Synthesis of Metal Nanoparticles under Microwave Irradiation: Get Much with Less Energy
Abstract
:1. Introduction
2. Noble Metals and Silver
2.1. Silver Nanoparticles (NPs)
2.2. Gold Particles
2.3. Palladium Particles
2.4. Platinum Particles
2.5. Other Noble Metal Nanoparticles
3. Non-Noble Metal Nanoparticles
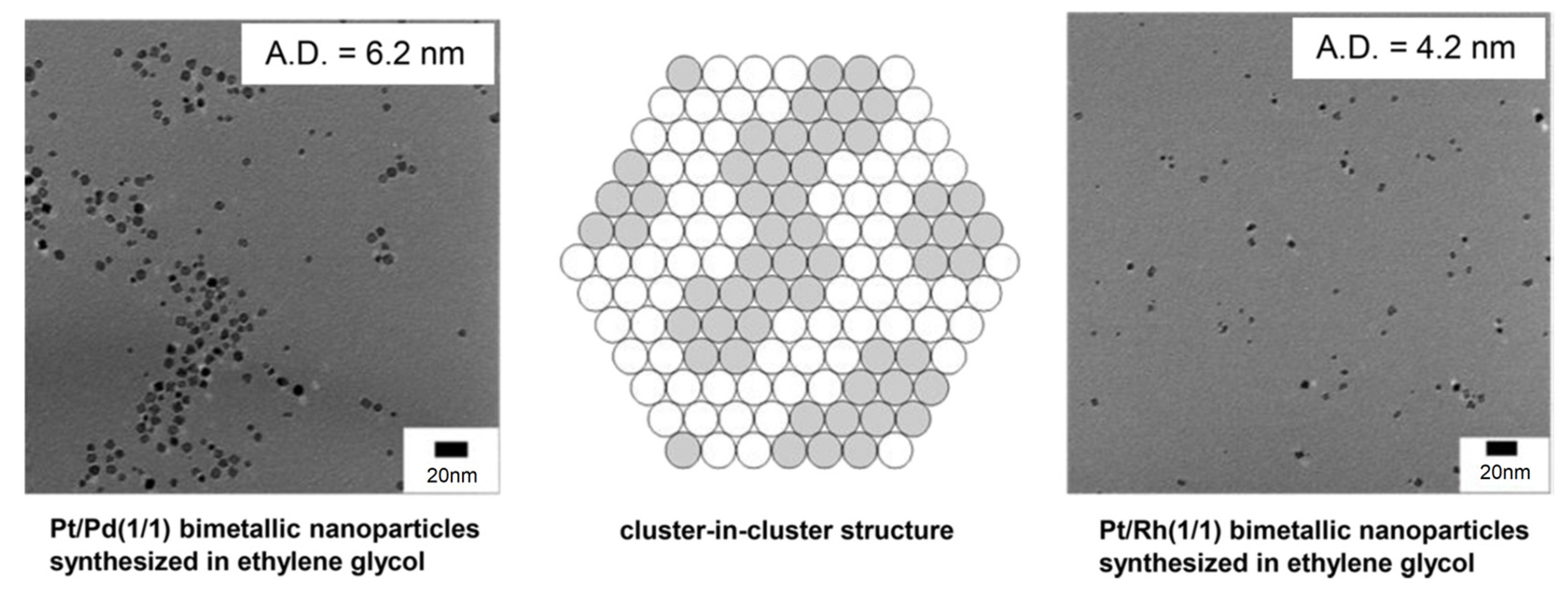
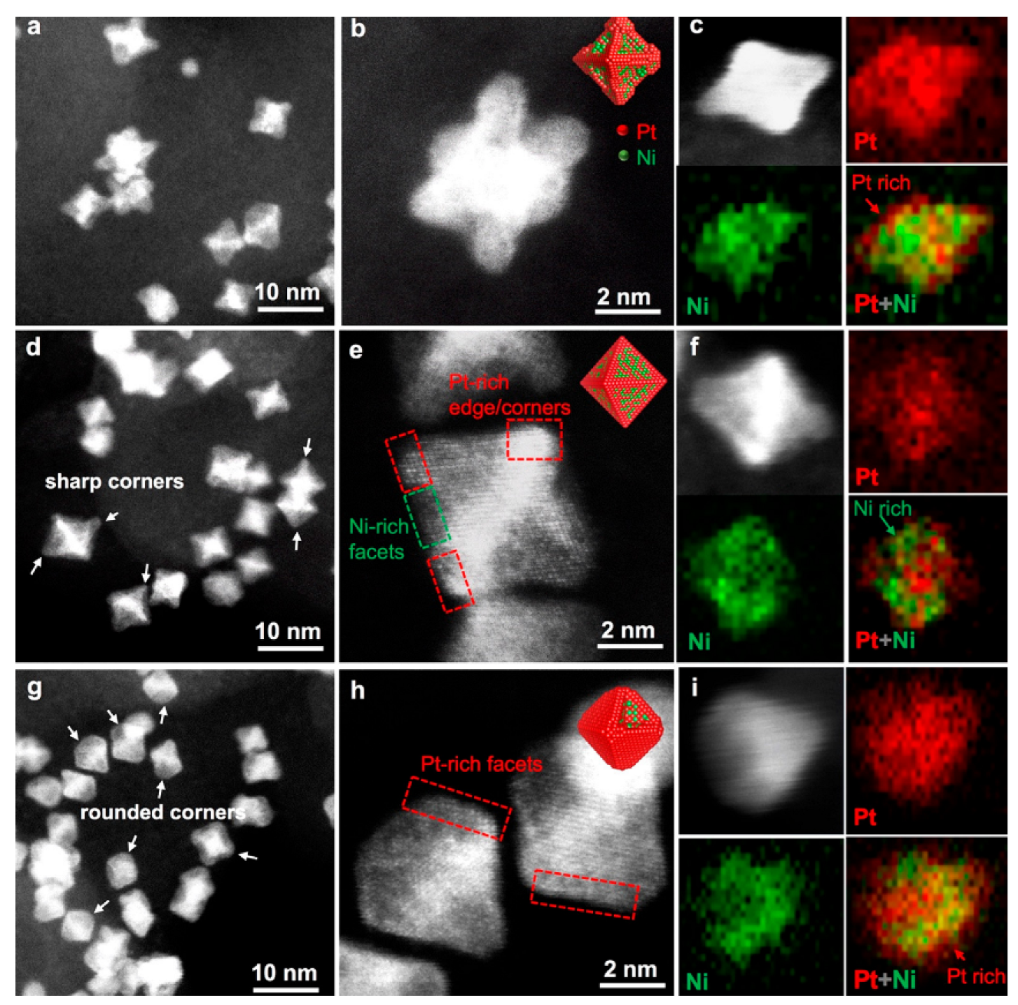
4. Bi- and Trimetallic Nanoparticles
5. Composites Based on Metal Nanoparticles
6. Comparison of the Microwave Method of Metal Nanoparticles Synthesis with Conventional Heating
7. Future Perspectives
- Continuous mode of operation (flow-type reactors for the synthesis of metal nanoparticles under microwave heating, suitable for unsupported nanoparticles only);
- In-depth exploration of specific or nonthermal effects in the course of metal nanoparticle preparation;
- Optimization of the frequency range beyond 2.45 GHz; there are some grounds to believe that higher frequencies are much more efficient in the microwave-assisted syntheses of nanomaterials, but this is a subject for a separate review;
- More inventive use of solvents with a strong dependence on the tangent of losses on temperature, like ionic liquids and deep eutectics solvents;
- More attention should be paid to the early stages of the synthesis (seconds to a minute); in many publications, it looks like the authors missed the very early stages and the consecutive transformations of the shapes from dots (spheres) to more complicated morphologies;
- Synthesis of metal carbides (underexplored so far);
- More inventive synthesis of hybrid nanomaterials;
- Synthesis of quasicrystals;
- Synthesis of immiscible phases (like immiscible metals);
- Heuristic syntheses of metastable phases (novel catalytically active materials);
- Original procedures of preparation of defect materials with control of the defect nature and concentration;
- Development of new MW-absorbing materials and compounds, including stimuli-responsive materials, in particular, materials and compounds with high sensitivity to MW frequency;
- Use of greener solvent mixtures;
- MW syntheses under elevated pressures (up to the supercritical region);
- Wide use of seeding in the synthesis of core–shell and other nanomaterials,
- Combination of the microwave and plasma-assisted preparation,
- MW syntheses combined with other unique methodologies (electrochemical, sonochemical, mechanochemical, photochemical, radiation-induced, and solid-state synthesis).
