Experimenting with Dimethyl Sulfoxide to Leach Gold from a Colombian Artisanal Gold Ore
Abstract
:1. Introduction
- To evaluate the efficacy of DMSO in comparison to other non-aqueous solvents for gold dissolution.
- To understand the mechanism behind gold dissolution in DMSO in the presence of halides and determine the optimal conditions for this.
- To assess the scalability and environmental implications of using DMSO for gold recovery, especially considering its interaction with various materials and its recycling potential.
2. Materials and Methods
2.1. Ore Sample
2.2. Gold Extraction Experiments Using DMSO
2.3. Yates Method
2.4. Gold Precipitation
3. Results and Discussion
3.1. Gold Extraction
3.2. Gold Precipitation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keane, S.; Bernaudat, L.; Davis, K.J.; Stylo, M.; Mutemeri, N.; Singo, P.; Twala, P.; Mutemeri, I.; Nakafeero, A.; Etui, I.D. Mercury and artisanal and small-scale gold mining: Review of global use estimates and considerations for promoting mercury-free alternatives. Ambio 2023, 52, 833–852. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Regufe, M.J.; Fiúza, A.; Leite, M.M.; Futuro, A. A systematic review of sustainable gold extraction from raw ores using alternative leaching reagents. Extr. Ind. Soc. 2022, 9, 101018. [Google Scholar] [CrossRef]
- Syed, S. A green technology for recovery of gold from non-metallic secondary sources. Hydrometallurgy 2006, 82, 48–53. [Google Scholar] [CrossRef]
- Kinsman, L.M.; Ngwenya, B.T.; Morrison, C.A.; Love, J.B. Tuneable separation of gold by selective precipitation using a simple and recyclable diamide. Nat. Commun. 2021, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Marsden, J.; House, I. The Chemistry of Gold Extraction; SME–Society for Mining, Metallurgy and Exploration Inc.: Littleton, CO, USA, 2006; p. 650. [Google Scholar]
- Zhang, Y.; Cui, M.; Wang, J.; Liu, X.; Lyu, X. A review of gold extraction using alternatives to cyanide: Focus on current status and future prospects of the novel eco-friendly synthetic gold lixiviants. Miner. Eng. 2022, 176, 107336. [Google Scholar] [CrossRef]
- Nakao, Y.; Sone, K. Reversible dissolution/deposition of gold in iodine–iodide–acetonitrile systems. Chem. Commun. 1996, 8, 897–898. [Google Scholar] [CrossRef]
- Mortier, T.; Persoons, A.; Verbiest, T. Oxidation of solid gold in chloroform solutions of cetyltrimethylammonium bromide. Inorg. Chem. Commun. 2005, 8, 1075–1077. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, R.W.; Jang, S.S.; Wong, C.P.; Hong, J.I. “Organic aqua regia”—Powerful liquids for dissolving noble metals. Angew. Chem. Int. Ed. 2010, 49, 7929–7932. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, G.R.; Al-Bassam, A.Z.; Harris, R.C.; Abbott, A.P.; Smith, D.J.; Holwell, D.A.; Chapman, R.J.; Stanley, C.J. The application of deep eutectic solvent ionic liquids for environmentally friendly dissolution and recovery of precious metals. Miner. Eng. 2016, 87, 18–24. [Google Scholar] [CrossRef]
- Eksteen, J.J.; Oraby, E.A. The leaching and adsorption of gold using low concentration amino acids and hydrogen peroxide: Effect of catalytic ions, sulphide minerals and amino acid type. Miner. Eng. 2015, 70, 36–42. [Google Scholar] [CrossRef]
- Liu, Z.W.; Guo, X.Y.; Tian, Q.H.; Zhang, L. A systematic review of gold extraction: Fundamentals, advancements, and challenges toward alternative lixiviants. J. Hazard. Mater. 2022, 440, 129778. [Google Scholar] [CrossRef] [PubMed]
- Dry, M. A preliminary evaluation of alkaline glycine as an alternative to cyanide for gold extraction. In Proceedings of the Alta Gold-PM Conference, Perth, Australia, 21–28 May 2016. [Google Scholar]
- Yoshimura, A.; Takai, M.; Matsuno, Y. Novel process for recycling gold from secondary sources: Leaching of gold by dimethyl sulfoxide solutions containing copper bromide and precipitation with water. Hydrometallurgy 2014, 149, 177–182. [Google Scholar] [CrossRef]
- ScienceLab.com. 21 May 2013. “Material Safety Data Sheet: Dimethyl Sulfoxide”. Available online: https://web.archive.org/web/20180919061701/http:/www.sciencelab.com/msds.php?msdsId=9927347 (accessed on 24 October 2023).
- Food and Drug Administratin (FDA). 2012. Available online: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073395.pdf (accessed on 30 September 2023).
