Holistic Investigation of the Inert Thermal Treatment of Industrially Shredded NMC 622 Lithium-Ion Batteries and Its Influence on Selective Lithium Recovery by Water Leaching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Used Materials
2.2. Thermal Treatment Trials
2.3. Water Leaching Trials
3. Results and Discussion
3.1. Off-Gas Analysis during Pyrolysis
3.2. Weight Loss and Particle Size Distribution after Pyrolysis
3.3. XRD Analysis of Pyrolyzed Active Mass

3.4. Results of Water Leaching Trials
3.5. Lithium Leaching over Time
3.6. Leaching Behavior of Other Battery Elements
3.7. Characterization of Salt Product
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Max. Temperature Pyrolysis [°C] | Holding Time Pyrolysis [min] | Weight Loss [%] |
|---|---|---|
| 555.3 | 40.3 | 11.79 |
| 489.5 | 34.8 | 11.13 |
| 572.6 | 15 | 11.41 |
| 612.2 | 42 | 14.33 |
| 534.7 | 48.3 | 12.4 |
| 454.3 | 21.7 | 11.32 |
| 547.3 | 45.3 | 12.21 |
| 524 | 33.3 | 13.59 |
| 463 | 30.3 | 9.84 |
| 576.4 | 30.7 | 12.75 |
| 593.8 | 100.6 | 13.1 |
| 583.1 | 76 | 12.84 |
| 518.2 | 86.3 | 11.5 |
| 518.2 | 79.3 | 10.74 |
| 517.3 | 49.3 | 11.38 |
| 469.8 | 71 | 11.06 |
| 504.7 | 32 | 11.2 |
| 638.5 | 37 | 15.95 |
| 646.3 | 36 | 14.89 |
| 376 | 30.2 | 8.61 |
| 719 | 51 | 16.75 |
| 717.9 | 40 | 16.34 |
| 597.7 | 63 | 14.24 |
| 563.8 | 114.7 | 12.94 |
| 601.6 | 115 | 13.35 |
| 403.6 | 72.6 | 9.15 |
| 445.6 | 101.7 | 9.69 |
References
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Schwich, L.; Schubert, T.; Friedrich, B. Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation. Metals 2021, 11, 177. [Google Scholar] [CrossRef]
- Harper, G.D.J.; Kendrick, E.; Anderson, P.A.; Mrozik, W.; Christensen, P.; Lambert, S.; Greenwood, D.; Das, P.K.; Ahmeid, M.; Milojevic, Z.; et al. Roadmap for a sustainable circular economy in lithium-ion and future battery technologies. J. Phys. Energy 2023, 5, 21501. [Google Scholar] [CrossRef]
- Hanisch, C. Recycling Method for Treating Used Batteries, in Particular Rechargeable Batteries, and Battery Processing Installation. U.S. Patent Application No. 2017076113, 12 October 2017. [Google Scholar]
- Primobius GmbH Process and Technology. Available online: https://www.primobius.com/process-technology (accessed on 3 April 2022).
