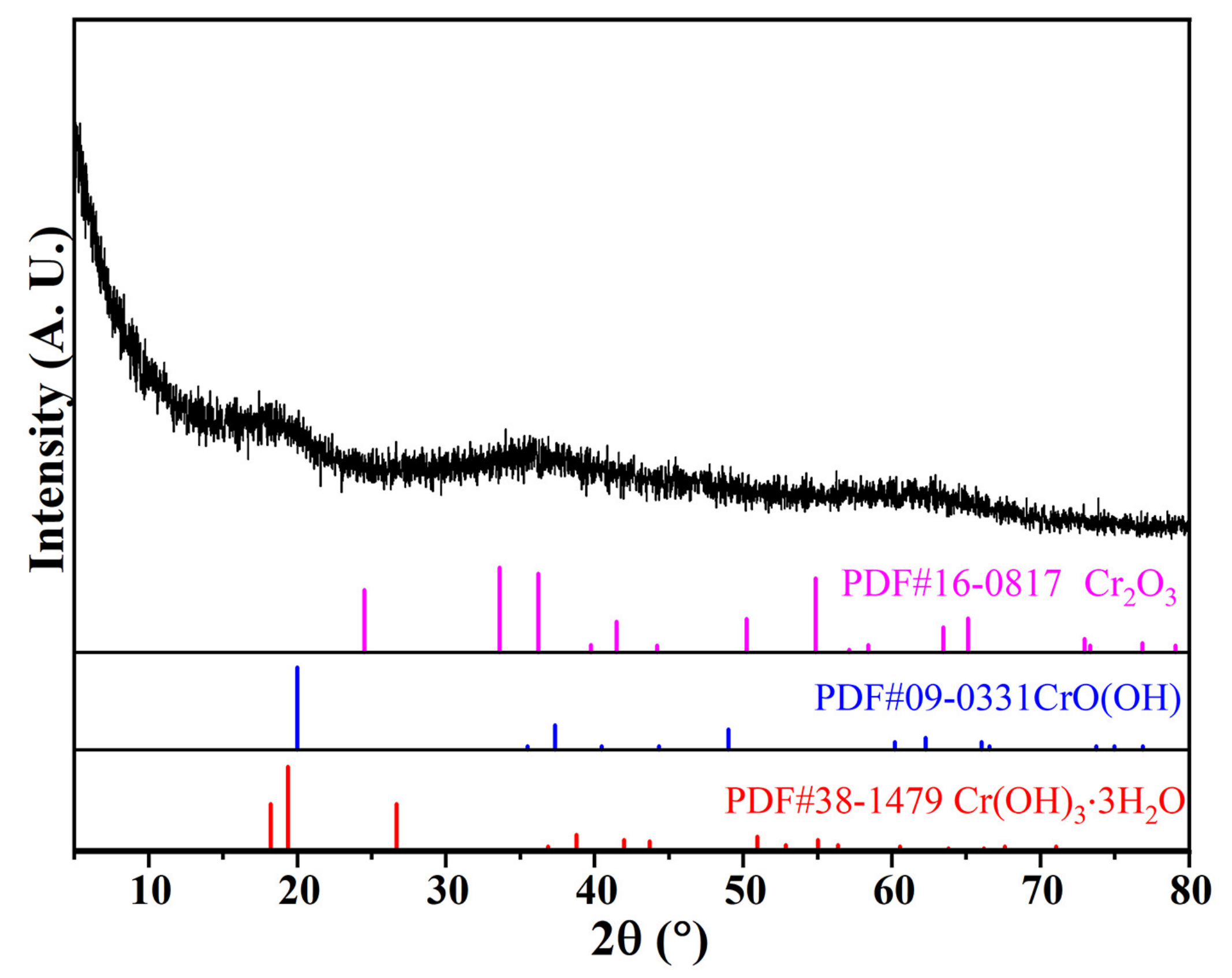
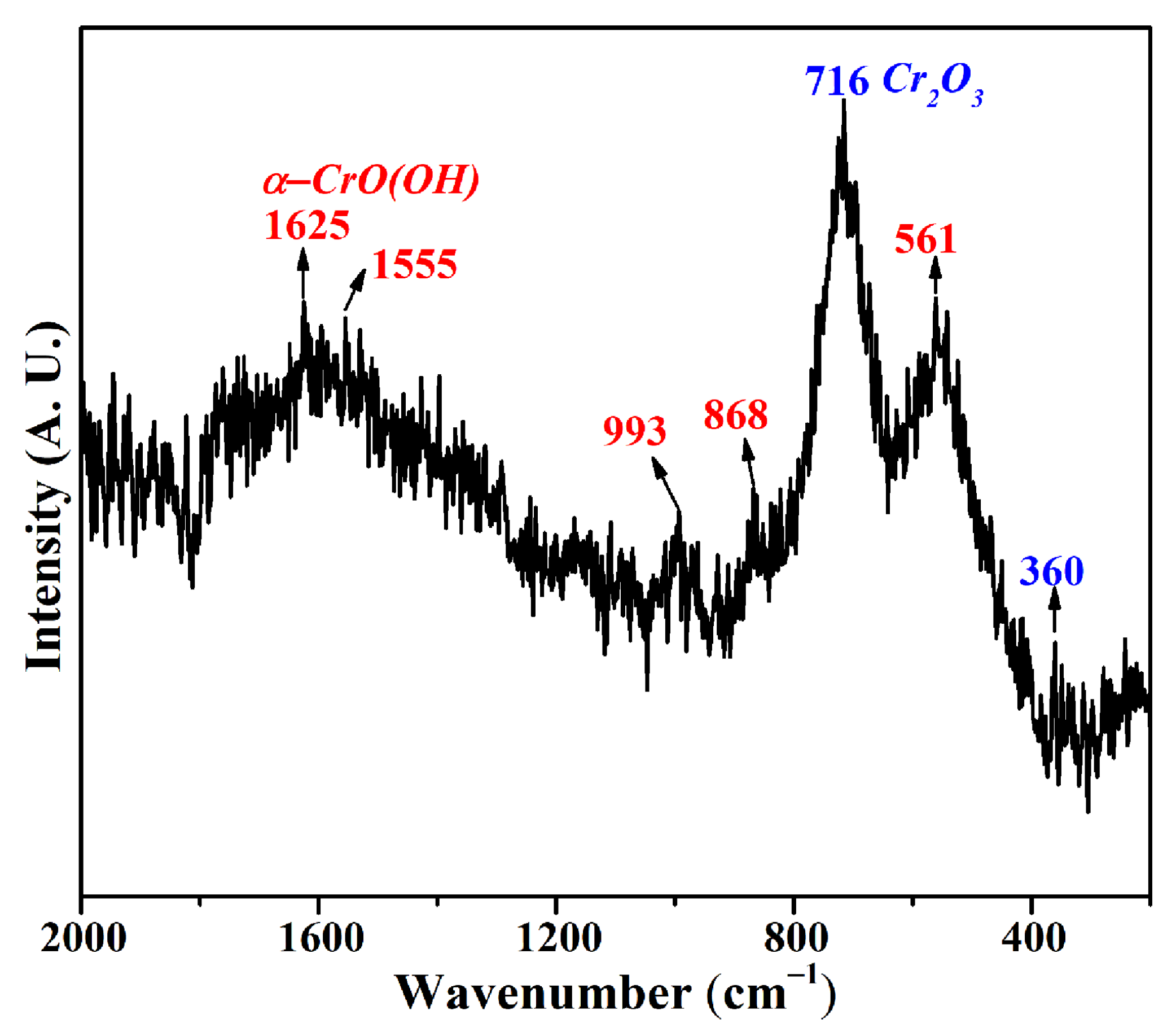
2.2.2. Chemical Composition and Phase Analysis
2.2.3. Permittivity Measurement
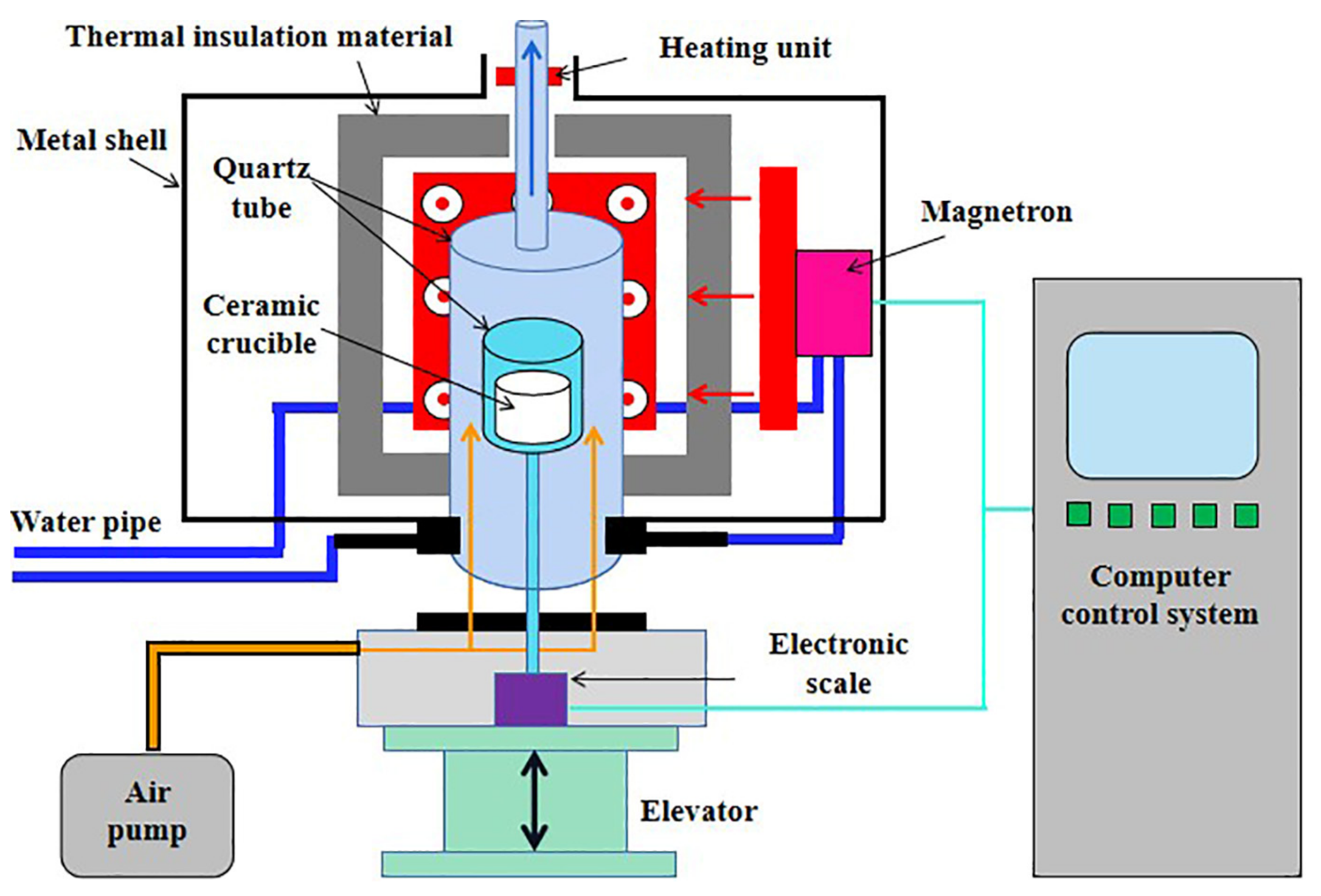
2.2.4. Microwave Drying
2.2.5. Conventional Drying
2.2.6. Determination of Drying Kinetics
Equations for Calculation of Drying Parameters
Kinetic Models
3. Results and Discussion
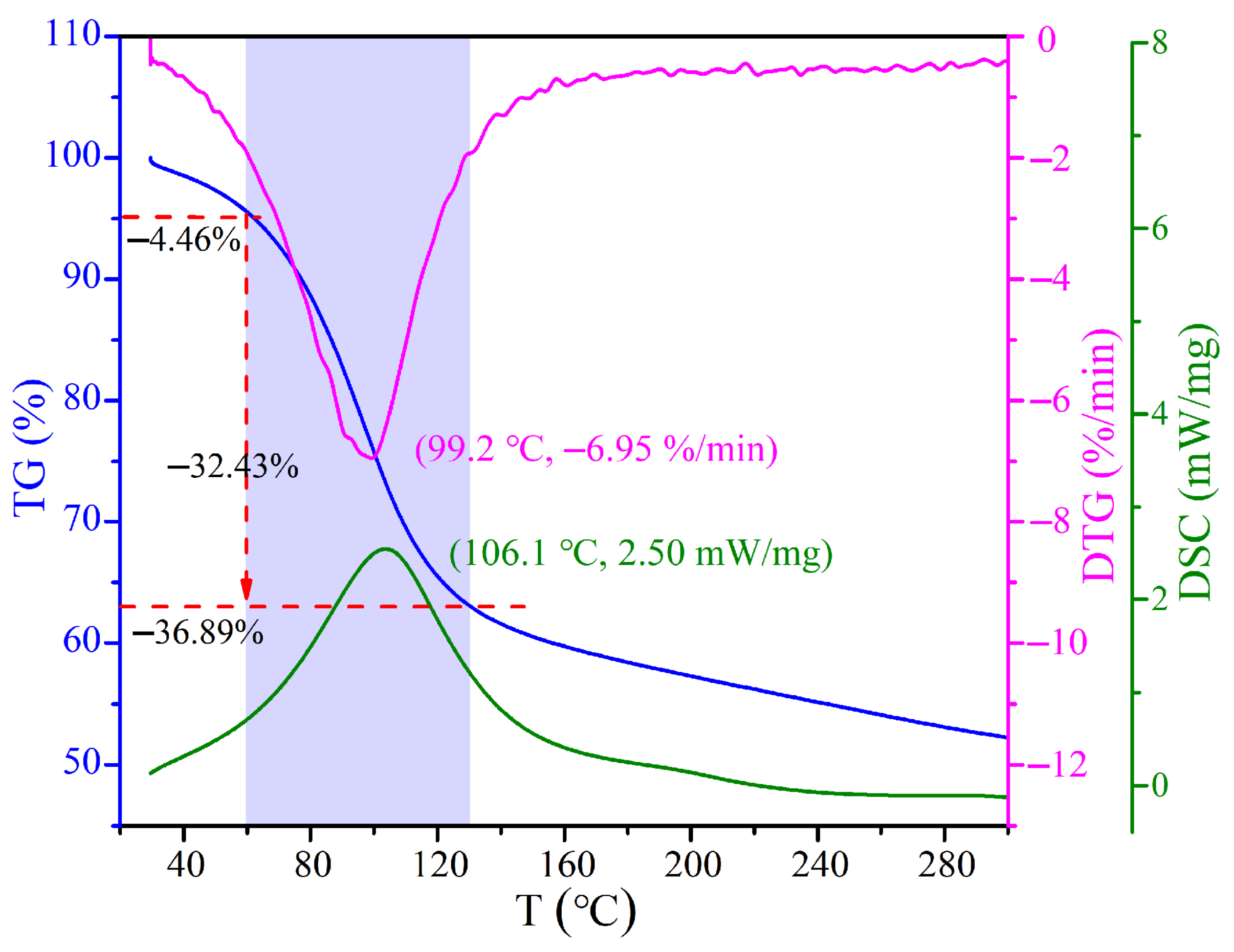
3.1. Physical and Chemical Properties
3.2. Microwave Response Characteristics of CRES
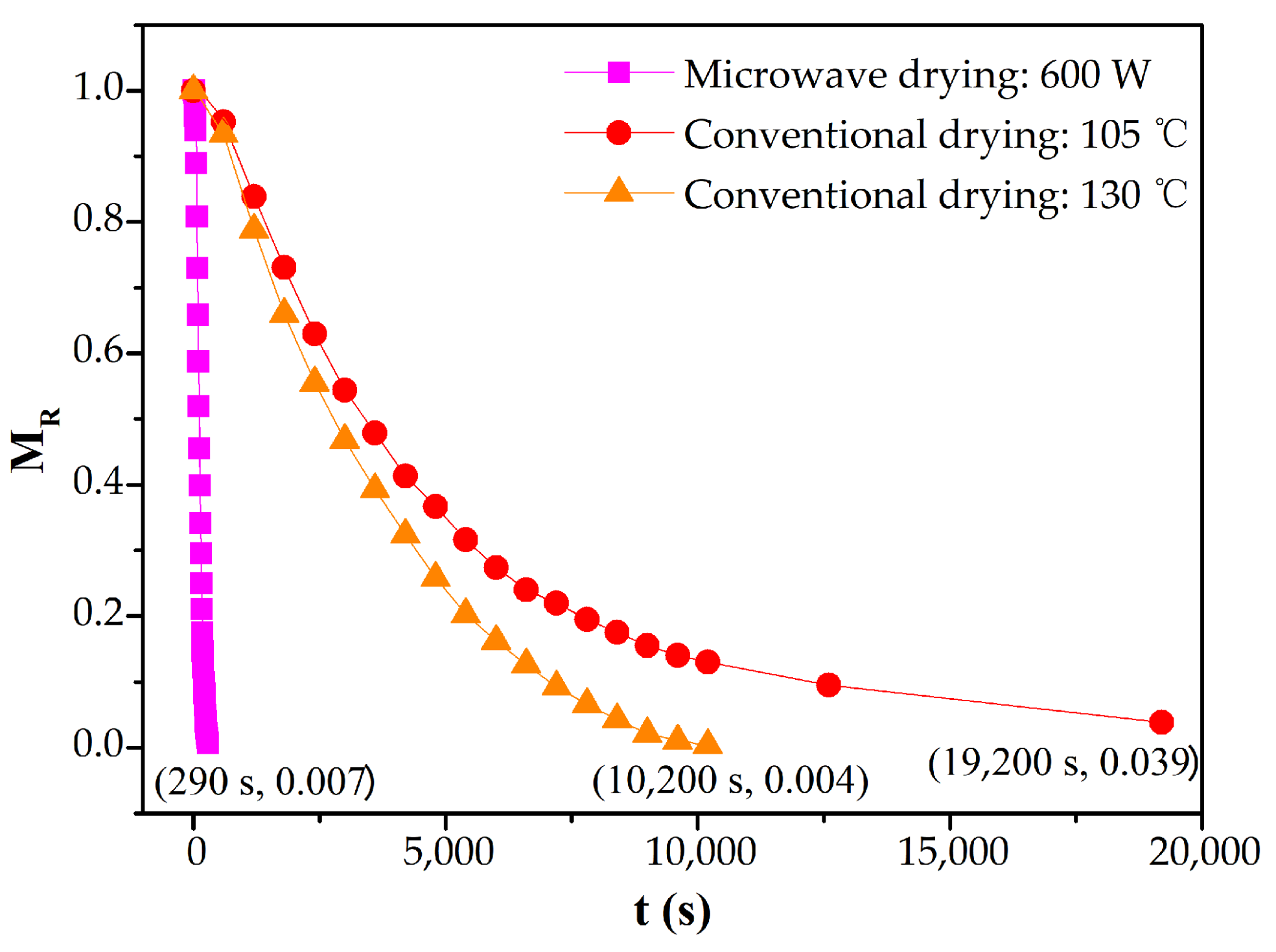
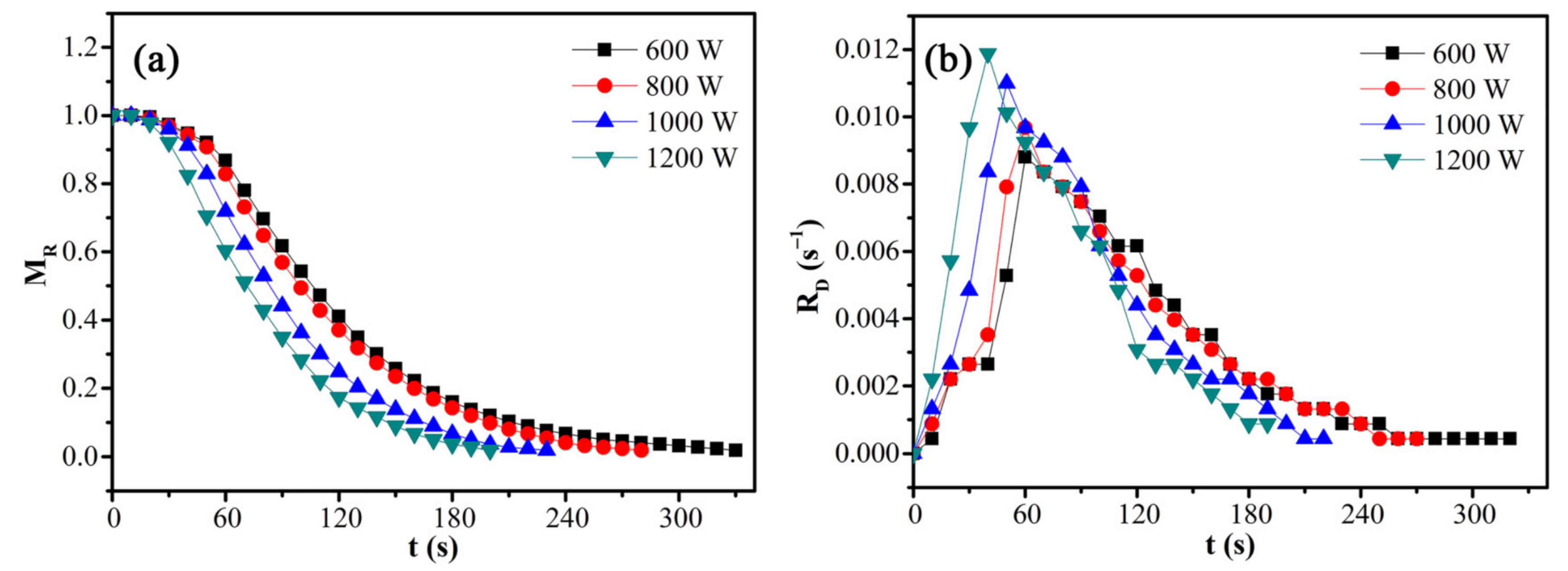
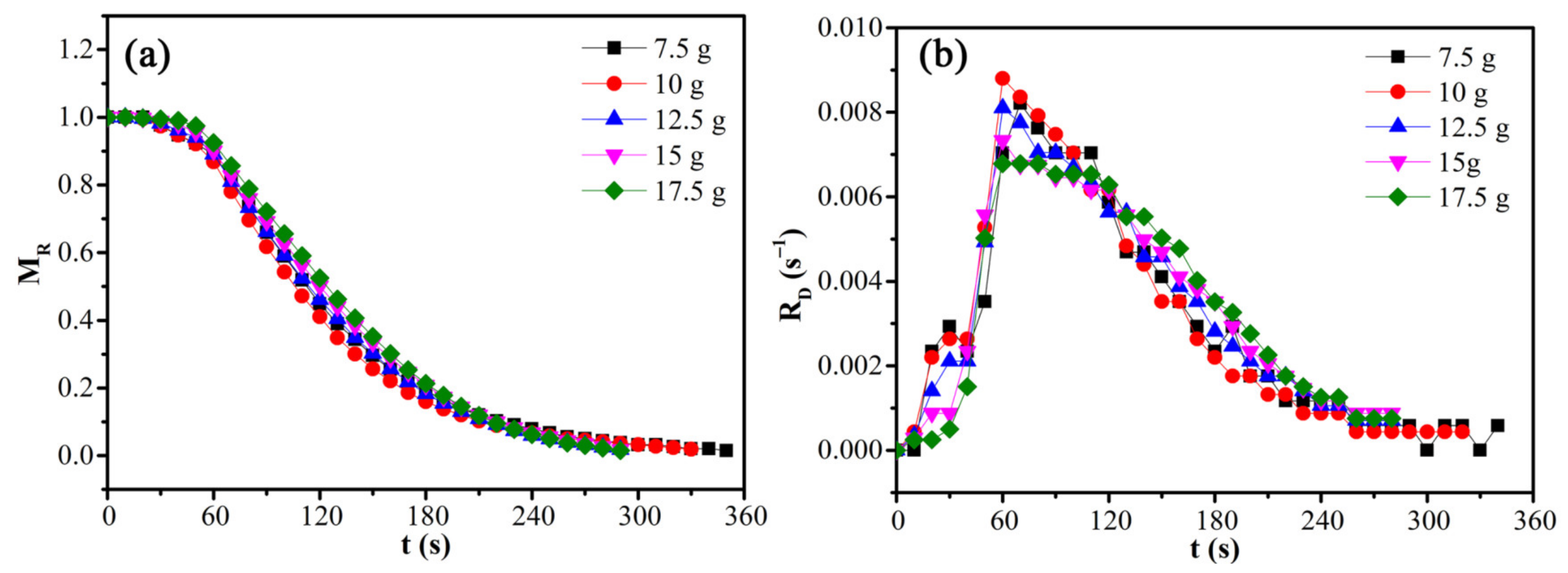
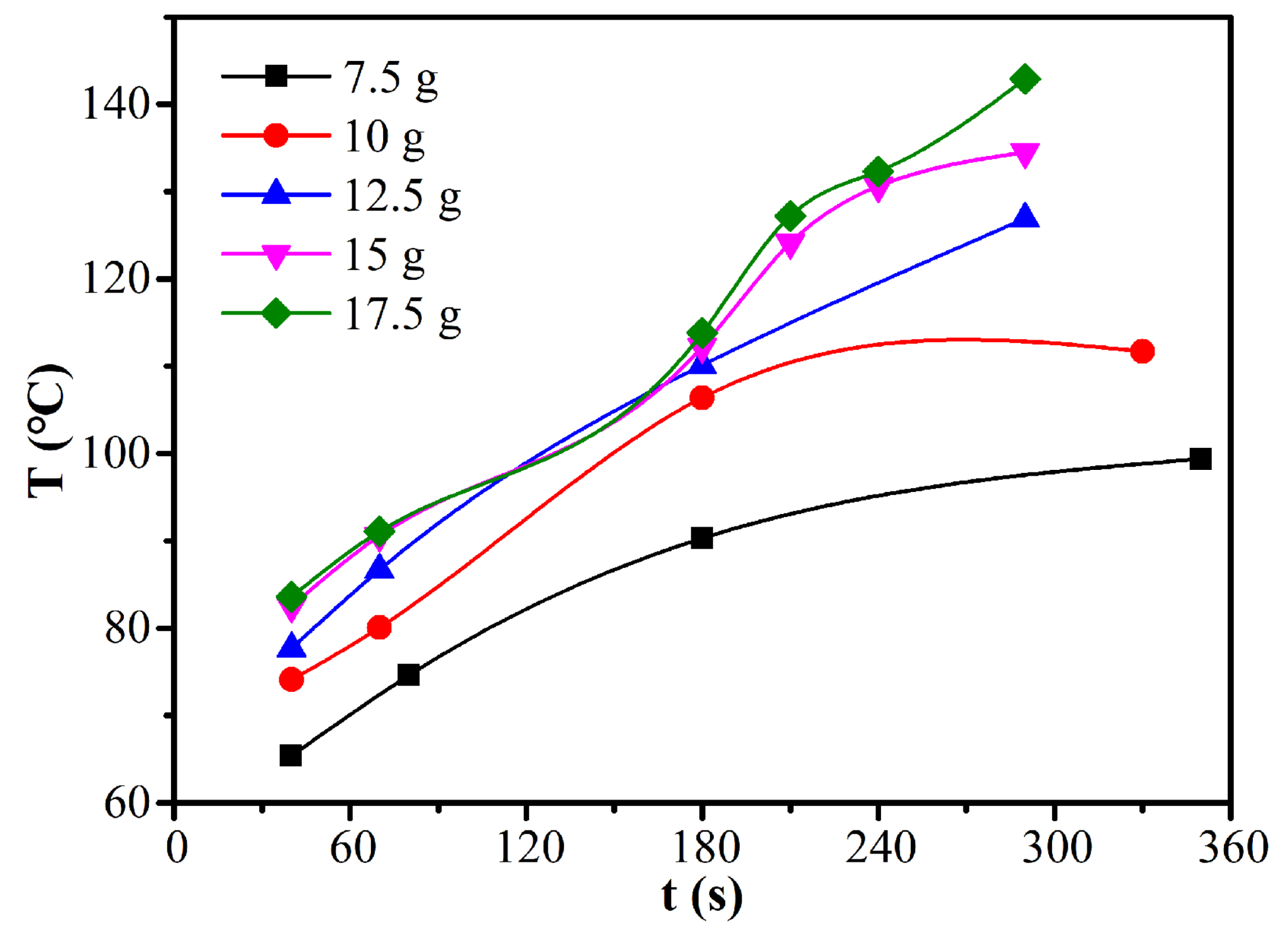
3.3. Microwave Drying Kinetics
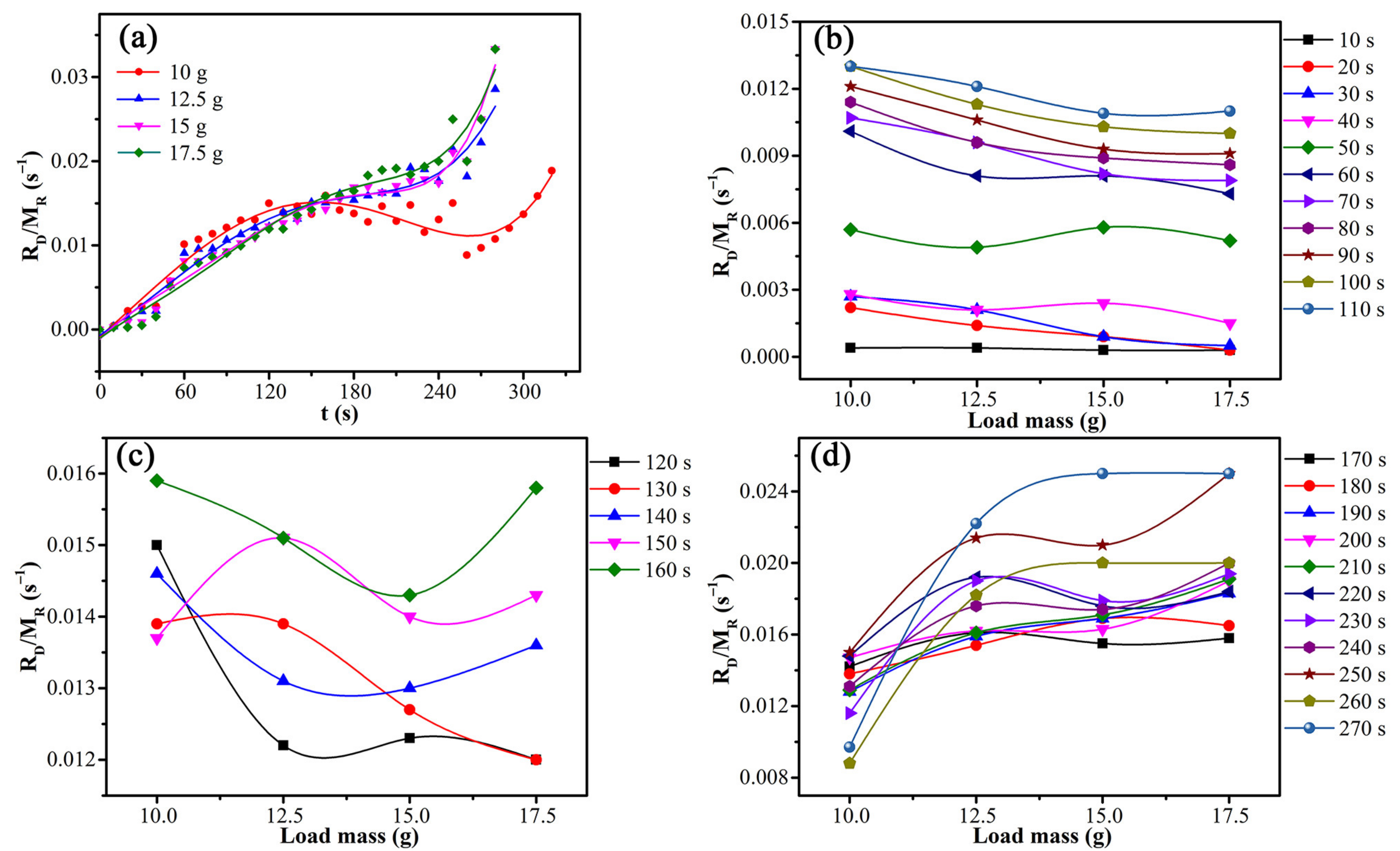
3.4. Analysis of the Rate-Limiting Step
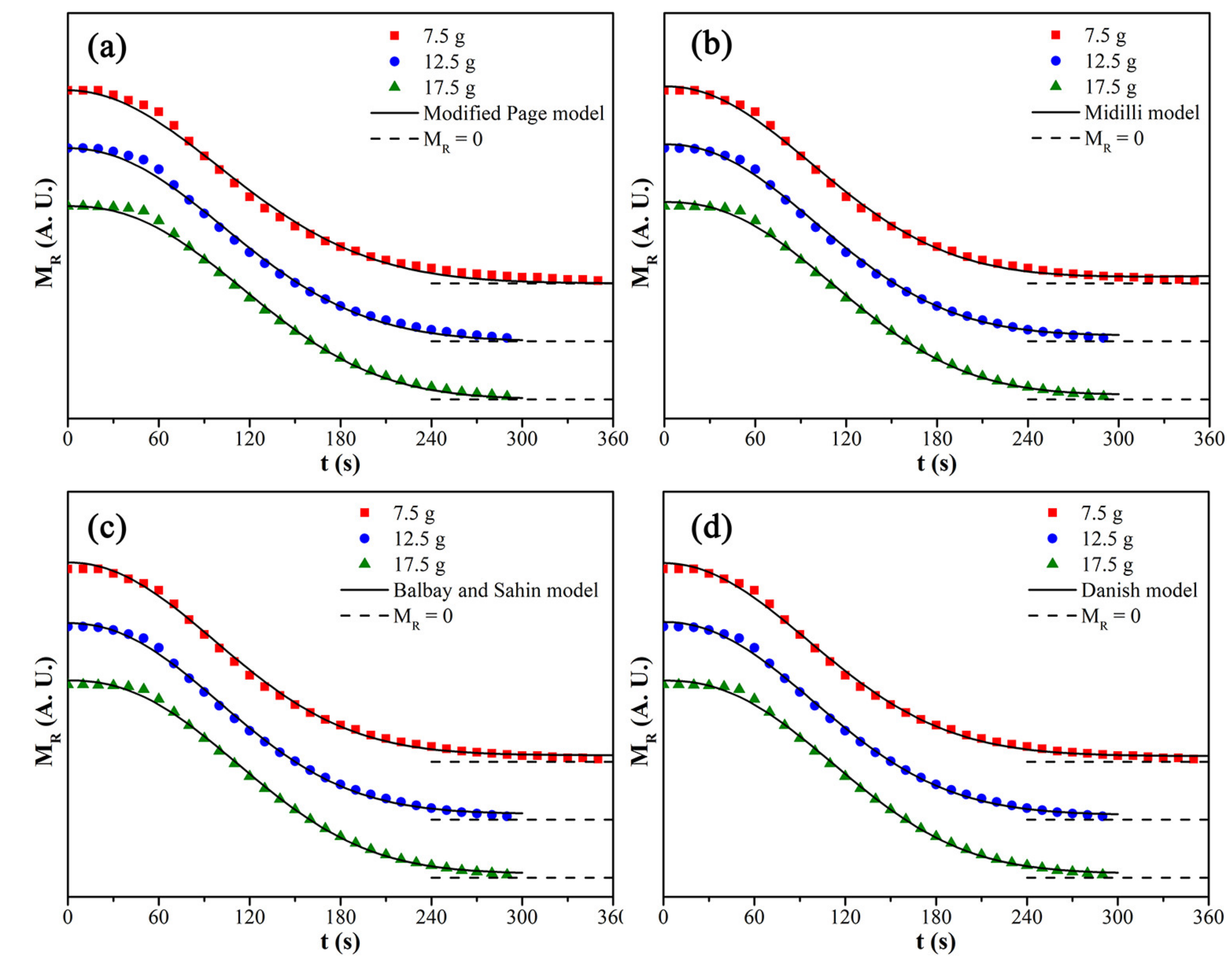
3.5. Fitting of Kinetic Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, K.; Liu, Z.; Li, Z.; Yue, R.; Guo, F.; Xu, Z. Selective separation of chromium from sulphuric acid leaching solutions of mixed electroplating sludge using phosphate precipitation. Hydrometallurgy 2019, 186, 42–49. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, J.; Zhang, J.; Zhang, J.; Chen, Z.; Ding, J.; Kong, F.; Qian, G.; Chen, J. Ferrite catalysts derived from electroplating sludge for high-calorie synthetic natural gas production. Appl. Catal. A-Gen. 2017, 534, 94–100. [Google Scholar] [CrossRef]
