Hydrogen Production Properties of Aluminum–Magnesium Alloy Presenting β-Phase Al3Mg2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparations
2.2. Characterizations
2.3. Hydrolysis
2.4. Modelling
3. Results and Discussions
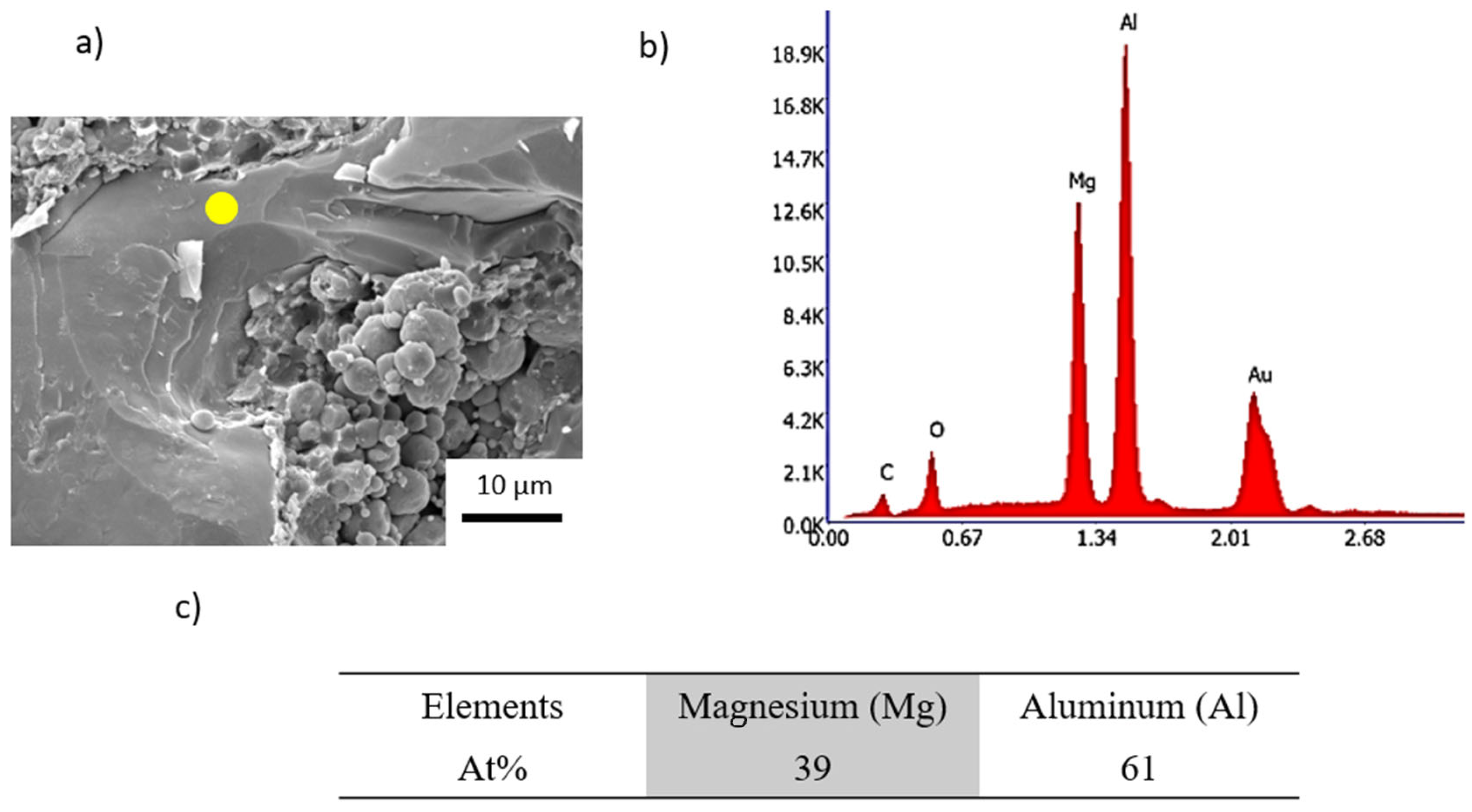
3.1. Fabrication of the Intermetallic Phase Al3Mg2
3.2. Hydrogen Production by Hydrolysis
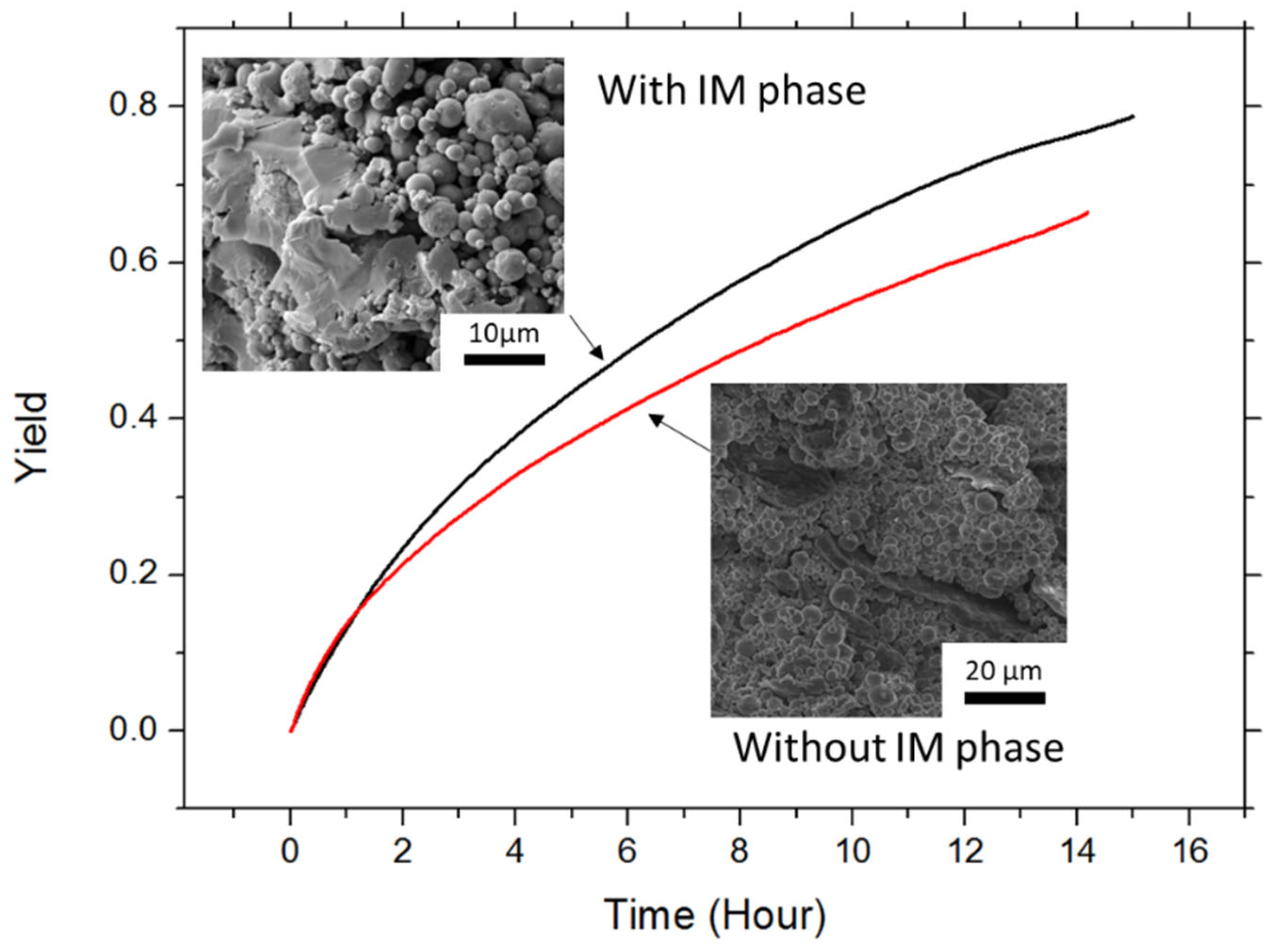
3.2.1. Hydrogen Production
3.2.2. Role of the Intermetallic Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vargel, C. Corrosion de L’aluminium; Technique et ingénierie Série Matériaux; Dunod: Paris, France, 1999; ISBN 978-2-10-006569-1. [Google Scholar]
- Vargel, C. Métallurgie de L’aluminium. Available online: https://www.techniques-ingenieur.fr/base-documentaire/materiaux-th11/metaux-et-alliages-non-ferreux-42357210/metallurgie-de-l-aluminium-m4663/ (accessed on 4 September 2023).