Author Contributions
Funding
Conflicts of Interest
References
- Ishii, T.K. Industrial Applications of Microwaves. In Handbook of Microwave Technology; Academic Press: San Diego, CA, USA, 1995; pp. 277–307. [Google Scholar]
- de la Hoz, A.; Díaz-Ortiz, À.; Moreno, A. Microwaves in Organic Synthesis. Thermal and Non-Thermal Microwave Effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.J.; Vaidhyanathan, B.; Ganguli, M.; Ramakrishnan, P.A. Synthesis of Inorganic Solids Using Microwaves. Chem. Mater. 1999, 11, 882–895. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-Assisted Synthesis of Colloidal Inorganic Nanocrystals. Angew. Chem.-Int. Ed. 2011, 50, 11312–11359. [Google Scholar]
- Tsukahara, Y.; Higashi, A.; Yamauchi, T.; Nakamura, T.; Yasuda, M.; Baba, A.; Wada, Y. In Situ Observation of Nonequilibrium Local Heating as an Origin of Special Effect of Microwave on Chemistry. J. Phys. Chem. C 2010, 114, 8965–8970. [Google Scholar] [CrossRef]
- Pathak, D.D.; Grover, V. Mechanochemistry: Synthesis That Uses Force. In Handbook on Synthesis Strategies for Advanced Materials; Tyagi, A.K., Ningthoujam, R.S., Eds.; Indian Institute of Metals Series; Springer: Singapore, 2021; pp. 657–682. [Google Scholar]
- Bilecka, I.; Niederberger, M. Microwave Chemistry for Inorganic Nanomaterials Synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar]
- Tsuji, M.; Hashimoto, M.; Nishizawa, Y.; Kubokawa, M.; Tsuji, T. Microwave-Assisted Synthesis of Metallic Nanostructures in Solution. Chem.-A Eur. J. 2005, 11, 440–452. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Cherepanova, V.A.; Ananikov, V.P. Dynamic Behavior of Metal Nanoparticles in Pd/C and Pt/C Catalytic Systems under Microwave and Conventional Heating. ACS Appl. Mater. Interfaces 2017, 9, 36723–36732. [Google Scholar] [CrossRef]
- Tu, W.; Liu, H. Continuous Synthesis of Colloidal Metal Nanoclusters by Microwave Irradiation. Chem. Mater. 2000, 12, 564–567. [Google Scholar] [CrossRef]
- Komarneni, S.; Li, D.; Newalkar, B.; Katsuki, H.; Bhalla, A.S. Microwave−Polyol Process for Pt and Ag Nanoparticles. Langmuir 2002, 18, 5959–5962. [Google Scholar] [CrossRef]
- Yu, W.; Tu, W.; Liu, H. Synthesis of Nanoscale Platinum Colloids by Microwave Dielectric Heating. Langmuir 1999, 15, 6–9. [Google Scholar] [CrossRef]
- Anumol, E.A.; Kundu, P.; Deshpande, P.A.; Madras, G.; Ravishankar, N. New Insights into Selective Heterogeneous Nucleation of Metal Nanoparticles on Oxides by Microwave-Assisted Reduction: Rapid Synthesis of High-Activity Supported Catalysts. ACS Nano 2011, 5, 8049–8061. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Liu, H. Rapid Synthesis of Nanoscale Colloidal Metal Clusters by Microwave Irradiation. J. Mater. Chem. 2000, 10, 2207–2211. [Google Scholar] [CrossRef]
- Kou, J.; Bennett-Stamper, C.; Varma, R.S. Green Synthesis of Noble Nanometals (Au, Pt, Pd) Using Glycerol under Microwave Irradiation Conditions. ACS Sustain. Chem. Eng. 2013, 1, 810–816. [Google Scholar] [CrossRef]
- Mallikarjuna, N.N.; Varma, R.S. Microwave-Assisted Shape-Controlled Bulk Synthesis of Noble Nanocrystals and Their Catalytic Properties. Cryst. Growth Des. 2007, 7, 686–690. [Google Scholar] [CrossRef]
- Zhu, J.F.; Zhu, Y.J. Microwave-Assisted One-Step Synthesis of Polyacrylamide-Metal (M = Ag, Pt, Cu) Nanocomposites in Ethylene Glycol. J. Phys. Chem. B 2006, 110, 8593–8597. [Google Scholar] [CrossRef]
- Harada, M.; Cong, C. Microwave-Assisted Polyol Synthesis of Polymer-Protected Monometallic Nanoparticles Prepared in Batch and Continuous-Flow Processing. Ind. Eng. Chem. Res. 2016, 55, 5634–5643. [Google Scholar] [CrossRef]
- Sumi, T.; Dillert, R.; Horikoshi, S. Utilization of the Microwave Electric or Magnetic Field in the Synthesis of Monometallic and Bimetallic Nanoparticles. RSC Adv. 2015, 5, 14637–14645. [Google Scholar] [CrossRef]
- Xu, S.; Zhong, G.; Chen, C.; Zhou, M.; Kline, D.J.; Jacob, R.J.; Xie, H.; He, S.; Huang, Z.; Dai, J.; et al. Uniform, Scalable, High-Temperature Microwave Shock for Nanoparticle Synthesis through Defect Engineering. Matter 2019, 1, 759–769. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Z.; Wang, L.; Xi, K.; Cao, Q.; Wang, D.; Yang, Y.; Du, Y. Excellent Microwave Absorption Property of Graphene-Coated Fe Nanocomposites. Sci. Rep. 2013, 3, 3421. [Google Scholar] [CrossRef]
- Qi, X.; Hu, Q.; Xu, J.; Xie, R.; Bai, Z.; Jiang, Y.; Qin, S.; Zhong, W.; Du, Y. Enhanced Microwave Absorption Properties and Mechanism of Core/Shell Structured Magnetic Nanoparticles/Carbon-Based Nanohybrids. Mater. Sci. Eng. B Solid. State Mater. Adv. Technol. 2016, 211, 3421. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver Nanoparticles: Green Synthesis and Their Antimicrobial Activities. Adv. Colloid. Interface Sci. 2009, 145, 83–96. [Google Scholar] [PubMed]
- Saloga, P.E.J.; Kästner, C.; Thünemann, A.F. High-Speed but Not Magic: Microwave-Assisted Synthesis of Ultra-Small Silver Nanoparticles. Langmuir 2018, 34, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, S.B.; Wang, K.; Zhang, M.; Yu, S.H. Microwave-Assisted Rapid Facile “Green” Synthesis of Uniform Silver Nanoparticles: Self-Assembly into Multilayered Films and Their Optical Properties. J. Phys. Chem. C 2008, 112, 11169–11174. [Google Scholar] [CrossRef]
- Singh, A.K.; Raykar, V.S. Microwave Synthesis of Silver Nanofluids with Polyvinylpyrrolidone (PVP) and Their Transport Properties. Colloid. Polym. Sci. 2008, 286, 1667–1673. [Google Scholar] [CrossRef]
- Pal, A.; Shah, S.; Devi, S. Microwave-Assisted Synthesis of Silver Nanoparticles Using Ethanol as a Reducing Agent. Mater. Chem. Phys. 2009, 114, 530–532. [Google Scholar] [CrossRef]
- Jiang, H.; Moon, K.S.; Zhang, Z.; Pothukuchi, S.; Wong, C.P. Variable Frequency Microwave Synthesis of Silver Nanoparticles. J. Nanoparticle Res. 2006, 8, 117–124. [Google Scholar] [CrossRef]
- Tsuji, M.; Nishizawa, Y.; Matsumoto, K.; Miyamae, N.; Tsuji, T.; Zhang, X. Rapid Synthesis of Silver Nanostructures by Using Microwave-Polyol Method with the Assistance of Pt Seeds and Polyvinylpyrrolidone. Colloids Surf. A Physicochem. Eng. Asp. 2007, 293, 185–194. [Google Scholar] [CrossRef]
- Liu, S.; Lu, F.; Zhu, J.J. Highly Fluorescent Ag Nanoclusters: Microwave-Assisted Green Synthesis and Cr3+ Sensing. Chem. Commun. 2011, 47, 2661–2663. [Google Scholar] [CrossRef]
- Manno, R.; Sebastian, V.; Mallada, R.; Santamaria, J. 110th Anniversary: Nucleation of Ag Nanoparticles in Helical Microfluidic Reactor. Comparison between Microwave and Conventional Heating. Ind. Eng. Chem. Res. 2019, 58, 12702–12711. [Google Scholar] [CrossRef]
- Horikoshi, S.; Sumi, T.; Serpone, N. A Hybrid Microreactor/Microwave High-Pressure Flow System of a Novel Concept Design and Its Application to the Synthesis of Silver Nanoparticles. Chem. Eng. Process. Process Intensif. 2013, 73, 59–66. [Google Scholar] [CrossRef]
- Horikoshi, S.; Abe, H.; Torigoe, K.; Abe, M.; Serpone, N. Access to Small Size Distributions of Nanoparticles by Microwave-Assisted Synthesis. Formation of Ag Nanoparticles in Aqueous Carboxymethylcellulose Solutions in Batch and Continuous-Flow Reactors. Nanoscale 2010, 2, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, M.; Miyakawa, M.; Kataoka, H.; Koda, H.; Sato, K.; Suzuki, T.M. Continuous Synthesis of Monodispersed Silver Nanoparticles Using a Homogeneous Heating Microwave Reactor System. Nanoscale 2011, 3, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Özkar, S.; Finke, R.G. Silver Nanoparticles Synthesized by Microwave Heating: A Kinetic and Mechanistic Re-Analysis and Re-Interpretation. J. Phys. Chem. C 2017, 121, 27643–27654. [Google Scholar] [CrossRef]
- Dzido, G.; Markowski, P.; Małachowska-Jutsz, A.; Prusik, K.; Jarzębski, A.B. Rapid Continuous Microwave-Assisted Synthesis of Silver Nanoparticles to Achieve Very High Productivity and Full Yield: From Mechanistic Study to Optimal Fabrication Strategy. J. Nanoparticle Res. 2015, 17, 27. [Google Scholar] [CrossRef]
- Tsuji, M.; Matsumoto, K.; Jiang, P.; Matsuo, R.; Tang, X.L.; Kamarudin, K.S.N. Roles of Pt Seeds and Chloride Anions in the Preparation of Silver Nanorods and Nanowires by Microwave-Polyol Method. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 266–277. [Google Scholar] [CrossRef]
- Gou, L.; Chipara, M.; Zaleski, J.M. Convenient, Rapid Synthesis of Ag Nanowires. Chem. Mater. 2007, 19, 1755–1760. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Synthesis of Silver Nanoprisms in DMF. Nano Lett. 2002, 2, 903–905. [Google Scholar] [CrossRef]