- Weng, C.; Sun, X.; Han, B.; Ye, X.; Zhong, Z.; Li, W.; Liu, W.; Deng, H.; Lin, Z. Targeted conversion of Ni in electroplating sludge to nickel ferrite nanomaterial with stable lithium storage performance. J. Hazard. Mater. 2020, 393, 122296. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, X.; Zhang, X.; Li, Y.; Song, X.; Wang, S. Electrochemical processes for the treatment of hazardous wastes exemplified by electroplating sludge leaching solutions. Water 2021, 13, 1576. [Google Scholar] [CrossRef]
- Rao, B.; Zhu, Y.; Yu, M.; Lu, X.; Wan, Y.; Huang, G.; Su, X.; Liu, X. High-dry dewatering of sludge based on different pretreatment conditions. Process Saf. Environ. Prot. 2019, 122, 288–297. [Google Scholar] [CrossRef]
- Fujimori, T.; Hayashi, H.; Nakajima, K. Phosphorus speciation in sludge from nickel electroplating. Mater. Trans. 2017, 58, 1337–1340. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Tan, Y.; Wang, L.; Chen, L.; Wu, Z.; Sasaki, K.; Mechtcherine, V.; Tsang, D.C.W. High-efficiency and low-carbon remediation of zinc contaminated sludge by magnesium oxysulfate cement. J. Hazard. Mater. 2021, 408, 124486. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, C.; Xie, J.; Wang, Z. The evolution of dehydration and thermal decomposition of nanocrystalline and amorphous chromium hydroxide. J. Anal. Appl. Pyrol. 2016, 118, 225–230. [Google Scholar] [CrossRef]