- Hajjari, E.; Divandari, M.; Razavi, S.H.; Homma, T.; Kamado, S. Intermetallic Compounds and Antiphase Domains in Al/Mg Compound Casting. Intermetallics 2012, 23, 182–186. [Google Scholar] [CrossRef]
- L’aluminium: Dossier Complet|Techniques de L’Ingénieur. Available online: https://www.techniques-ingenieur.fr/base-documentaire/43804210-techniques-du-batiment-connaitre-les-materiaux-de-la-construction/download/tba1066/l-aluminium.html (accessed on 22 September 2021).
- Murray, J.L. ASM Handbook, Volume 3, Alloy Phase Diagrams; ASM International: Novelty, OH, USA, 1992. [Google Scholar]
- Al Bacha, S.; Thienpont, A.; Zakhour, M.; Nakhl, M.; Bobet, J.-L. Clean Hydrogen Production by the Hydrolysis of Magnesium-Based Material: Effect of the Hydrolysis Solution. J. Clean. Prod. 2021, 282, 124498. [Google Scholar] [CrossRef]
- Bauer, E.; Kaldarar, H.; Lackner, R.; Michor, H.; Steiner, W.; Scheidt, E.-W.; Galatanu, A.; Marabelli, F.; Wazumi, T.; Kumagai, K.; et al. Superconductivity in the Complex Metallic Alloy β−Al3Mg2. Phys. Rev. B 2007, 76, 014528. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Liu, W.; Huang, Y.; Wu, L.; Wang, T.; Liu, C.; Yang, L. The Mechanical Properties and Formation Mechanism of Al/Mg Composite Interface Prepared by Spark Plasma Sintering under Different Sintering Pressures. Vacuum 2020, 176, 109300. [Google Scholar] [CrossRef]
- Bhargava, N.R.M.R.; Samajdar, I.; Ranganathan, S.; Surappa, M.K. Role of Cold Work and SiC Reinforcements on the Β′/β Precipitation in Al-10 Pct Mg Alloy. Metall. Mater. Trans. A 1998, 29, 2835–2842. [Google Scholar] [CrossRef]
- Ding, Y.; Gao, K.; Huang, H.; Wen, S.; Wu, X.; Nie, Z.; Guo, S.; Shao, R.; Huang, C.; Zhou, D. Nucleation and Evolution of β Phase and Corresponding Intergranular Corrosion Transition at 100–230 °C in 5083 Alloy Containing Er and Zr. Mater. Des. 2019, 174, 107778. [Google Scholar] [CrossRef]
- Scudino, S.; Liu, G.; Sakaliyska, M.; Surreddi, K.B.; Eckert, J. Powder Metallurgy of Al-Based Metal Matrix Composites Reinforced with β-Al3Mg2 Intermetallic Particles: Analysis and Modeling of Mechanical Properties. Acta Mater. 2009, 57, 4529–4538. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, X.; Zhang, D. Making Strong Al(Mg)-Al3Mg2 Composites. Materialia 2021, 16, 101099. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, J.; Tang, Y.; Wan, P.; Yuan, Q.; Hu, H.; Coulon, F.; Hu, Q.; Yang, X.J. Production of High-Purity Hydrogen and Layered Doubled Hydroxide by Hydrolysis of Mg-Al Alloys. Chem. Eng. Technol. 2021, 44, 797–803. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Marbán, G.; Valdés-Solís, T. Towards the Hydrogen Economy? Int. J. Hydrogen Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Midilli, A.; Ay, M.; Dincer, I.; Rosen, M.A. On Hydrogen and Hydrogen Energy Strategies: I: Current Status and Needs. Renew. Sustain. Energy Rev. 2005, 9, 255–271. [Google Scholar] [CrossRef]