- He, R.; Qian, X.; Yin, J.; Zhu, Z. Preparation of Polychrome Silver Nanoparticles in Different Solvents. J. Mater. Chem. 2002, 12, 3783–3786. [Google Scholar] [CrossRef]
- He, R.; Qian, X.; Yin, J.; Zhu, Z. Formation of Silver Dendrites under Microwave Irradiation. Chem. Phys. Lett. 2003, 369, 454–458. [Google Scholar] [CrossRef]
- Yamamoto, T.; Wada, Y.; Sakata, T.; Mori, H.; Goto, M.; Hibino, S.; Yanagida, S. Microwave-Assisted Preparation of Silver Nanoparticles. Chem. Lett. 2004, 33, 158–159. [Google Scholar] [CrossRef]
- Tsuji, M.; Hashimoto, M.; Nishizawa, Y.; Tsuji, T. Synthesis of Gold Nanorods and Nanowires by a Microwave-Polyol Method. Mater. Lett. 2004, 58, 2326–2330. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yin, H.; Wada, Y.; Kitamura, T.; Sakata, T.; Mori, H.; Yanagida, S. Morphology-Control in Microwave-Assisted Synthesis of Silver Particles in Aqueous Solutions. Bull. Chem. Soc. Jpn. 2004, 77, 757–761. [Google Scholar] [CrossRef]
- Tsuji, M.; Nishizawa, Y.; Hashimoto, M.; Tsuji, T. Syntheses of Silver Nanofilms, Nanorods, and Nanowires by a Microwave-Polyol Method in the Presence of Pt Seeds and Polyvinylpyrrolidone. Chem. Lett. 2004, 33, 370–371. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Hu, X.-L. Microwave-Assisted Polythiol Reduction Method: A New Solid–Liquid Route to Fast Preparation of Silver Nanowires. Mater. Lett. 2004, 58, 1517–1519. [Google Scholar] [CrossRef]
- Yin, H.; Yamamoto, T.; Wada, Y.; Yanagida, S. Large-Scale and Size-Controlled Synthesis of Silver Nanoparticles under Microwave Irradiation. Mater. Chem. Phys. 2004, 83, 66–70. [Google Scholar] [CrossRef]
- Nirmala Grace, A.; Pandian, K. One Pot Synthesis of Polymer Protected Pt, Pd, Ag and Ru Nanoparticles and Nanoprisms under Reflux and Microwave Mode of Heating in Glycerol-A Comparative Study. Mater. Chem. Phys. 2007, 104, 191–198. [Google Scholar] [CrossRef]
- Hebbalalu, D.; Lalley, J.; Nadagouda, M.N.; Varma, R.S. Greener Techniques for the Synthesis of Silver Nanoparticles Using Plant Extracts, Enzymes, Bacteria, Biodegradable Polymers, and Microwaves. ACS Sustain. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Zhang, X.; Jin, Y. Microwave-Assisted Green Synthesis of Silver Nanoparticles by Carboxymethyl Cellulose Sodium and Silver Nitrate. Mater. Chem. Phys. 2008, 108, 421–424. [Google Scholar] [CrossRef]
- Tohidi, M.; Ghanbari, A.; Honarasa, F. Synthesis of Copper and Silver Nanoparticles by Using Microwave-Assisted Ionic Liquid Crystal Method and Their Application for Nonenzymatic Hydrogen Peroxide Determination. Electrocatalysis 2021, 12, 350–361. [Google Scholar] [CrossRef]
- Torras, M.; Roig, A. From Silver Plates to Spherical Nanoparticles: Snapshots of Microwave-Assisted Polyol Synthesis. ACS Omega 2020, 5, 5731–5738. [Google Scholar] [CrossRef] [PubMed]
- Darmanin, T.; Nativo, P.; Gilliland, D.; Ceccone, G.; Pascual, C.; De Berardis, B.; Guittard, F.; Rossi, F. Microwave-Assisted Synthesis of Silver Nanoprisms/Nanoplates Using a “Modified Polyol Process”. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 145–151. [Google Scholar] [CrossRef]
- Korkmaz, N.; Karadağ, A. Microwave Assisted Green Synthesis of Ag, Ag2O, and Ag2O3 Nanoparticles. J. Turk. Chem. Soc. Sect. A: Chem. 2021, 8, 585–592. [Google Scholar] [CrossRef]
- Ragunathan, V.; Chithra, K. Sequential Microwave-Ultrasound-Assisted Silver Nanoparticles Synthesis: A Swift Approach, Their Antioxidant, Antimicrobial, and in-Silico Studies. J. Mol. Liq. 2022, 347, 117954. [Google Scholar] [CrossRef]
- Daublytė, E.; Zdaniauskienė, A.; Talaikis, M.; Drabavičius, A.; Charkova, T. A Facile Microwave-Assisted Synthesis of Ag@SiO2 Nanoparticles for Raman Spectroscopy. New J. Chem. 2021, 45, 10952–10958. [Google Scholar] [CrossRef]
- Manno, R.; Ranjan, P.; Sebastian, V.; Mallada, R.; Irusta, S.; Sharma, U.K.; Van der Eycken, E.V.; Santamaria, J. Continuous Microwave-Assisted Synthesis of Silver Nanoclusters Confined in Mesoporous SBA-15: Application in Alkyne Cyclizations. Chem. Mater. 2020, 32, 2874–2883. [Google Scholar] [CrossRef]
- Souza, H.T.S.; Oliveira, S.A.A.; Souza, J.S. Modulating the Photocatalytic Activity of Ag Nanoparticles-Titanate Nanotubes Heterojunctions through Control of Microwave-Assisted Synthesis Conditions. J. Photochem. Photobiol. A Chem. 2020, 390, 112264. [Google Scholar] [CrossRef]
- Shkir, M.; Khan, M.T.; Ashraf, I.M.; AlFaify, S.; El-Toni, A.M.; Aldalbahi, A.; Ghaithan, H.; Khan, A. Rapid Microwave-Assisted Synthesis of Ag-Doped PbS Nanoparticles for Optoelectronic Applications. Ceram. Int. 2019, 45, 21975–21985. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Wongwiwat, N.; Thongtem, T.; Thongtem, S. Microwave-Assisted Solution Synthesis and Photocatalytic Activity of Ag Nanoparticles Supported on ZnO Nanostructure Flowers. Res. Chem. Intermed. 2018, 44, 7427–7436. [Google Scholar] [CrossRef]
- Ajay Rakkesh, R.; Durgalakshmi, D.; Karthe, P.; Balakumar, S. Anisotropic Growth and Strain-Induced Tunable Optical Properties of Ag–ZnO Hierarchical Nanostructures by a Microwave Synthesis Method. Mater. Chem. Phys. 2020, 244, 122720. [Google Scholar] [CrossRef]
- Porrawatkul, P.; Pimsen, R.; Kuyyogsuy, A.; Teppaya, N.; Noypha, A.; Chanthai, S.; Nuengmatcha, P. Microwave-Assisted Synthesis of Ag/ZnO Nanoparticles Using Averrhoa Carambola Fruit Extract as the Reducing Agent and Their Application in Cotton Fabrics with Antibacterial and UV-Protection Properties. RSC Adv. 2022, 12, 15008–15019. [Google Scholar] [CrossRef]
- Kumar, S.V.; Bafana, A.P.; Pawar, P.; Rahman, A.; Dahoumane, S.A.; Jeffryes, C.S. High Conversion Synthesis of <10 Nm Starch-Stabilized Silver Nanoparticles Using Microwave Technology. Sci. Rep. 2018, 8, 5106. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, W.; Cheng, B. Microwave-Assisted Synthesis of Silver Nanoparticles in Alkalic Carboxymethyl Chitosan Solution. Engineering 2010, 2, 387–390. [Google Scholar] [CrossRef]
- Tsuji, M.; Hashimoto, M.; Nishizawa, Y.; Tsuji, T. Preparation of Gold Nanoplates by a Microwave-Polyol Method. Chem. Lett. 2003, 32, 1114–1115. [Google Scholar] [CrossRef]
- Liu, F.-K.; Ker, C.-J.; Chang, Y.-C.; Ko, F.-H.; Chu, T.-C.; Dai, B.-T. Microwave Heating for the Preparation of Nanometer Gold Particles. Jpn. J. Appl. Phys. 2003, 42, 4152–4158. [Google Scholar] [CrossRef]
- Kundu, S.; Peng, L.; Liang, H. A New Route to Obtain High-Yield Multiple-Shaped Gold Nanoparticles in Aqueous Solution Using Microwave Irradiation. Inorg. Chem. 2008, 47, 6344–6352. [Google Scholar] [CrossRef]
- Ren, L.; Meng, L.; Lu, Q.; Fei, Z.; Dyson, P.J. Fabrication of Gold Nano- and Microstructures in Ionic Liquids—A Remarkable Anion Effect. J. Colloid. Interface Sci. 2008, 323, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Redel, E.; Walter, M.; Thomann, R.; Vollmer, C.; Hussein, L.; Scherer, H.; Krüger, M.; Janiak, C. Synthesis, Stabilization, Functionalization and, DFT Calculations of Gold Nanoparticles in Fluorous Phases (PTFE and Ionic Liquids). Chem.-A Eur. J. 2009, 15, 10047–10059. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Zhang, J.; Han, B.; Du, J.; Gao, Y.; Jiang, T. Synthesis of Single-Crystal Gold Nanosheets of Large Size in Ionic Liquids. J. Phys. Chem. B 2005, 109, 14445–14448. [Google Scholar] [CrossRef]
- Ren, L.; Meng, L.; Lu, Q. Fabrication of Octahedral Gold Nanostructures Using an Alcoholic Ionic Liquid. Chem. Lett. 2008, 37, 106–107. [Google Scholar] [CrossRef]
- Joseph, S.; Mathew, B. Microwave Assisted Facile Green Synthesis of Silver and Gold Nanocatalysts Using the Leaf Extract of Aerva Lanata. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Gangapuram, B.R.; Bandi, R.; Alle, M.; Dadigala, R.; Kotu, G.M.; Guttena, V. Microwave Assisted Rapid Green Synthesis of Gold Nanoparticles Using Annona Squamosa L Peel Extract for the Efficient Catalytic Reduction of Organic Pollutants. J. Mol. Struct. 2018, 1167, 305–315. [Google Scholar] [CrossRef]
- Thanayutsiri, T.; Patrojanasophon, P.; Opanasopit, P.; Ngawhirunpat, T.; Laiwattanapaisal, W.; Rojanarata, T. Rapid and Efficient Microwave-Assisted Extraction of Caesalpinia Sappan Linn. Heartwood and Subsequent Synthesis of Gold Nanoparticles. Green. Process. Synth. 2023, 12, 20228109. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Le Trung, H.; Nguyen, T.H.; Hoang, D.; Tran, T.H. Advancement of Microwave-Assisted Biosynthesis for Preparing Au Nanoparticles Using Ganoderma Lucidum Extract and Evaluation of Their Catalytic Reduction of 4-Nitrophenol. ACS Omega 2021, 6, 32198–32207. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.A.; Reddy, G.B.; Mittapalli, V. Microwave Assisted Synthesis of Gold Nanoparticles with Phyla Nodiflora (L.) Greene Leaves Extract and Its Studies of Catalytic Reduction of Organic Pollutants. Mater. Today Proc. 2020, 27, 1449–1454. [Google Scholar] [CrossRef]