- Yang, Y.; Huang, G.; Xu, S.; He, Y.; Liu, X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy 2016, 165, 390–396. [Google Scholar] [CrossRef]
- Lombardo, G.; Ebin, B.; Steenari, B.-M.; Alemrajabi, M.; Karlsson, I.; Petranikova, M. Comparison of the effects of incineration, vacuum pyrolysis and dynamic pyrolysis on the composition of NMC-lithium battery cathode-material production scraps and separation of the current collector. Resour. Conserv. Recycl. 2021, 164, 105142. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 97th ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2017; ISBN 978-1-4987-5429-3. [Google Scholar]
- Zhang, G.; Du, Z.; He, Y.; Wang, H.; Xie, W.; Zhang, T. A Sustainable Process for the Recovery of Anode and Cathode Materials Derived from Spent Lithium-Ion Batteries. Sustainability 2019, 11, 2363. [Google Scholar] [CrossRef]
- Petranikova, M.; Miskufova, A.; Havlik, T.; Vojtko, M. Recovery of Cobalt and Lithium from Spent Portable Lithium Accumulators After Incineration. In Proceedings of the Kammel’s Quo Vadis Hydrometallurgy, Herlany, Slowakia, 4–7 June 2012; pp. 155–162. [Google Scholar]
- Vieceli, N.; Casasola, R.; Lombardo, G.; Ebin, B.; Petranikova, M. Hydrometallurgical recycling of EV lithium-ion batteries: Effects of incineration on the leaching efficiency of metals using sulfuric acid. Waste Manag. 2021, 125, 192–203. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, L.; Tang, Y.; Chen, Y.; Ye, L.; Zhu, Y. Study on the reduction roasting of spent LiNixCoyMnzO2 lithium-ion battery cathode materials. J. Therm. Anal. Calorim. 2019, 136, 1323–1332. [Google Scholar] [CrossRef]
- Yue, Y.; Wei, S.; Yongjie, B.; Chenyang, Z.; Shaole, S.; Yuehua, H. Recovering Valuable Metals from Spent Lithium Ion Battery via a Combination of Reduction Thermal Treatment and Facile Acid Leaching. ACS Sustain. Chem. Eng. 2018, 6, 10445–10453. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Weyhe, R.; Friedrich, B. Gas generation measurement and evaluation during mechanical processing and thermal treatment of spent Li-ion batteries. Waste Manag. 2019, 84, 102–111. [Google Scholar] [CrossRef]
- Lombardo, G.; Ebin, B.; Foreman, M.R.S.J.; Steenari, B.-M.; Petranikova, M. Incineration of EV Lithium-ion batteries as a pretreatment for recycling—Determination of the potential formation of hazardous by-products and effects on metal compounds. J. Hazard. Mater. 2020, 393, 122372. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qiu, K. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Balachandran, S.; Forsberg, K.; Lemaître, T.; Vieceli, N.; Lombardo, G.; Petranikova, M. Comparative Study for Selective Lithium Recovery via Chemical Transformations during Incineration and Dynamic Pyrolysis of EV Li-Ion Batteries. Metals 2021, 11, 1240. [Google Scholar] [CrossRef]
- Rouquette, L.M.; Lemaître, T.; Vieceli, N.; Petranikova, M. Intensification of lithium carbonation in the thermal treatment of spent EV Li-ion batteries via waste utilization and selective recovery by water leaching. Resour. Conserv. Recycl. Adv. 2023, 17, 200125. [Google Scholar] [CrossRef]
- Wang, J.-P.; Pyo, J.-J.; Ahn, S.-H.; Choi, D.-H.; Lee, B.-W.; Lee, D.-W. A Study on the Recovery of Li2CO3 from Cathode Active Material NCM(LiNiCoMnO2) of Spent Lithium Ion Batteries. J. Korean Powder Metall. Inst. 2018, 25, 296–301. [Google Scholar] [CrossRef]
- Vishvakarma, S.; Dhawan, N. Recovery of Cobalt and Lithium Values from Discarded Li-Ion Batteries. J. Sustain. Metall. 2019, 5, 204–209. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Xu, Z. Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy. J. Hazard. Mater. 2017, 338, 124–131. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Xu, Z. Novel Approach for in Situ Recovery of Lithium Carbonate from Spent Lithium Ion Batteries Using Vacuum Metallurgy. Environ. Sci. Technol. 2017, 51, 11960–11966. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhu, X. Pyrolysis-Ultrasonic-Assisted Flotation Technology for Recovering Graphite and LiCoO 2 from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10896–10904. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, M. Development of a Two-Stage Pyrolysis Process for the End-Of-Life Nickel Cobalt Manganese Lithium Battery Recycling from Electric Vehicles. Sustainability 2020, 12, 9164. [Google Scholar] [CrossRef]