- Hanley, E.S.; Deane, J.; Gallachóir, B.Ó. The Role of Hydrogen in Low Carbon Energy Futures–A Review of Existing Perspectives. Renew. Sustain. Energy Rev. 2018, 82, 3027–3045. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an Energy Carrier: Prospects and Challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Sci. 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An Overview of Hydrogen Production Technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Konieczny, A.; Mondal, K.; Wiltowski, T.; Dydo, P. Catalyst Development for Thermocatalytic Decomposition of Methane to Hydrogen. Int. J. Hydrogen Energy 2008, 33, 264–272. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M. Political, Economic and Environmental Impacts of Biomass-Based Hydrogen. Int. J. Hydrogen Energy 2009, 34, 3589–3603. [Google Scholar] [CrossRef]
- Donadey, G.; Caillaud, S.; Coeuret, P.; Moussa, M.; Cuzacq, L.; Bobet, J.-L. Hydrogen Generation from Mg Wastes by Cold Rolling and Ball Milling by Hydrolysis Reaction: Elektron 21 (UNS-M12310) in Simulated Seawater. Metals 2022, 12, 1821. [Google Scholar] [CrossRef]
- Legrée, M.; Bobet, J.-L.; Mauvy, F.; Sabatier, J. Modeling Hydrolysis Kinetics of Dual Phase α-Mg/LPSO Alloys. Int. J. Hydrogen Energy 2022, 47, 23084–23093. [Google Scholar] [CrossRef]
- Alinejad, B.; Mahmoodi, K. A Novel Method for Generating Hydrogen by Hydrolysis of Highly Activated Aluminum Nanoparticles in Pure Water. Int. J. Hydrogen Energy 2009, 34, 7934–7938. [Google Scholar] [CrossRef]
- Soler, L.; Macanás, J.; Muñoz, M.; Casado, J. Aluminum and Aluminum Alloys as Sources of Hydrogen for Fuel Cell Applications. J. Power Sources 2007, 169, 144–149. [Google Scholar] [CrossRef]
- Zou, H.; Chen, S.; Zhao, Z.; Lin, W. Hydrogen Production by Hydrolysis of Aluminum. J. Alloys Compd. 2013, 578, 380–384. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Chen, X.; Yang, S.; Wang, C. Hydrogen Generation from Hydrolysis of Activated Al-Bi, Al-Sn Powders Prepared by Gas Atomization Method. Int. J. Hydrogen Energy 2017, 42, 10943–10951. [Google Scholar] [CrossRef]
- Liu, S.; Fan, M.; Wang, C.; Huang, Y.; Chen, D.; Bai, L.; Shu, K. Hydrogen Generation by Hydrolysis of Al–Li–Bi–NaCl Mixture with Pure Water. Int. J. Hydrogen Energy 2012, 37, 1014–1020. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Z.; Liu, X.; Hao, M.; Chen, A.; Tang, Z. Hydrogen Generation by the Hydrolysis Reaction of Ball-Milled Aluminium–Lithium Alloys. J. Power Sources 2014, 254, 345–352. [Google Scholar] [CrossRef]
- du Preez, S.P.; Bessarabov, D.G. Hydrogen Generation by the Hydrolysis of Mechanochemically Activated Aluminum-Tin-Indium Composites in Pure Water. Int. J. Hydrogen Energy 2018, 43, 21398–21413. [Google Scholar] [CrossRef]