- Yenikaya, S.; Salihoglu, G.; Salihoglu, N.K.; Yenikaya, G. Microwave drying of automotive industry paint sludge. J. Hazard. Toxic Radioact. Waste 2018, 22, 04018015. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Martínez-Martínez, S.; Carrasco-Hurtado, B.; Eliche-Quesada, D.; Ureña-Nieto, C.; Sánchez-Soto, P.J. Valorization and inertization of galvanic sludge waste in clay bricks. Appl. Clay Sci. 2015, 105, 89–99. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q. Review on microwave-matter interaction fundamentals and efficient microwave-associated heating strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yan, B.; Yang, Y.; Zhu, H.; Huang, K. Accordion microwave oven for uniformity and efficiency heating. Int. J. RF Microw. Comput.-Aided Eng. 2019, 30, 22190. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, L.; Li, Z. Microwave drying behavior, energy consumption, and mathematical modeling of sewage sludge in a novel pilot-scale microwave drying system. Sci. Total Environ. 2021, 777, 146109. [Google Scholar] [CrossRef]
- Eom, H.; Jang, Y.H.; Lee, D.Y.; Kim, S.S.; Lee, S.M.; Cho, E.M. Optimization of a hybrid sludge drying system with flush drying and microwave drying technology. Chem. Eng. Res. Des. 2019, 148, 68–74. [Google Scholar] [CrossRef]
- Kocbek, E.; Garcia, H.A.; Hooijmans, C.M.; Mijatović, I.; Kržišnik, D.; Humar, M.; Brdjanovic, D. Effects of the sludge physical-chemical properties on its microwave drying performance. Sci. Total. Environ. 2022, 828, 154142. [Google Scholar] [CrossRef]
- Yang, B.; Yuan, W.; Ma, B.; Zhang, Y.; Yang, Y. Study on the experiment in solution of electroplating pretreatment by microwave drying. Environ. Sci. Technol. 2013, 26, 1–3. [Google Scholar]
- Kocbek, E.; Garcia, H.A.; Hooijmans, C.M.; Mijatović, I.; Lah, B.; Brdjanovic, D. Microwave treatment of municipal sewage sludge: Evaluation of the drying performance and energy demand of a pilot-scale microwave drying system. Sci. Total. Environ. 2020, 742, 140541. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, L.; Peng, J.; Ge, Y.; Tian, Z.; Sun, J.; Cheng, H.; Zhou, H. Cleaner utilization of electroplating sludge by bioleaching with a moderately thermophilic consortium: A pilot study. Chemosphere 2019, 232, 345–355. [Google Scholar] [CrossRef]
- Peng, Z.; Hwang, J.Y. Microwave-assisted metallurgy. Int. Mater. Rev. 2015, 60, 30–63. [Google Scholar] [CrossRef]