- Putri, S.E.; Pratiwi, D.E.; Side, S. The Effect Of Microwave Irradiation on Synthesis of Gold Nanoparticles Using Ethanol Extract of White Bol Guava Leaves. J. Phys. Conf. Ser. 2021, 1752, 012058. [Google Scholar] [CrossRef]
- Hussein, J.; El-Naggar, M.E.; Fouda, M.M.G.; Othman, S.I.; Allam, A.A.; Nadwa, E.H.; Rashwan, E.K.; Hendawy, O.M. Eco-Friendly Microwave Synthesis of Gold Nanoparticles for Attenuation of Brain Dysfunction in Diabetic Rats. J. Clust. Sci. 2021, 32, 423–435. [Google Scholar] [CrossRef]
- Madhavan, V.; Gangadharan, P.K.; Ajayan, A.; Chandran, S.; Raveendran, P. Microwave-Assisted Solid-State Synthesis of Au Nanoparticles, Size-Selective Speciation, and Their Self-Assembly into 2D-Superlattice. Nano-Struct. Nano-Objects 2019, 17, 218–222. [Google Scholar] [CrossRef]
- Zhong, G.; Xu, S.; Cui, M.; Dong, Q.; Wang, X.; Xia, Q.; Gao, J.; Pei, Y.; Qiao, Y.; Pastel, G.; et al. Rapid, High-Temperature, In Situ Microwave Synthesis of Bulk Nanocatalysts. Small 2019, 15, 1904881. [Google Scholar] [CrossRef]
- Yu, S.; Hachtel, J.A.; Chisholm, M.F.; Pantelides, S.T.; Laromaine, A.; Roig, A. Magnetic Gold Nanotriangles by Microwave-Assisted Polyol Synthesis. Nanoscale 2015, 7, 14039–14046. [Google Scholar] [CrossRef]
- Shah, K.W.; Zheng, L. Microwave-Assisted Synthesis of Hexagonal Gold Nanoparticles Reduced by Organosilane (3-Mercaptopropyl)Trimethoxysilane. Materials 2019, 12, 1680. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Wing, C.; Esparza, R.; Vargas-Hernández, C.; Fernández García, M.E.; José-Yacamán, M. Microwave-Assisted Synthesis of Gold Nanoparticles Self-Assembled into Self-Supported Superstructures. Nanoscale 2012, 4, 2281. [Google Scholar] [CrossRef] [PubMed]
- Morad, M.; Karim, M.A.; Altass, H.M.; Khder, A.E.R.S. Microwave-Assisted Synthesis of Gold Nanoparticles Supported on Mn3O4 Catalyst for Low Temperature CO Oxidation. Environ. Technol. 2021, 42, 2680–2689. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Prapassornwattana, P.; Thongtem, S.; Thongtem, T. Synthesis of Heterostructure Au/ZnO Nanocomposites by Microwave-Assisted Deposition Method and Their Photocatalytic Activity in Methylene Blue Degradation. Russ. J. Phys. Chem. A 2020, 94, 1464–1470. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.; Qi, S. Synthesis of Nitrogen-Doped, Graphene-Supported Gold Nanoparticles via a Microwave Irradiation Method and Their Electrochemical Properties. Res. Chem. Intermed. 2020, 46, 2017–2024. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z. Rapid Synthesis of Hexagon-Shaped Gold Nanoplates by Microwave Assistant Method. Mater. Lett. 2007, 61, 4149–4151. [Google Scholar] [CrossRef]
- Horikoshi, S.; Abe, H.; Sumi, T.; Torigoe, K.; Sakai, H.; Serpone, N.; Abe, M. Microwave Frequency Effect in the Formation of Au Nanocolloids in Polar and Non-Polar Solvents. Nanoscale 2011, 3, 1697. [Google Scholar] [CrossRef]
- Mohamed, M.B.; AbouZeid, K.M.; Abdelsayed, V.; Aljarash, A.A.; El-Shall, M.S. Growth Mechanism of Anisotropic Gold Nanocrystals via Microwave Synthesis: Formation of Dioleamide by Gold Nanocatalysis. ACS Nano 2010, 4, 2766–2772. [Google Scholar] [CrossRef]
- Tsuji, M.; Miyamae, N.; Hashimoto, M.; Nishio, M.; Hikino, S.; Ishigami, N.; Tanaka, I. Shape and Size Controlled Synthesis of Gold Nanocrystals Using Oxidative Etching by AuCl4− and Cl− Anions in Microwave-Polyol Process. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 587–598. [Google Scholar] [CrossRef]
- Shang, L.; Yang, L.; Stockmar, F.; Popescu, R.; Trouillet, V.; Bruns, M.; Gerthsen, D.; Nienhaus, G.U. Microwave-Assisted Rapid Synthesis of Luminescent Gold Nanoclusters for Sensing Hg2+ in Living Cells Using Fluorescence Imaging. Nanoscale 2012, 4, 4155. [Google Scholar] [CrossRef] [PubMed]
- May-Masnou, A.; Soler, L.; Torras, M.; Salles, P.; Llorca, J.; Roig, A. Fast and Simple Microwave Synthesis of TiO2/Au Nanoparticles for Gas-Phase Photocatalytic Hydrogen Generation. Front. Chem. 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Arshi, N.; Ahmed, F.; Kumar, S.; Anwar, M.S.; Lu, J.; Koo, B.H.; Lee, C.G. Microwave Assisted Synthesis of Gold Nanoparticles and Their Antibacterial Activity against Escherichia coli (E. coli). Curr. Appl. Phys. 2011, 11, S360–S363. [Google Scholar] [CrossRef]
- Dahal, N.; García, S.; Zhou, J.; Humphrey, S.M. Beneficial Effects of Microwave-Assisted Heating versus Conventional Heating in Noble Metal Nanoparticle Synthesis. ACS Nano 2012, 6, 9433–9446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Y.; Zhang, X.-Q.; Yao, X.-H.; Wan, Y.; Song, P.; Liu, Z.-Y.; Fu, Y.-J. Microwave-Assisted Synthesis of PdNPs by Cellulose Solution to Prepare 3D Porous Microspheres Applied on Dyes Discoloration. Carbohydr. Polym. 2020, 247, 116569. [Google Scholar] [CrossRef]
- Heinrich, F.; Keßler, M.T.; Dohmen, S.; Singh, M.; Prechtl, M.H.G.; Mathur, S. Molecular Palladium Precursors for Pd0 Nanoparticle Preparation by Microwave Irradiation: Synthesis, Structural Characterization and Catalytic Activity. Eur. J. Inorg. Chem. 2012, 2012, 6027–6033. [Google Scholar] [CrossRef]
- Miyakawa, M.; Hiyoshi, N.; Koda, H.; Watanabe, K.; Kunigami, H.; Kunigami, H.; Miyazawa, A.; Nishioka, M. Continuous Syntheses of Carbon-Supported Pd and Pd@Pt Core–Shell Nanoparticles Using a Flow-Type Single-Mode Microwave Reactor. RSC Adv. 2020, 10, 6571–6575. [Google Scholar] [CrossRef] [PubMed]
- Sikeyi, L.L.; Ntuli, T.D.; Mongwe, T.H.; Maxakato, N.W.; Carleschi, E.; Doyle, B.P.; Coville, N.J.; Maubane-Nkadimeng, M.S. Microwave Assisted Synthesis of Nitrogen Doped and Oxygen Functionalized Carbon Nano Onions Supported Palladium Nanoparticles as Hybrid Anodic Electrocatalysts for Direct Alkaline Ethanol Fuel Cells. Int. J. Hydrog. Energy 2021, 46, 10862–10875. [Google Scholar] [CrossRef]
- Kumar, R.; da Silva, E.T.S.G.; Singh, R.K.; Savu, R.; Alaferdov, A.V.; Fonseca, L.C.; Carossi, L.C.; Singh, A.; Khandka, S.; Kar, K.K.; et al. Microwave-Assisted Synthesis of Palladium Nanoparticles Intercalated Nitrogen Doped Reduced Graphene Oxide and Their Electrocatalytic Activity for Direct-Ethanol Fuel Cells. J. Colloid. Interface Sci. 2018, 515, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, R.; Han, G.; Tian, Y.; Chang, Y.; Xiao, Y. Facile Synthesis of Pd-Ni Nanoparticles on Reduced Graphene Oxide under Microwave Irradiation for Formic Acid Oxidation. Chin. J. Chem. 2017, 35, 1405–1410. [Google Scholar] [CrossRef]
- Fatmawati, D.A.; Triyono, T.; Trisunaryanti, W.; Chasanah, U. Microwave-Assisted Chemical Co-Reduction of Pd Nanoparticles Anchored on Reduced Graphene Oxide with Different Loading Amounts. Indones. J. Chem. 2022, 22, 1282. [Google Scholar] [CrossRef]
- Ameri, A.; Shakibaie, M.; Rahimi, H.-R.; Adeli-Sardou, M.; Raeisi, M.; Najafi, A.; Forootanfar, H. Rapid and Facile Microwave-Assisted Synthesis of Palladium Nanoparticles and Evaluation of Their Antioxidant Properties and Cytotoxic Effects Against Fibroblast-Like (HSkMC) and Human Lung Carcinoma (A549) Cell Lines. Biol. Trace Elem. Res. 2020, 197, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Elazab, H.A.; Sadek, M.A.; El-Idreesy, T.T. Microwave-Assisted Synthesis of Palladium Nanoparticles Supported on Copper Oxide in Aqueous Medium as an Efficient Catalyst for Suzuki Cross-Coupling Reaction. Adsorpt. Sci. Technol. 2018, 36, 1352–1365. [Google Scholar] [CrossRef]
- Elazab, H.A.; Moussa, S.; Gupton, B.F.; El-Shall, M.S. Microwave-Assisted Synthesis of Pd Nanoparticles Supported on Fe3O4, Co3O4, and Ni(OH)2 Nanoplates and Catalysis Application for CO Oxidation. J. Nanoparticle Res. 2014, 16, 2477. [Google Scholar] [CrossRef]
- Walls, J.M.; Sagu, J.S.; Upul Wijayantha, K.G. Microwave Synthesised Pd–TiO2 for Photocatalytic Ammonia Production. RSC Adv. 2019, 9, 6387–6394. [Google Scholar] [CrossRef]
- Kwon, J.; Choi, K.; Tervoort, E.; Niederberger, M. One-Pot Microwave Synthesis of Pd Modified Titanium Dioxide Nanocrystals for 3D Aerogel Monoliths with Efficient Visible-Light Photocatalytic Activity in a Heated Gas Flow Reactor. J. Mater. Chem. A Mater. 2022, 10, 18383–18395. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Klangnoi, T.; Patiphatpanya, P.; Dumrongrojthanath, P.; Thongtem, S.; Thongtem, T. Synthesis of Pd Nanoparticles Modified Bi2MoO6 Nanoplates by Microwave-Assisted Deposition with Their Enhanced Visible-Light-Driven Photocatalyst. Optik 2020, 212, 164674. [Google Scholar] [CrossRef]