- Transparency Market Research. Sodium Persulphate Market. 2022. Available online: https://www.transparencymarketresearch.com/sodium-persulfate-market.html (accessed on 23 April 2023).
- Yoshimura, A.; Matsuno, Y. A Novel Process for the Production of Gold Micrometer-Sized Particles from Secondary Sources. Mater. Trans. 2016, 57, 357–361. [Google Scholar] [CrossRef]
- Dodge, Y. The Concise Encyclopedia of Statistics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; 622p. [Google Scholar]
- Riedwyl, H. Modifying and using Yates’ algorithm. Stat. Pap. 1998, 39, 41–60. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2005; 655p. [Google Scholar]
- QY Research. Global Dimethyl Sulfoxide (DMSO) Sales Market Report 2020. 2020. 139p. Available online: https://www.qyresearch.com/index/detail/2195777/global-dimethyl-sulfoxide-dmso-sales-market (accessed on 30 September 2023).
- ReportLinker. Global Dimethyl Sulfoxide (DMSO) Industry. 2023. 215p. Available online: https://www.reportlinker.com/p05817800/Global-Dimethyl-Sulfoxide-DMSO-Industry.html?utm_source=GNW (accessed on 30 September 2023).
- Future Market Insights. DiMethyl Sulfoxide (DMSO) Market by Raw Materials, Application & Region—Forecast 2022–2032. 2022. Available online: https://www.futuremarketinsights.com/reports/dimethyl-sulfoxide-dmso-market (accessed on 30 September 2023).
- Torkaman, P.; Veiga, M.M. Comparing Cyanidation with Amalgamation of a Colombian Artisanal Gold Mining Sample: Suggestion of a Modified Merrill-Crowe Process. Extr. Ind. Soc. 2023, 13, 101208. [Google Scholar]
- Takatori, K.; Kato, H.; Yoshimura, A.; Matsuno, Y. Chalcopyrite Leaching in a Dimethyl Sulfoxide Solution Containing Copper Chloride. Min. Metall. Explor. 2021, 38, 1477–1485. [Google Scholar] [CrossRef]
- Gaylord Chemical. DMSO—Environmental Health and Safety. 2022. Available online: https://www.gaylordchemical.com/environmental-health-safety/ (accessed on 2 October 2023).
| Acronym | Variable | Levels | |
|---|---|---|---|
| S | S:L (Ore:DMSO) | 10% (S0.1) | 30% (S0.3) |
| N | NaCl | 0.6 g (N1) | 1.16 g (N2) |
| C | CuCl2 | 1.34 g (C1) | 2.7 g (C2) |
| T | Time | 8 h (T1) | 24 h (T2) |
| Sample ID | Combination | S:L | Time (h) | NaCl (g) | CuCl2 (g) |