- Silvain, J.-F.; Heintz, J.-M.; Veillere, A.; Constantin, L.; Lu, Y.F. A Review of Processing of Cu/C Base Plate Composites for Interfacial Control and Improved Properties. Int. J. Extrem. Manuf. 2020, 2, 012002. [Google Scholar] [CrossRef]
- Buryakovskaya, O.A.; Kurbatova, A.I.; Vlaskin, M.S.; Valyano, G.E.; Grigorenko, A.V.; Ambaryan, G.N.; Dudoladov, A.O. Waste to Hydrogen: Elaboration of Hydroreactive Materials from Magnesium-Aluminum Scrap. Sustainability 2022, 14, 4496. [Google Scholar] [CrossRef]
- Hardie, D.; Parkins, R.N. Lattice Spacing Relationships in Magnesium Solid Solutions. Philos. Mag. 1959, 4, 815–825. [Google Scholar] [CrossRef]
- Huang, X.; Gao, T.; Pan, X.; Wei, D.; Lv, C.; Qin, L.; Huang, Y. A Review: Feasibility of Hydrogen Generation from the Reaction between Aluminum and Water for Fuel Cell Applications. J. Power Sources 2013, 229, 133–140. [Google Scholar] [CrossRef]
- Pourbaix, M.J.N. Atlas D’équilibres Électrochimiques; Gauthier-Villars & Cie: Paris, France, 1963. [Google Scholar]
- Jones, R.H.; Baer, D.R.; Danielson, M.J.; Vetrano, J.S. Role of Mg in the Stress Corrosion Cracking of an Al-Mg Alloy. Metall. Mater. Trans. A 2001, 32, 1699–1711. [Google Scholar] [CrossRef]
- Legrée, M. Étude d’un Procédé de Production d’Hydrogène In-Situ Sous Haute Pression Par Hydrolyse En Présence de Magnésium. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 2022. [Google Scholar]

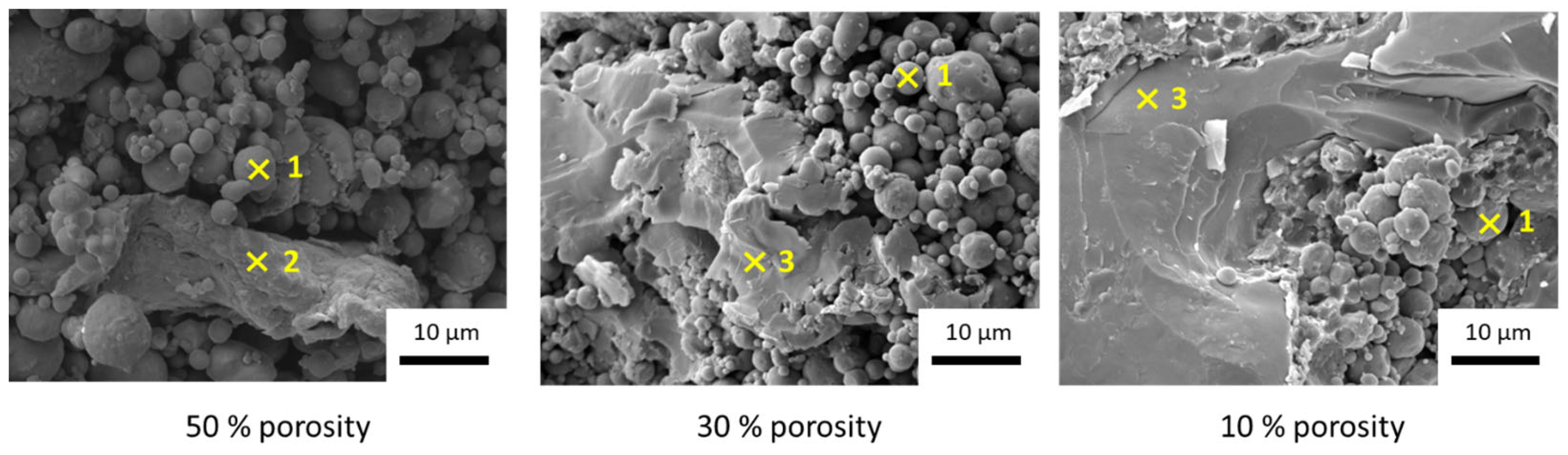

| Temperature (°C) | Pressure (MPa) | Time (min) | Porosity (%) |
|---|---|---|---|
| 400 | 0 | 10 | 50 |
| 400 | 15 | 10 | 30 |
| 400 | 40 | 10 | 10 |
| Avrami–Erofeev Parameters | 50% | 30% | 10% |
|---|---|---|---|
| n | 0.94 (1) | 0.88 (1) | 0.79 (1) |
| k | 0.25 (0.133) | 0.14 (0.109) | 0.12 (0.076) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuzacq, L.; Polido, C.; Silvain, J.-F.; Bobet, J.-L. Hydrogen Production Properties of Aluminum–Magnesium Alloy Presenting β-Phase Al3Mg2. Metals 2023, 13, 1868. https://doi.org/10.3390/met13111868
Cuzacq L, Polido C, Silvain J-F, Bobet J-L. Hydrogen Production Properties of Aluminum–Magnesium Alloy Presenting β-Phase Al3Mg2. Metals. 2023; 13(11):1868. https://doi.org/10.3390/met13111868
Chicago/Turabian StyleCuzacq, Laurent, Chloé Polido, Jean-François Silvain, and Jean-Louis Bobet. 2023. "Hydrogen Production Properties of Aluminum–Magnesium Alloy Presenting β-Phase Al3Mg2" Metals 13, no. 11: 1868. https://doi.org/10.3390/met13111868
APA StyleCuzacq, L., Polido, C., Silvain, J.-F., & Bobet, J.-L. (2023). Hydrogen Production Properties of Aluminum–Magnesium Alloy Presenting β-Phase Al3Mg2. Metals, 13(11), 1868. https://doi.org/10.3390/met13111868