- Luo, Y. A Simple Microwave-Based Route for Size-Controlled Preparation of Colloidal Pt Nanoparticles. Mater. Lett. 2007, 61, 1873–1875. [Google Scholar] [CrossRef]
- Chin, C.D.-W.; Treadwell, L.J.; Wiley, J.B. Microwave Synthetic Routes for Shape-Controlled Catalyst Nanoparticles and Nanocomposites. Molecules 2021, 26, 3647. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ling, X.Y.; Lee, J.Y.; Su, X.; Gan, L.M. Nanosized Pt and PtRu Colloids as Precursors for Direct Methanol Fuel Cell Catalysts. J. Mater. Chem. 2003, 13, 3049. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Z.; Han, B.; Zhang, J.; Huang, J.; Du, J.; Miao, S. Microwave-Assisted Synthesis of Pt Nanocrystals and Deposition on Carbon Nanotubes in Ionic Liquids. J. Nanosci. Nanotechnol. 2006, 6, 175–179. [Google Scholar] [CrossRef]
- Wojnicki, M.; Luty-Błocho, M.; Kwolek, P.; Gajewska, M.; Socha, R.P.; Pędzich, Z.; Csapó, E.; Hessel, V. The Influence of Dielectric Permittivity of Water on the Shape of PtNPs Synthesized in High-Pressure High-Temperature Microwave Reactor. Sci. Rep. 2021, 11, 4851. [Google Scholar] [CrossRef] [PubMed]
- Kalyva, M.; Sunding, M.F.; Gunnæs, A.E.; Diplas, S.; Redekop, E.A. Correlation between Surface Chemistry and Morphology of PtCu and Pt Nanoparticles during Oxidation-Reduction Cycle. Appl. Surf. Sci. 2020, 532, 147369. [Google Scholar] [CrossRef]
- Sandström, R.; Gracia-Espino, E.; Hu, G.; Shchukarev, A.; Ma, J.; Wågberg, T. Yttria Stabilized and Surface Activated Platinum (PtxYOy) Nanoparticles through Rapid Microwave Assisted Synthesis for Oxygen Reduction Reaction. Nano Energy 2018, 46, 141–149. [Google Scholar] [CrossRef]
- Tsuji, M.; Uto, K.; Nagami, T.; Muto, A.; Fukushima, H.; Hayashi, J. Synthesis of Carbon-Supported Pt-YOx and PtY Nanoparticles with High Catalytic Activity for the Oxygen Reduction Reaction Using a Microwave-Based Polyol Method. ChemCatChem 2017, 9, 962–970. [Google Scholar] [CrossRef]
- Song, S.; Wang, Y.; Shen, P.K. Pulse-Microwave Assisted Polyol Synthesis of Highly Dispersed High Loading Pt/C Electrocatalyst for Oxygen Reduction Reaction. J. Power Sources 2007, 170, 46–49. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Swiegers, G.F.; Ma, Z.-F.; Wallace, G.G. Microwave-Assisted Synthesis of Pt/CNT Nanocomposite Electrocatalysts for PEM Fuel Cells. Nanoscale 2010, 2, 282–286. [Google Scholar] [CrossRef]
- Bharti, A.; Cheruvally, G.; Muliankeezhu, S. Microwave Assisted, Facile Synthesis of Pt/CNT Catalyst for Proton Exchange Membrane Fuel Cell Application. Int. J. Hydrog. Energy 2017, 42, 11622–11631. [Google Scholar] [CrossRef]
- Harish, S.; Baranton, S.; Coutanceau, C.; Joseph, J. Microwave Assisted Polyol Method for the Preparation of Pt/C, Ru/C and PtRu/C Nanoparticles and Its Application in Electrooxidation of Methanol. J. Power Sources 2012, 214, 33–39. [Google Scholar] [CrossRef]
- Sharma, R.; Wang, Y.; Li, F.; Chamier, J.; Andersen, S.M. Synthesis of a Pt/C Electrocatalyst from a User-Friendly Pt Precursor (Ammonium Hexachloroplatinate) through Microwave-Assisted Polyol Synthesis. ACS Appl. Energy Mater. 2019, 2, 6875–6882. [Google Scholar] [CrossRef]
- Sharma, R.; Gyergyek, S.; Andersen, S.M. Microwave-Assisted Scalable Synthesis of Pt/C: Impact of the Microwave Irradiation and Carrier Solution Polarity on Nanoparticle Formation and Aging of the Support Carbon. ACS Appl. Energy Mater. 2022, 5, 705–716. [Google Scholar] [CrossRef]
- Sharma, R.; Gyergyek, S.; Chamier, J.; Morgen, P.; Andersen, S.M. Pt/C Electrocatalyst Durability Enhancement by Inhibition of Pt Nanoparticle Growth Through Microwave Pretreatment of Carbon Support. ChemElectroChem 2021, 8, 1183–1195. [Google Scholar] [CrossRef]
- Shakoorioskooie, M.; Menceloglu, Y.Z.; Unal, S.; Hayat Soytas, S. Rapid Microwave-Assisted Synthesis of Platinum Nanoparticles Immobilized in Electrospun Carbon Nanofibers for Electrochemical Catalysis. ACS Appl. Nano Mater. 2018, 1, 6236–6246. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, M.; Liu, H.; Wang, Y.; Pan, D. Synthesis of Novel Three-Dimensional Mesoporous Nitrogen Doped Graphene Supported Pt Nanoparticles as Superior Catalyst for Hydrogen Generation. Int. J. Hydrog. Energy 2018, 43, 19327–19335. [Google Scholar] [CrossRef]
- Marinoiu, A.; Carcadea, E.; Sacca, A.; Carbone, A.; Sisu, C.; Dogaru, A.; Raceanu, M.; Varlam, M. One-Step Synthesis of Graphene Supported Platinum Nanoparticles as Electrocatalyst for PEM Fuel Cells. Int. J. Hydrog. Energy 2021, 46, 12242–12253. [Google Scholar] [CrossRef]
- Sridhar, V.; Jeon, J.-H.; Oh, I.-K. Synthesis of Graphene Nano-Sheets Using Eco-Friendly Chemicals and Microwave Radiation. Carbon 2010, 48, 2953–2957. [Google Scholar] [CrossRef]
- Eren, E.O.; Özkan, N.; Devrim, Y. Polybenzimidazole-Modified Carbon Nanotubes as a Support Material for Platinum-Based High-Temperature Proton Exchange Membrane Fuel Cell Electrocatalysts. Int. J. Hydrog. Energy 2021, 46, 29556–29567. [Google Scholar] [CrossRef]
- Xin, Y.; Nagata, T.; Kato, K.; Shirai, T. Microwave-Assisted Synthesis of Pt Nanoparticles via Liquid-Phase Polyol Reaction for Catalytic Volatile Organic Compound Elimination. ACS Appl. Nano Mater. 2022, 5, 4305–4315. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Yang, F.-L.; Chang, C.-H.; Chen-Yang, Y.W. Microwave-Assisted Synthesis of Silica Aerogel Supported Pt Nanoparticles for Self-Humidifying Proton Exchange Membrane Fuel Cell. Int. J. Hydrog. Energy 2012, 37, 7669–7676. [Google Scholar] [CrossRef]
- Chu, C.-H.; Cheng, S.-H.; Chen, N.-T.; Liao, W.-N.; Lo, L.-W. Microwave-Synthesized Platinum-Embedded Mesoporous Silica Nanoparticles as Dual-Modality Contrast Agents: Computed Tomography and Optical Imaging. Int. J. Mol. Sci. 2019, 20, 1560. [Google Scholar] [CrossRef]
- Ano, T.; Maitani, M.M.; Sato, Y.; Tsubaki, S.; Wada, Y. Drastic Microwave Heating of Percolated Pt Metal Nanoparticles Supported on Al2O3 Substrate. Processes 2020, 8, 72. [Google Scholar] [CrossRef]
- Lebègue, E.; Baranton, S.; Coutanceau, C. Polyol Synthesis of Nanosized Pt/C Electrocatalysts Assisted by Pulse Microwave Activation. J. Power Sources 2011, 196, 920–927. [Google Scholar] [CrossRef]
- Wang, H.-W.; Dong, R.-X.; Chang, H.-Y.; Liu, C.-L.; Chen-Yang, Y.-W. Preparation and Catalytic Activity of Pt/C Materials via Microwave Irradiation. Mater. Lett. 2007, 61, 830–833. [Google Scholar] [CrossRef]
- Kim, T.H.; Yoo, J.H.; Yi, S.C. Graphene Supported Platinum for Oxygen Reduction Reaction Electrocatalyst through a Facile Microwave-Assisted Polyol Synthesis. J. Ceram. Process. Res. 2017, 18, 261–264. [Google Scholar]
- Zhang, F.; Wang, Z.; Zhang, Y.; Zheng, Z.; Wang, C.; Du, Y.; Ye, W. Microwave-Assisted Synthesis of Pt/Graphene Nanocomposites for Nonenzymatic Hydrogen Peroxide Sensor. Int. J. Electrochem. Sci. 2012, 7, 1968–1977. [Google Scholar] [CrossRef]
- Kundu, P.; Nethravathi, C.; Deshpande, P.A.; Rajamathi, M.; Madras, G.; Ravishankar, N. Ultrafast Microwave-Assisted Route to Surfactant-Free Ultrafine Pt Nanoparticles on Graphene: Synergistic Co-Reduction Mechanism and High Catalytic Activity. Chem. Mater. 2011, 23, 2772–2780. [Google Scholar] [CrossRef]
- Sakthivel, M.; Schlange, A.; Kunz, U.; Turek, T. Microwave Assisted Synthesis of Surfactant Stabilized Platinum/Carbon Nanotube Electrocatalysts for Direct Methanol Fuel Cell Applications. J. Power Sources 2010, 195, 7083–7089. [Google Scholar] [CrossRef]
- Liu, S.-J.; Huang, C.-H.; Huang, C.-K.; Hwang, W.-S. Chelating Agent Assisted Microwave Synthesis of Carbon Supported Pt Nanoparticles for Low Temperature Polymer Electrolyte Fuel Cells. Electrochem. Commun. 2009, 11, 1792–1795. [Google Scholar] [CrossRef]
- Chu, Y.-Y.; Wang, Z.-B.; Gu, D.-M.; Yin, G.-P. Performance of Pt/C Catalysts Prepared by Microwave-Assisted Polyol Process for Methanol Electrooxidation. J. Power Sources 2010, 195, 1799–1804. [Google Scholar] [CrossRef]
- Song, S.; Liu, J.; Shi, J.; Liu, H.; Maragou, V.; Wang, Y.; Tsiakaras, P. The Effect of Microwave Operation Parameters on the Electrochemical Performance of Pt/C Catalysts. Appl. Catal. B 2011, 103, 287–293. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Wei, L.; Li, J.; Zhao, X. Catalytic Performance and Synthesis of a Pt/Graphene-TiO2 Catalyst Using an Environmentally Friendly Microwave-Assisted Solvothermal Method. Chin. J. Catal. 2017, 38, 1680–1687. [Google Scholar] [CrossRef]