|---|---|---|---|---|---|
| A1 | S0.3T2N2C2 | 30 | 8 | 1.16 | 2.7 |
| A2 | S0.3T2N1C1 | 30 | 24 | 0.6 | 1.34 |
| A3 | S0.3T2N2C1 | 30 | 24 | 1.16 | 1.34 |
| A4 | S0.3T2N1C2 | 30 | 24 | 0.6 | 2.7 |
| A5 | S0.1T1N1C2 | 10 | 8 | 0.6 | 2.7 |
| A6 | S0.1T1N2C1 | 10 | 8 | 1.16 | 1.34 |
| A7 | S0.1T1N2C2 | 10 | 8 | 1.16 | 2.7 |
| A8 | S0.1T1N1C1 | 10 | 8 | 0.6 | 1.34 |
| A9 | S0.3T1N1C1 | 30 | 8 | 0.6 | 1.34 |
| A10 | S0.3T1N2C1 | 30 | 8 | 1.16 | 1.34 |
| A11 | S0.3T1N2C2 | 30 | 8 | 1.16 | 2.7 |
| A12 | S0.3T1N1C2 | 30 | 8 | 0.6 | 2.7 |
| A13 | S0.1T2N2C2 | 10 | 24 | 1.16 | 2.7 |
| A14 | S0.1T2N1C2 | 10 | 24 | 0.6 | 2.7 |
| A15 | S0.1T2N1C1 | 10 | 24 | 0.6 | 1.34 |
| A16 | S0.1T2N2C1 | 10 | 24 | 1.16 | 1.34 |
| Sample ID | Ore (g) | DMSO (mL) | Time (h) | KBr (g/L) | CuBr2 (g/L) | Acid Used for Precipitation |
|---|---|---|---|---|---|---|
| LM-1 | 20 | 100 | 12 | 11.6 | 32.7 | Commercial vinegar |
| LM-2 | 20 | 100 | 12 | 5.8 | 16.4 | H2SO4 (1.0 M) |
| LM-3 | 20 | 100 | 12 | 2.9 | 8.2 | H2SO4 (1.0 M) |
| LM-4 | 20 | 100 | 12 | 5.8 | 16.4 | Diluted (half) lemon juice |
| LM-5 | 20 | 100 | 12 | 5.8 | 16.4 | Undiluted lemon juice |
| Sample ID | % Gold Extracted by DMSO |
|---|---|
| A1 | 49.52 |
| A2 | 62.14 |
| A3 | 79.84 |
| A4 | 30.16 |
| A5 | 86.31 |
| A6 | 78.84 |
| A7 | 80.66 |
| A8 | 82.50 |
| A9 | 89.91 |
| A10 | 65.95 |
| A11 | 59.87 |
| A12 | 81.03 |
| A13 | 94.68 |
| A14 | 93.72 |
| A15 | 91.28 |
| A16 | 91.20 |
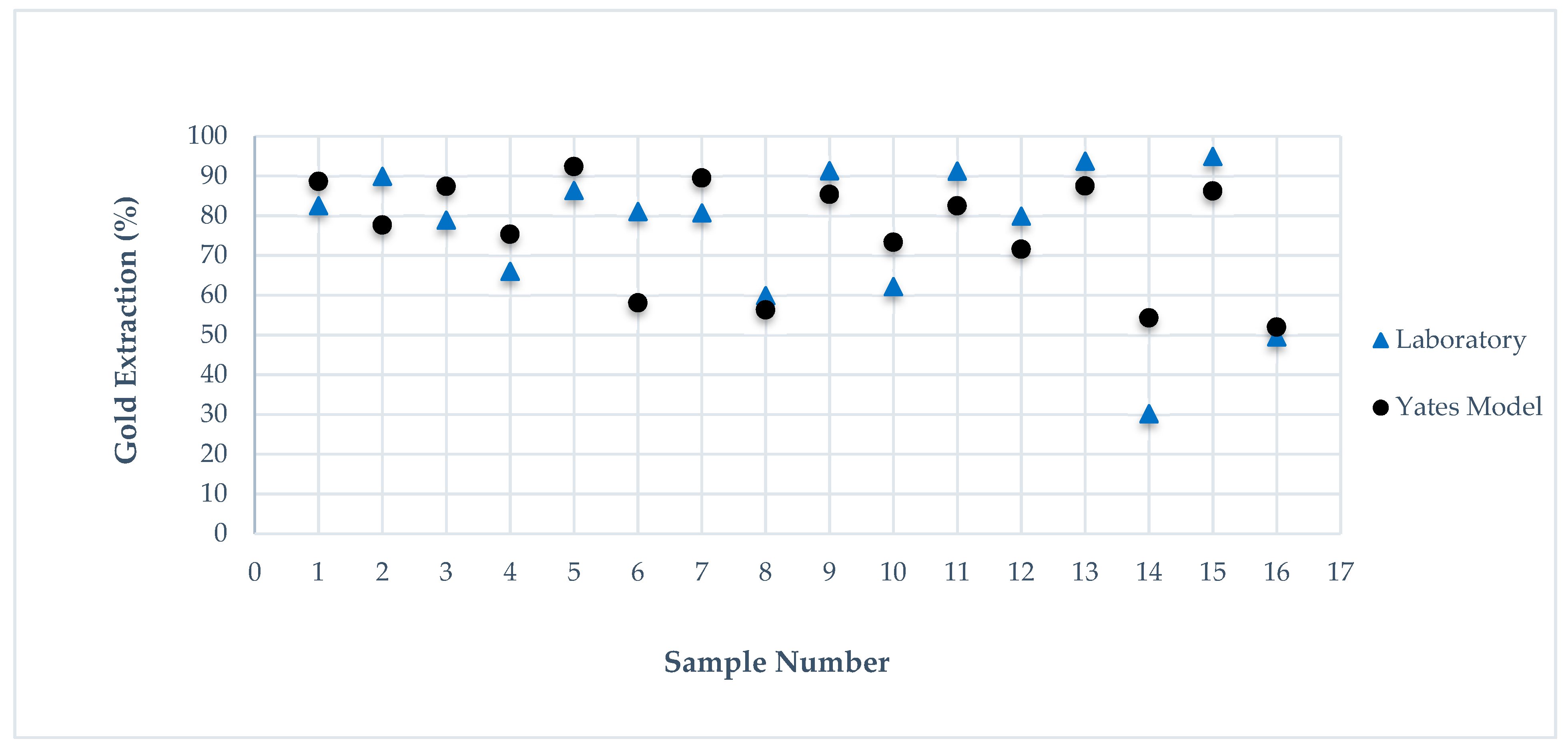
| Au Extracted % | Pred. 1 | Error % | Pred. 2 (No S) | Error % | Pred. 3 (No N) | Error % | Pred. 4 (No C) | Error % | Pred. 5 (No T) | Error % |
|---|---|---|---|---|---|---|---|---|---|---|
| 82.5 | 88.62 | 1.52 | 86.21 | 4.50 | 80.67 | 2.22 | 84.40 | 2.30 | 86.89 | 5.32 |
| 89.91 | 77.64 | 5.45 | 86.21 | 4.12 | 77.93 | 13.32 | 85.48 | 4.93 | 76.03 | 15.44 |
| 78.84 | 87.36 | 1.46 | 72.41 | 8.16 | 80.67 | 2.32 | 79.76 | 1.17 | 85.03 | 7.85 |
| 65.95 | 75.34 | 25.41 | 72.41 | 9.80 | 77.93 | 18.17 | 62.92 | 4.59 | 72.89 | 10.52 |
| 86.31 | 92.34 | 1.55 | 83.67 | 3.06 | 83.47 | 3.29 | 84.40 | 2.21 | 90.01 | 4.29 |
| 81.03 | 58.04 | 19.28 | 83.67 | 3.26 | 70.45 | 13.06 | 85.48 | 5.49 | 55.59 | 31.40 |