- Du, J.; Gao, L.; Yang, Y.; Guo, S.; Chen, J.; Omran, M.; Chen, G. Modeling and kinetics study of microwave heat drying of low grade manganese ore. Adv. Powder Technol. 2020, 31, 2901–2911. [Google Scholar] [CrossRef]
- Titze, T.; Lauerer, A.; Heinke, L.; Chmelik, C.; Zimmermann, N.E.R.; Keil, F.J.; Ruthven, D.M.; Kärger, J. Transport in nanoporous materials including MOFs: The applicability of Fick’s laws. Angew. Chem. Int. Ed. 2015, 54, 14580–14583. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Cai, C. Chemical Mass Transfer and Separation Process; Chemical Industry Press: Beijing, China, 2001; pp. 32–33. [Google Scholar]
- Zhou, Y.; Jian, Y. Mathematical modeling of thin-layer infrared drying of dewatered municipal sewage sludge (DWMSS). Procedia Environ. Sci. 2016, 31, 758–766. [Google Scholar] [CrossRef] [Green Version]
- Midilli, A.; Kucuk, H.; Yapar, Z. A new model for single-layer drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Balbay, A.; Sahin, O. Microwave drying kinetics of a thin-layer liquorice root. Dry. Technol. 2012, 30, 1503–1513. [Google Scholar] [CrossRef]
- Danish, M.; Hu, J.; Zhou, P.; Lou, Z.; Qian, P. A new drying kinetic model for sewage sludge drying in presence of CaO and NaClO. Appl. Therm. Eng. 2016, 106, 141–152. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Yang, J.; Martens, W.N.; Frost, R.L. Transition of chromium oxyhydroxide nanomaterials to chromium oxide: A hot-stage Raman spectroscopic study. J. Raman Spectrosc. 2011, 42, 1142–1146. [Google Scholar] [CrossRef]