- Ling, X.Y.; Liu, Z.; Lee, J.Y. Microwave-Assisted Synthesis of Platinum Nanoparticles. J. Metastable Nanocrystalline Mater. 2005, 23, 199–202. [Google Scholar]
- Pradhan, M.; Sarkar, S.; Sinha, A.K.; Basu, M.; Pal, T. High-Yield Synthesis of 1D Rh Nanostructures from Surfactant Mediated Reductive Pathway and Their Shape Transformation. J. Phys. Chem. C 2010, 114, 16129–16142. [Google Scholar] [CrossRef]
- Marquardt, D.; Vollmer, C.; Thomann, R.; Steurer, P.; Mülhaupt, R.; Redel, E.; Janiak, C. The Use of Microwave Irradiation for the Easy Synthesis of Graphene-Supported Transition Metal Nanoparticles in Ionic Liquids. Carbon 2011, 49, 1326–1332. [Google Scholar] [CrossRef]
- He, B.; Chen, Y.; Liu, H.; Liu, Y. Synthesis of Solvent-Stabilized Colloidal Nanoparticles of Platinum, Rhodium, and Rutheniumby Microwave-Polyol Process. J. Nanosci. Nanotechnol. 2005, 5, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lin, R.; Beuerle, C.; Jackson, J.E.; Obare, S.O.; Ofoli, R.Y. Effects of Surface Activation on the Structural and Catalytic Properties of Ruthenium Nanoparticles Supported on Mesoporous Silica. Nanotechnology 2014, 25, 045701. [Google Scholar] [CrossRef] [PubMed]
- Raspolli Galletti, A.M.; Antonetti, C.; Longo, I.; Capannelli, G.; Venezia, A.M. A Novel Microwave Assisted Process for the Synthesis of Nanostructured Ruthenium Catalysts Active in the Hydrogenation of Phenol to Cyclohexanone☆. Appl. Catal. A Gen. 2008, 350, 46–52. [Google Scholar] [CrossRef]
- Ugalde, M.; Chavira, E.; Figueroa, I.A.; Quintanar, C.; Espinosa-Magaña, F.; Zaragoza-Contreras, E.A.; Ochoa-Lara, M.T. Preparation of Rhodium Nano-Particles Using Microwaves. J. Solgel Sci. Technol. 2013, 65, 311–317. [Google Scholar] [CrossRef]
- Suryawanshi, Y.R.; Chakraborty, M.; Jauhari, S.; Mukhopadhyay, S.; Shenoy, K.T.; Shridharkrishna, R. Microwave Irradiation Solvothermal Technique: An Optimized Protocol for Size-Control Synthesis of Ru Nanoparticles. Cryst. Res. Technol. 2013, 48, 69–74. [Google Scholar] [CrossRef]
- Karimi, F.; Peppley, B.A. Comparison of Conventional versus Microwave Heating for Polyol Synthesis of Supported Iridium Based Electrocatalyst for Polymer Electrolyte Membrane Water Electrolysis. Int. J. Hydrog. Energy 2017, 42, 5083–5094. [Google Scholar] [CrossRef]
- Valodkar, M.; Modi, S.; Pal, A.; Thakore, S. Synthesis and Anti-Bacterial Activity of Cu, Ag and Cu–Ag Alloy Nanoparticles: A Green Approach. Mater. Res. Bull. 2011, 46, 384–389. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Zhang, M.; Wang, F.-X.; Pan, G.-B. Facile Microwave-Assisted Synthesis of Uniform Single-Crystal Copper Nanowires with Excellent Electrical Conductivity. RSC Adv. 2012, 2, 11235. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, C.; Yin, Y. Rapid Synthesis of Copper Nanoparticles by Sodium Hypophosphite Reduction in Ethylene Glycol under Microwave Irradiation. J. Cryst. Growth 2004, 270, 722–728. [Google Scholar] [CrossRef]
- Kawasaki, H.; Kosaka, Y.; Myoujin, Y.; Narushima, T.; Yonezawa, T.; Arakawa, R. Microwave-Assisted Polyol Synthesis of Copper Nanocrystals without Using Additional Protective Agents. Chem. Commun. 2011, 47, 7740. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, Y.; Yin, Y. A Novel One-Step Chemical Method for Preparation of Copper Nanofluids. J. Colloid. Interface Sci. 2004, 277, 100–103. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, C.; Yin, Y. Novel Synthesis of Copper Nanoparticles: Influence of the Synthesis Conditions on the Particle Size. Nanotechnology 2005, 16, 3079–3083. [Google Scholar] [CrossRef]
- Blosi, M.; Albonetti, S.; Dondi, M.; Martelli, C.; Baldi, G. Microwave-Assisted Polyol Synthesis of Cu Nanoparticles. J. Nanoparticle Res. 2011, 13, 127–138. [Google Scholar] [CrossRef]
- Jung, Y.; Son, Y.-H.; Lee, J.-K. 3-D Self-Assembly of Flower-like Particles via Microwave Irradiation for Water Treatment. RSC Adv. 2012, 2, 5877. [Google Scholar] [CrossRef]
- Cheng, W.-T.; Cheng, H.W. Synthesis and Characterization of Cobalt Nano-Particles through Microwave Polyol Process. AIChE J. 2009, 55, 1383–1389. [Google Scholar] [CrossRef]
- Wada, Y.; Kuramoto, H.; Sakata, T.; Mori, H.; Sumida, T.; Kitamura, T.; Yanagida, S. Preparation of Nano-Sized Nickel Metal Particles by Microwave Irradiation. Chem. Lett. 1999, 28, 607–608. [Google Scholar] [CrossRef]
- Eluri, R.; Paul, B. Microwave Assisted Greener Synthesis of Nickel Nanoparticles Using Sodium Hypophosphite. Mater. Lett. 2012, 76, 36–39. [Google Scholar] [CrossRef]
- Li, D.; Komarneni, S. Microwave-Assisted Polyol Process for Synthesis of Ni Nanoparticles. J. Am. Ceram. Soc. 2006, 89, 1510–1517. [Google Scholar] [CrossRef]
- Liu, X.; Meridor, U.; Zhao, P.; Song, G.; Frydman, A.; Gedanken, A. The Synthesis and Magnetic Properties of Monodispersed Single-Domain Nickel Nanospheres and Highly Globular Nanostructures of NicoreNiOshell. J. Magn. Magn. Mater. 2006, 301, 13–21. [Google Scholar] [CrossRef]
- Xu, W.; Liew, K.Y.; Liu, H.; Huang, T.; Sun, C.; Zhao, Y. Microwave-Assisted Synthesis of Nickel Nanoparticles. Mater. Lett. 2008, 62, 2571–2573. [Google Scholar] [CrossRef]
- Donegan, K.P.; Godsell, J.F.; Tobin, J.M.; O’Byrne, J.P.; Otway, D.J.; Morris, M.A.; Roy, S.; Holmes, J.D. Microwave-Assisted Synthesis of Icosahedral Nickel Nanocrystals. CrystEngComm 2011, 13, 2023. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.C. High-Yield Synthesis of Nickel and Nickel Phosphide Nanowires via Microwave-Assisted Processes. Chem. Mater. 2008, 20, 6743–6749. [Google Scholar] [CrossRef]
- Zhou, B.; Ren, T.; Zhu, J.-J. A Rapid Preparation of Bismuth Nanowires Via A Microwave-Assisted Polyol Method. Int. J. Mod. Phys. B 2005, 19, 2829–2834. [Google Scholar] [CrossRef]
- Fouad, O.A.; El-Shall, M.S. Microwave Irradiation Assisted Growth of Cu, Ni, Co Metals and/or Oxides Nanoclusters and Their Catalytic Performance. Nano 2012, 07, 1250034. [Google Scholar] [CrossRef]
- Zhou, B.; Hong, J.-M.; Zhu, J.-J. Microwave-Assisted Rapid Synthesis of Antimony Dendrites. Mater. Lett. 2005, 59, 3081–3084. [Google Scholar] [CrossRef]
- Liu, J.-W.; Chen, F.; Zhang, M.; Qi, H.; Zhang, C.-L.; Yu, S.-H. Rapid Microwave-Assisted Synthesis of Uniform Ultralong Te Nanowires, Optical Property, and Chemical Stability. Langmuir 2010, 26, 11372–11377. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, G.; Su, X.; Chen, X.; Wang, D.; Qin, J. Tellurium Nanotubes Synthesized with Microwave-Assisted Monosaccharide Reduction Method. J. Nanosci. Nanotechnol. 2007, 7, 2500–2505. [Google Scholar] [CrossRef]
- Gao, F.; Lu, Q.; Meng, X.; Komarneni, S. Synthesis of Nanorods and Nanowires Using Biomolecules under Conventional- and Microwave-Hydrothermal Conditions. J. Mater. Sci. 2008, 43, 2377–2386. [Google Scholar] [CrossRef]
- Marquardt, D.; Xie, Z.; Taubert, A.; Thomann, R.; Janiak, C. Microwave Synthesis and Inherent Stabilization of Metal Nanoparticles in 1-Methyl-3-(3-Carboxyethyl)-Imidazolium Tetrafluoroborate. Dalton Trans. 2011, 40, 8290. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, C.; Redel, E.; Abu-Shandi, K.; Thomann, R.; Manyar, H.; Hardacre, C.; Janiak, C. Microwave Irradiation for the Facile Synthesis of Transition-Metal Nanoparticles (NPs) in Ionic Liquids (ILs) from Metal-Carbonyl Precursors and Ru-, Rh-, and Ir-NP/IL Dispersions as Biphasic Liquid-Liquid Hydrogenation Nanocatalysts for Cyclohexene. Chem.-A Eur. J. 2010, 16, 3849–3858. [Google Scholar] [CrossRef]
- Jacob, D.S.; Genish, I.; Klein, L.; Gedanken, A. Carbon-Coated Core Shell Structured Copper and Nickel Nanoparticles Synthesized in an Ionic Liquid. J. Phys. Chem. B 2006, 110, 17711–17714. [Google Scholar] [CrossRef]
- Pande, J.V.; Bindwal, A.B.; Pakade, Y.B.; Biniwale, R.B. Application of Microwave Synthesized Ag-Rh Nanoparticles in Cyclohexane Dehydrogenation for Enhanced H2 Delivery. Int. J. Hydrog. Energy 2018, 43, 7411–7423. [Google Scholar] [CrossRef]
- Guo, H.; Li, H.; Jarvis, K.; Wan, H.; Kunal, P.; Dunning, S.G.; Liu, Y.; Henkelman, G.; Humphrey, S.M. Microwave-Assisted Synthesis of Classically Immiscible Ag–Ir Alloy Nanoparticle Catalysts. ACS Catal. 2018, 8, 11386–11397. [Google Scholar] [CrossRef]
- García, S.; Zhang, L.; Piburn, G.W.; Henkelman, G.; Humphrey, S.M. Microwave Synthesis of Classically Immiscible Rhodium–Silver and Rhodium–Gold Alloy Nanoparticles: Highly Active Hydrogenation Catalysts. ACS Nano 2014, 8, 11512–11521. [Google Scholar] [CrossRef]