| 80.66 | 89.48 | 1.80 | 70.27 | 12.88 | 83.47 | 3.48 | 79.76 | 1.12 | 87.75 | 8.79 |
| 59.87 | 56.3 | 6.35 | 70.27 | 17.37 | 70.45 | 17.67 | 62.92 | 5.09 | 54.69 | 8.65 |
| 91.28 | 85.36 | 1.59 | 76.71 | 15.96 | 91.23 | 0.05 | 92.50 | 1.34 | 86.89 | 4.81 |
| 62.14 | 73.3 | 6.10 | 76.71 | 23.45 | 70.97 | 14.21 | 46.14 | 25.75 | 76.03 | 22.35 |
| 91.2 | 82.5 | 1.46 | 85.51 | 6.24 | 91.23 | 0.03 | 93.02 | 2.00 | 85.03 | 6.77 |
| 79.84 | 71.56 | 19.60 | 85.51 | 7.10 | 70.97 | 11.11 | 64.66 | 19.01 | 72.89 | 8.70 |
| 93.72 | 87.48 | 1.21 | 61.93 | 33.92 | 94.27 | 0.59 | 92.50 | 1.30 | 90.01 | 3.96 |
| 30.16 | 54.26 | 55.47 | 61.93 | 105.34 | 39.81 | 32.00 | 46.14 | 52.98 | 55.59 | 84.32 |
| 94.86 | 86.22 | 1.34 | 72.17 | 23.92 | 94.27 | 0.62 | 93.02 | 1.94 | 87.75 | 7.50 |
| 49.52 | 51.96 | 9.96 | 72.17 | 45.74 | 39.81 | 19.61 | 64.66 | 30.57 | 54.69 | 10.44 |
| AVERAGE | 9.97 | 20.30 | 9.48 | 10.11 | 15.07 |
| Sample ID | Time (h) | Temperature | Au Extracted by DMSO (%) |
|---|---|---|---|
| 1 | 2 | 40 °C | 98.81 |
| 2 | 4 | 40 °C | 97.12 |
| 3 | 6 | 40 °C | 97.36 |
| 4 | 8 | 40 °C | 97.91 |
| 5 | 2 | Ambient | 96.5 |
| 6 | 4 | Ambient | 96.17 |
| 7 | 6 | Ambient | 95.42 |
| 8 | 8 | Ambient | 95.4 |
| Sample ID | Acid Used for Precipitation | Gold Precipitation (%) |
|---|---|---|
| LM-1 | Commercial vinegar | 25 |
| LM-2 | H2SO4 (1.0 M) | 100 |
| LM-3 | H2SO4 (1.0 M) | 89.6 |
| LM-4 | diluted (half) lemon juice | 100 |
| LM-5 | undiluted lemon juice | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torkaman, P.; Yoshimura, A.; Lavkulich, L.M.; Veiga, M.M. Experimenting with Dimethyl Sulfoxide to Leach Gold from a Colombian Artisanal Gold Ore. Metals 2023, 13, 1855. https://doi.org/10.3390/met13111855
Torkaman P, Yoshimura A, Lavkulich LM, Veiga MM. Experimenting with Dimethyl Sulfoxide to Leach Gold from a Colombian Artisanal Gold Ore. Metals. 2023; 13(11):1855. https://doi.org/10.3390/met13111855
Chicago/Turabian StyleTorkaman, Pariya, Akihiro Yoshimura, Leslie M. Lavkulich, and Marcello M. Veiga. 2023. "Experimenting with Dimethyl Sulfoxide to Leach Gold from a Colombian Artisanal Gold Ore" Metals 13, no. 11: 1855. https://doi.org/10.3390/met13111855