| No. | Model | Equation | Reference |
|---|---|---|---|
| 1 | Modified Page | MR = exp (−(kt)n) | [20,23] |
| 2 | Midilli | MR = a∙exp (−ktn) + bt | [24] |
| 3 | Balbay and Sahin | MR = (1−a)∙exp (−ktn) + b | [25] |
| 4 | Danish | MR = exp (−ktn) + b | [26] |
| Element | Cr | Fe | Ca | S | Cu | Zn | Pb | Ni |
|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 36.85 | 4.64 | 2.32 | 2.03 | 0.24 | 0.24 | 0.22 | 0.03 |
| Element | Na | Cl | Mg | Al | Si | P | K | |
| Content (wt%) | 0.03 | 0.01 | 0.31 | 0.17 | 0.22 | 0.08 | 0.01 |
| MR | tanδε | Dp (mm) | ||
| 1.000 | 4.574 | 1.705 | 0.373 | 34.72 |
| 0.474 | 3.299 | 0.513 | 0.156 | 96.69 |
| 0.374 | 2.961 | 0.347 | 0.117 | 135.25 |
| 0.256 | 2.590 | 0.312 | 0.120 | 140.70 |
| 0 | 2.687 | 0.097 | 0.036 | 460.20 |
| Microwave Power (W) | 600 | 800 | 1000 | 1200 |
|---|---|---|---|---|
| Average drying rate (s−1) | 0.00297 | 0.00350 | 0.00427 | 0.00491 |
| Maximum drying rate (s−1) | 0.00870 | 0.00957 | 0.0109 | 0.0117 |
| Temperature at the maximum drying rate (°C) | 80.1 | 81.8 | 83.9 | 84.2 |
| End-point temperature (°C) | 113.7 | 113.3 | 113.5 | 115.3 |