- Jia, X.; Yao, Y.; Yu, G.; Qu, L.; Li, T.; Li, Z.; Xu, C. Synthesis of Gold-Silver Nanoalloys under Microwave-Assisted Irradiation by Deposition of Silver on Gold Nanoclusters/Triple Helix Glucan and Antifungal Activity. Carbohydr. Polym. 2020, 238, 116169. [Google Scholar] [CrossRef]
- Cabello, G.; Davoglio, R.A.; Hartl, F.W.; Marco, J.F.; Pereira, E.C.; Biaggio, S.R.; Varela, H.; Cuesta, A. Microwave-Assisted Synthesis of Pt-Au Nanoparticles with Enhanced Electrocatalytic Activity for the Oxidation of Formic Acid. Electrochim. Acta 2017, 224, 56–63. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, Y.; Hu, Y.; Li, C.; Wu, P.; Wei, S.; Cai, C. Pd@Pt Core−Shell Nanostructures with Controllable Composition Synthesized by a Microwave Method and Their Enhanced Electrocatalytic Activity toward Oxygen Reduction and Methanol Oxidation. J. Phys. Chem. C 2010, 114, 11861–11867. [Google Scholar] [CrossRef]
- Song, P.; Lei, Y.; Hu, X.; Wang, C.; Wang, J.; Tang, Y. Rapid One-Step Synthesis of Carbon-Supported Platinum–Copper Nanoparticles with Enhanced Electrocatalytic Activity via Microwave-Assisted Heating. J. Colloid. Interface Sci. 2020, 574, 421–429. [Google Scholar] [CrossRef]
- Liu, F.-K.; Huang, P.-W.; Chang, Y.-C.; Ko, C.-J.; Ko, F.-H.; Chu, T.-C. Formation of Silver Nanorods by Microwave Heating in the Presence of Gold Seeds. J. Cryst. Growth 2005, 273, 439–445. [Google Scholar] [CrossRef]
- Niu, X.; Wang, F.; Wang, W.; Wang, Y.; Huang, Y.; Zhang, J. Microwave-Assisted Synthesis of Pd3Ag Nanocomposite via Nature Polysaccharide Applied to Glucose Detection. Int. J. Biol. Macromol. 2018, 118, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Harpeness, R.; Gedanken, A. Microwave Synthesis of Core−Shell Gold/Palladium Bimetallic Nanoparticles. Langmuir 2004, 20, 3431–3434. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.G.R.; Miedziak, P.J.; Morgan, D.J.; He, Q.; Strasser, P.; Edwards, J.K. One Pot Microwave Synthesis of Highly Stable AuPd@Pd Supported Core–Shell Nanoparticles. Faraday Discuss. 2018, 208, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Cong, C.; Nakayama, S.; Maenosono, S.; Harada, M. Microwave-Assisted Polyol Synthesis of Pt/Pd and Pt/Rh Bimetallic Nanoparticles in Polymer Solutions Prepared by Batch and Continuous-Flow Processing. Ind. Eng. Chem. Res. 2018, 57, 179–190. [Google Scholar] [CrossRef]
- Patel, K.; Kapoor, S.; Dave, D.P.; Mukherjee, T. Synthesis of Pt, Pd, Pt/Ag and Pd/Ag Nanoparticles by Microwave-Polyol Method. J. Chem. Sci. 2005, 117, 311–316. [Google Scholar] [CrossRef]
- Guo, D.-J. Novel Synthesis of PtRu/Multi-Walled Carbon Nanotube Catalyst via a Microwave-Assisted Imidazolium Ionic Liquid Method for Methanol Oxidation. J. Power Sources 2010, 195, 7234–7237. [Google Scholar] [CrossRef]
- Kunal, P.; Li, H.; Dewing, B.L.; Zhang, L.; Jarvis, K.; Henkelman, G.; Humphrey, S.M. Microwave-Assisted Synthesis of Pd x Au 100– x Alloy Nanoparticles: A Combined Experimental and Theoretical Assessment of Synthetic and Compositional Effects upon Catalytic Reactivity. ACS Catal. 2016, 6, 4882–4893. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, C.; Wang, X.; Guo, G.; Sun, Y. Synthesis of PtAu Alloy Nanocrystals in Micelle Nanoreactors Enabled by Flash Heating and Cooling. Part. Part. Syst. Charact. 2018, 35, 1700413. [Google Scholar] [CrossRef]
- Bensebaa, F.; Patrito, N.; Le Page, Y.; L’Ecuyer, P.; Wang, D. Tunable Platinum–Ruthenium Nanoparticle Properties Using Microwave Synthesis. J. Mater. Chem. 2004, 14, 3378–3384. [Google Scholar] [CrossRef]
- Mathe, N.R.; Scriba, M.R.; Rikhotso, R.S.; Coville, N.J. Microwave-Irradiation Polyol Synthesis of PVP-Protected Pt–Ni Electrocatalysts for Methanol Oxidation Reaction. Electrocatalysis 2018, 9, 388–399. [Google Scholar] [CrossRef]
- Mathe, N.R.; Scriba, M.R.; Coville, N.J. Methanol Oxidation Reaction Activity of Microwave-Irradiated and Heat-Treated Pt/Co and Pt/Ni Nano-Electrocatalysts. Int. J. Hydrog. Energy 2014, 39, 18871–18881. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Aljarash, A.; El-Shall, M.S.; Al Othman, Z.A.; Alghamdi, A.H. Microwave Synthesis of Bimetallic Nanoalloys and CO Oxidation on Ceria-Supported Nanoalloys. Chem. Mater. 2009, 21, 2825–2834. [Google Scholar] [CrossRef]
- Peng, X.; Chen, D.; Yang, X.; Wang, D.; Li, M.; Tseng, C.-C.; Panneerselvam, R.; Wang, X.; Hu, W.; Tian, J.; et al. Microwave-Assisted Synthesis of Highly Dispersed PtCu Nanoparticles on Three-Dimensional Nitrogen-Doped Graphene Networks with Remarkably Enhanced Methanol Electrooxidation. ACS Appl. Mater. Interfaces 2016, 8, 33673–33680. [Google Scholar] [CrossRef]
- El-Deeb, H.; Bron, M. Microwave-Assisted Polyol Synthesis of PtCu/Carbon Nanotube Catalysts for Electrocatalytic Oxygen Reduction. J. Power Sources 2015, 275, 893–900. [Google Scholar] [CrossRef]
- Lin, R.; Cai, X.; Hao, Z.; Pu, H.; Yan, H. Rapid Microwave-Assisted Solvothermal Synthesis of Shape-Controlled Pt-Ni Alloy Nanoparticles for PEMFC. Electrochim. Acta 2018, 283, 764–771. [Google Scholar] [CrossRef]
- Ma, Y.; Miao, L.; Guo, W.; Yao, X.; Qin, F.; Wang, Z.; Du, H.; Li, J.; Kang, F.; Gan, L. Modulating Surface Composition and Oxygen Reduction Reaction Activities of Pt–Ni Octahedral Nanoparticles by Microwave-Enhanced Surface Diffusion during Solvothermal Synthesis. Chem. Mater. 2018, 30, 4355–4360. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Y.; Wang, X.; Zeng, L.; Luo, F.; Liu, A. Amorphous Nickel Coating on Carbon Nanotubes Supported Pt Nanoparticles as a Highly Durable and Active Electrocatalyst for Methanol Oxidation Reaction. J. Electroanal. Chem. 2020, 856, 113739. [Google Scholar] [CrossRef]
- Sandström, R.; Ekspong, J.; Gracia-Espino, E.; Wågberg, T. Oxidatively Induced Exposure of Active Surface Area during Microwave Assisted Formation of Pt 3 Co Nanoparticles for Oxygen Reduction Reaction. RSC Adv. 2019, 9, 17979–17987. [Google Scholar] [CrossRef]
- Higgins, D.C.; Ye, S.; Knights, S.; Chen, Z. Highly Durable Platinum-Cobalt Nanowires by Microwave Irradiation as Oxygen Reduction Catalyst for PEM Fuel Cell. Electrochem. Solid-State Lett. 2012, 15, B83. [Google Scholar] [CrossRef]
- Kepenienė, V.; Tamašauskaitė-Tamašiūnaitė, L.; Jablonskienė, J.; Vaičiūnienė, J.; Kondrotas, R.; Juškėnas, R.; Norkus, E. Investigation of Graphene Supported Platinum-Cobalt Nanocomposites as Electrocatalysts for Ethanol Oxidation. J. Electrochem. Soc. 2014, 161, F1354–F1359. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Yan, Z.; Jing, J.; Xie, J.; Chen, M. Improved Catalytic Activity of Cobalt Core–Platinum Shell Nanoparticles Supported on Surface Functionalized Graphene for Methanol Electro-Oxidation. Electrochim. Acta 2015, 158, 81–88. [Google Scholar] [CrossRef]
- Du, J.-Q.; Zhang, Y.; Tian, T.; Yan, S.-C.; Wang, H.-T. Microwave Irradiation Assisted Rapid Synthesis of Fe–Ru Bimetallic Nanoparticles and Their Catalytic Properties in Water-Gas Shift Reaction. Mater. Res. Bull. 2009, 44, 1347–1351. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Howard, L.E.M.; Giblin, S.R.; Tanner, B.K.; Terry, I.; Hughes, A.K.; Ross, I.M.; Serres, A.; Bürckstümmer, H.; Evans, J.S.O. Synthesis of Monodispersed Fcc and Fct FePt/FePd Nanoparticles by Microwave Irradiation. J. Mater. Chem. 2005, 15, 5136. [Google Scholar] [CrossRef]
- Harpeness, R.; Gedanken, A. The Microwave-Assisted Polyol Synthesis of Nanosized Hard Magnetic Material, FePt. J. Mater. Chem. 2005, 15, 698. [Google Scholar] [CrossRef]
- Minami, R.; Kitamoto, Y.; Chikata, T.; Kato, S. Direct Synthesis of L10 Type Fe–Pt Nanoparticles Using Microwave-Polyol Method. Electrochim. Acta 2005, 51, 864–866. [Google Scholar] [CrossRef]
- Köhler, D.; Heise, M.; Baranov, A.I.; Luo, Y.; Geiger, D.; Ruck, M.; Armbrüster, M. Synthesis of BiRh Nanoplates with Superior Catalytic Performance in the Semihydrogenation of Acetylene. Chem. Mater. 2012, 24, 1639–1644. [Google Scholar] [CrossRef]
- Jia, J.; Yu, J.C.; Wang, Y.-X.J.; Chan, K.M. Magnetic Nanochains of FeNi 3 Prepared by a Template-Free Microwave-Hydrothermal Method. ACS Appl. Mater. Interfaces 2010, 2, 2579–2584. [Google Scholar] [CrossRef]
- GUO, X.; LI, Y.; LIU, Q.; SHEN, W. Microwave-Assisted Polyol-Synthesis of CoNi Nanomaterials. Chin. J. Catal. 2012, 33, 645–650. [Google Scholar] [CrossRef]