| Load Mass (g) | 7.5 | 10 | 12.5 | 15 | 17.5 |
|---|---|---|---|---|---|
| Material layer thickness (mm) | 7.80 | 10.39 | 12.99 | 15.59 | 18.19 |
| Average drying rate (s−1) | 0.00282 | 0.00297 | 0.00339 | 0.00339 | 0.00340 |
| Maximum drying rate (s−1) | 0.00812 | 0.00870 | 0.00800 | 0.00725 | 0.00671 |
| Particle Size (mm) | 0.15–0.3 | 0.3–0.6 | 0.6–1.25 | 1.25–2.5 |
|---|---|---|---|---|
| Average drying rate (s−1) | 0.00379 | 0.00364 | 0.00339 | 0.00339 |
| Maximum drying rate (s−1) | 0.00912 | 0.00870 | 0.00800 | 0.00780 |
| Temperature at maximum drying rate (°C) | 89.4 | 89.1 | 86.7 | 84 |
| End-point temperature (°C) | 135.4 | 131.3 | 126.9 | 117.4 |
| Model | Load Mass (g) | Parameters | R2 | RSS | AIC | |
|---|---|---|---|---|---|---|
| Modified Page | 7.5 | k = 0.00713, n = 2.00734 | 0.996 | 0.01968 | 5.788 × 10−4 | −266.4 |
| 12.5 | k = 0.00717, n = 2.11124 | 0.997 | 0.01066 | 3.806 × 10−4 | −234.3 | |
| 17.5 | k = 0.00673, n = 2.30727 | 0.998 | 0.00926 | 3.307 × 10−4 | −238.5 | |
| Midilli | 7.5 | a = 1.01993, b = 1.04033 × 10−4, k = 4.04004 × 10−5, n = 2.06505 | 0.998 | 0.00847 | 2.646 × 10−4 | −292.8 |
| 12.5 | a = 1.02011, b = 9.60889 × 10−5, k = 3.01866 × 10−5, n = 2.11995 | 0.998 | 0.00537 | 2.066 × 10−4 | −250.8 | |
| 17.5 | a = 1.02095, b = 7.14532 × 10−5, k = 1.18911 × 10−5, n = 2.28123 | 0.999 | 0.00502 | 1.930 × 10−4 | −252.9 | |
| Balbay and Sahin | 7.5 | a = 0.01243, b = 0.0342, k = 3.65816 × 10−5, n = 2.0911 | 0.998 | 0.00751 | 2.348 × 10−4 | −297.1 |
| 12.5 | a = 0.01169, b = 0.03036, k = 2.41858 × 10−5, n = 2.17321 | 0.999 | 0.00528 | 2.030 × 10−4 | −251.4 | |
| 17.5 | a = −7.50411 × 10−4, b = 0.02087, k = 1.08217 × 10−5, n = 2.30206 | 0.999 | 0.00504 | 1.937 × 10−4 | −252.7 | |
| Danish | 7.5 | b = 0.02988, k = 4.65995 × 10−5, n = 2.04148 | 0.998 | 0.00784 | 2.377 × 10−4 | −297.6 |
| 12.5 | b = 0.0244, k = 3.1433 × 10−5, n = 2.11821 | 0.999 | 0.00533 | 1.976 × 10−4 | −253.1 | |
| 17.5 | b = 0.02126, k = 1.06884 × 10−5, n = 2.30461 | 0.999 | 0.00504 | 1.866 × 10−4 | −254.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Peng, Z.; Luo, G.; Tian, R.; Rao, M. Microwave Drying Kinetics of Chromium-Rich Electroplating Sludge. Metals 2023, 13, 87. https://doi.org/10.3390/met13010087
Zhang J, Peng Z, Luo G, Tian R, Rao M. Microwave Drying Kinetics of Chromium-Rich Electroplating Sludge. Metals. 2023; 13(1):87. https://doi.org/10.3390/met13010087
Chicago/Turabian StyleZhang, Jian, Zhiwei Peng, Guanwen Luo, Ran Tian, and Mingjun Rao. 2023. "Microwave Drying Kinetics of Chromium-Rich Electroplating Sludge" Metals 13, no. 1: 87. https://doi.org/10.3390/met13010087