- Li, C.; Sui, J.; Zhang, Z.; Jiang, X.; Zhang, Z.; Yu, L. Microwave-Assisted Synthesis of Tremella-like NiCo/C Composites for Efficient Broadband Electromagnetic Wave Absorption at 2–40 GHz. Chem. Eng. J. 2019, 375, 122017. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, R.R.; Dey, K.K. Microwave Assisted Formation of Trimetallic AuPtCu Nanoparticles from Bimetallic Nano-Islands: Why It Is a Superior New Age Biocidal Agent Compared to Monometallic & Bimetallic Nanoparticles. J. Alloys Compd. 2022, 896, 163073. [Google Scholar] [CrossRef]
- Hu, X.; Song, P.; Yang, X.; Wang, C.; Wang, J.; Tang, Y.; Zhang, J.; Mao, Z. One-Step Microwave-Assisted Synthesis of Carbon-Supported Ternary Pt-Sn-Rh Alloy Nanoparticles for Fuel Cells. J. Taiwan. Inst. Chem. Eng. 2020, 115, 272–278. [Google Scholar] [CrossRef]
- Womiloju, A.A.; Höppener, C.; Schubert, U.S.; Hoeppener, S. Microwave-Assisted Synthesis of Core–Shell Nanoparticles—Insights into the Growth of Different Geometries. Part. Part. Syst. Charact. 2020, 37, 2000019. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tsukahara, Y.; Sakata, T.; Mori, H.; Yanagida, T.; Kawai, T.; Wada, Y. Magnetic Cu–Ni (Core–Shell) Nanoparticles in a One-Pot Reaction under Microwave Irradiation. Nanoscale 2010, 2, 515. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Ren, Y.; Wei, Q.; Sun, Y. Microwave Synthesis of Single-Phase Nanoparticles Made of Multi-Principal Element Alloys. Nano Res. 2022, 15, 4886–4892. [Google Scholar] [CrossRef]
- Kalyva, M.; Wragg, D.S.; Fjellvåg, H.; Sjåstad, A.O. Engineering Functions into Platinum and Platinum-Rhodium Nanoparticles in a One-Step Microwave Irradiation Synthesis. ChemistryOpen 2017, 6, 273–281. [Google Scholar] [CrossRef]
- Chen, Z.; Mochizuki, D.; Maitani, M.M.; Wada, Y. Facile Synthesis of Bimetallic Cu–Ag Nanoparticles under Microwave Irradiation and Their Oxidation Resistance. Nanotechnology 2013, 24, 265602. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, H.; Yu, G.; Yuan, M.; Yang, J.; Xu, D.; Hou, Y.; Dong, Z. Pt Coated Co Nanoparticles Supported on N-Doped Mesoporous Carbon as Highly Efficient, Magnetically Recyclable and Reusable Catalyst for Hydrogen Generation from Ammonia Borane. Int. J. Hydrog. Energy 2017, 42, 27055–27065. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, B.; Hong, L.; Lim, T.H. Microwave Heated Polyol Synthesis of Carbon-Supported PtSn Nanoparticles for Methanol Electrooxidation. Electrochem. Commun. 2006, 8, 83–90. [Google Scholar] [CrossRef]
- Sarkar, A.; Vadivel Murugan, A.; Manthiram, A. Rapid Microwave-Assisted Solvothermal Synthesis of Methanol Tolerant Pt-Pd-Co Nanoalloy Electrocatalysts. Fuel Cells 2010, 10, 375–383. [Google Scholar] [CrossRef]
- Maulana, A.L.; Chen, P.-C.; Shi, Z.; Yang, Y.; Lizandara-Pueyo, C.; Seeler, F.; Abruña, H.D.; Muller, D.; Schierle-Arndt, K.; Yang, P. Understanding the Structural Evolution of IrFeCoNiCu High-Entropy Alloy Nanoparticles under the Acidic Oxygen Evolution Reaction. Nano Lett. 2023, 23, 6637–6644. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, C.-G.; Liu, C.; Duan, X.; Guo, Q.; Shen, Y.; Liu, J.; Chen, Y. Microwave-Assisted Continuous Flow Phytosynthesis of Silver Nanoparticle/Reduced Graphene Oxide Composites and Related Visible Light Catalytic Performance. J. Environ. Sci. 2022, 115, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Amri, F.; Kasim, W.; Rochliadi, A.; Patah, A. Facile One-Pot Microwave-Assisted Synthesis of Rod-like and Hexagonal Plate-like AgNP@Ni-BTC Composites for a Potential Salivary Glucose Sensor. Sens. Actuators Rep. 2023, 5, 100141. [Google Scholar] [CrossRef]
- Prabhakar Vattikuti, S.V.; Nagajyothi, P.C.; Devarayapalli, K.C.; Yoo, K.; Dang Nam, N.; Shim, J. Hybrid Ag/MoS2 Nanosheets for Efficient Electrocatalytic Oxygen Reduction. Appl. Surf. Sci. 2020, 526, 146751. [Google Scholar] [CrossRef]
- Venishetty, S.K.; Kummari, S.; Karingula, S.; Moru, S.; Gobi, K.V. Design and Synthesis of Pd Decorated RGO-MoSe2 2D Hybrid Network as High Performance Electrocatalyst toward Methanol Electrooxidation. Int. J. Hydrog. Energy 2023, 48, 21487–21498. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Gao, J.; Zhang, X.; Sun, H.; Wang, G. Microwave-Regulated Bi Nanoparticles on Carbon Nanotube Networks as a Freestanding Electrode for Flexible Sodium-Ion Capacitors. J. Colloid. Interface Sci. 2023, 643, 420–427. [Google Scholar] [CrossRef]
- Jia, P.; Sun, J.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y. Study on the Underpinning Mechanisms of Microwave-Induced Synthesis of Carbon-Coated Metal Nanoparticles. Front. Energy Res. 2023, 10, 1044283. [Google Scholar] [CrossRef]





| Advantages | Disadvantages |
|---|---|
| Economy of energy | Difficulties with measurements and control of temperature |
| Mild reaction conditions | Sometimes nonequilibrium processes are difficult to control |
| Reduction of time of synthesis | Sometimes the processes are too fast |
| Fast heating | Difficulties with scale-up |
| Fast change of regimes | Typically, a small-scale preparation |
| Fast cooling | Limited pressure range |
| Nonequilibrium process | Limited geometry of the reactor |
| High rate of nucleation | Possible electric arching or plasma effects |
| Accelerated growth of particles (temporally separated from the nucleation stage) | Not all solvents are suitable |
| Selective heating | Temperature dependences of loss tangents are rarely known |
| No need to use capping agents or surfactants | Difficulties with in situ monitoring of the process |
| Possibility to use pulse mode of operation | |
| Narrow particle-size distribution | |
| Small size of nanoparticles | |
| Hot spots/hot surfaces/superheated areas | |
| Realization of metastable phases | |
| Elimination of barrier effects caused by inverted temperature gradients | |
| Reduced temperature gradient | |
| Reduced wall effects | |
| No need to heat the walls, gases |
| Table of Nanoparticles | Microwave (MW) Heating Conditions | Conventional Heating (CH) Conditions | Notes | Relative Gain in the Energy Consumption of MW vs. CH (Rough Estimate) | ||
|---|---|---|---|---|---|---|
| Power/Temperature | Synthesis Time | Temperature | Synthesis Time | |||
| Ag nanoclusters [31] | 200 W | 60–90 s | No information | 120 min | The quantum yield for MW-synthesized NPs is 6%, while the quantum yield for NPs prepared by thermal heating is 1% | 80–120 |
| Ag nanorods [46] | - | 1–3 min | - | 50 min | Ag nanosheets are formed only at a very short MW treatment, and they are not produced at all under conventional heating | 15–50 |
| Au nanoparticles [76] | 400 W | 10 min | 85 °C | 6 h | The size of MW synthesized NPs was 22 nm, while the size of CH NPs was 33 nm | 36 |
| Pd nanoparticles [96] | 300 W, 60 °C | 4 h | 60 °C | 8 h | Twice faster process under MW conditions | 2 |
| Pt nanoparticles [129] | 160 °C | 200 s | 160 °C | 2000 s | The size of MW synthesized NPs was 3.6 nm, while the size of CH (oil-bath) NPs was 10.1 nm | 10 |
| Ir nanoparticles on antimony tin oxide [151] | 170 °C | 30 s | 170 °C | 2.5 h | Obtained NPs characterized by a similar size, surface area, and morphology | 300 |
| Cu nanofluids [156] | 350 W | 5 min | 120 °C | 1 h | The size of MW-synthesized NPs was <20 nm, while the size of CH NPs was 30–80 nm. MW-obtained nanofluids were stable for 3 weeks, while CH-synthesized ones were only for 1 week | 12 |
| Metal nanoparticles [175] | Just 10 W | 3 min | UV irradiation—1000 W, CH—180–250 °C | UV irradiation—15 min, CH—6–12 h | NPs produced in the microwave regime were smaller (<5 nm) and more uniform than those prepared by reference methods. | A gain of 500 compared to UV irradiation and 360–720 compared to CH |
| Pt-Ni nanoparticles [195] | 750 W 200 °C | 30 min | 200 °C | 4 h | Electrocatalytic activity of MW synthesized NPs is higher than CH-synthesized NPs | 8 |
| Fe-Pt nanoparticles [209] | 800 W 250 °C | 1–2.5 h | 290 °C | 3 h | Coercivity of NPs obtained by the MW method was almost 10 times higher than that of CH-obtained NPs | <3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kustov, L.; Vikanova, K. Synthesis of Metal Nanoparticles under Microwave Irradiation: Get Much with Less Energy. Metals 2023, 13, 1714. https://doi.org/10.3390/met13101714
Kustov L, Vikanova K. Synthesis of Metal Nanoparticles under Microwave Irradiation: Get Much with Less Energy. Metals. 2023; 13(10):1714. https://doi.org/10.3390/met13101714
Chicago/Turabian StyleKustov, Leonid, and Kseniia Vikanova. 2023. "Synthesis of Metal Nanoparticles under Microwave Irradiation: Get Much with Less Energy" Metals 13, no. 10: 1714. https://doi.org/10.3390/met13101714