- Lombardo, G.; Ebin, B.; Foreman, M.R.S.J.; Steenari, B.-M.; Petranikova, M. Chemical Transformations in Li-Ion Battery Electrode Materials by Carbothermic Reduction. ACS Sustain. Chem. Eng. 2019, 7, 13668–13679. [Google Scholar] [CrossRef]
- Lombardo, G. Effects of Pyrolysis and Incineration on the Chemical Composition of Li-Ion Batteries and Analysis of the By-Products; Chalmers University of Technology Nuclear Chemistry/Industrial Materials Recycling: Göteborg, Sweden, 2019. [Google Scholar]
- Stallmeister, C.; Friedrich, B. Influence of Flow-Gas Composition on Reaction Products of Thermally Treated NMC Battery Black Mass. Metals 2023, 13, 923. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Stallmeister, C.; Schwich, L.; Friedrich, B. Early-Stage Li-Removal—Vermeidung von Lithiumverlusten im Zuge der Thermischen und Chemischen Recyclingrouten von Batterien. In Recycling und Rohstoffe; Holm, O., Thomé-Kozmiensky, E., Goldmann, D., Friedrich, B., Eds.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2020; pp. 544–557. ISBN 978-3-944310-51-0. [Google Scholar]
- Wang, H.; Friedrich, B. Development of a Highly Efficient Hydrometallurgical Recycling Process for Automotive Li–Ion Batteries. J. Sustain. Metall. 2015, 1, 168–178. [Google Scholar] [CrossRef]
- Peschel, C.; van Wickeren, S.; Preibisch, Y.; Naber, V.; Werner, D.; Frankenstein, L.; Horsthemke, F.; Peuker, U.; Winter, M.; Nowak, S. Comprehensive Characterization of Shredded Lithium-Ion Battery Recycling Material. Chemistry 2022, 28, e202200485. [Google Scholar] [CrossRef] [PubMed]
- Bernd, F.; Christin, S.; Claudia, S.; Lilian, P. Process Alternatives for Li-Recovery from Spent Batteries. Copenhagen. 2019. Available online: https://www.researchgate.net/publication/336086375_Process_Alternatives_for_Li-recovery_from_spent_batteries (accessed on 3 April 2022).
- Gravritchev, K.S.; Sharpataya, G.A.; Smagin, A.A.; Malyi, E.N.; Matyukha, V.A. Calorimetric study of thermal decomposition of lithium hexafluorophosphate. J. Therm. Anal. Calorim. 2003, 73, 71–83. [Google Scholar] [CrossRef]
- Weber, N.; Schuhmann, S.; Tübke, J.; Nirschl, H. Chemical Thermal Runaway Modeling of Lithium-Ion Batteries for Prediction of Heat and Gas Generation. Energy Technol. 2023, 11, 2300565. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J.; Roth, E.P.; Langendorf, J. Studies on the Thermal Breakdown of Common Li-Ion Battery Electrolyte Components. J. Electrochem. Soc. 2015, 162, A2131–A2135. [Google Scholar] [CrossRef]
- Kriston, A.; Adanouj, I.; Ruiz, V.; Pfrang, A. Quantification and simulation of thermal decomposition reactions of Li-ion battery materials by simultaneous thermal analysis coupled with gas analysis. J. Power Sources 2019, 435, 226774. [Google Scholar] [CrossRef]
- Jakab, E.; Mészáros, E.; Borsa, J. Effect of slight chemical modification on the pyrolysis behavior of cellulose fibers. J. Anal. Appl. Pyrolysis 2010, 87, 117–123. [Google Scholar] [CrossRef]
- Tebbe, J.L.; Fuerst, T.F.; Musgrave, C.B. Mechanism of hydrofluoric acid formation in ethylene carbonate electrolytes with fluorine salt additives. J. Power Sources 2015, 297, 427–435. [Google Scholar] [CrossRef]
- Stich, M.; Göttlinger, M.; Kurniawan, M.; Schmidt, U.; Bund, A. Hydrolysis of LiPF 6 in Carbonate-Based Electrolytes for Lithium-Ion Batteries and in Aqueous Media. J. Phys. Chem. C 2018, 122, 8836–8842. [Google Scholar] [CrossRef]
- de Jesus Silva, A.J.; Contreras, M.M.; Nascimento, C.R.; Da Costa, M.F. Kinetics of thermal degradation and lifetime study of poly(vinylidene fluoride) (PVDF) subjected to bioethanol fuel accelerated aging. Heliyon 2020, 6, e04573. [Google Scholar] [CrossRef] [PubMed]
- O’shea, M.L.; Morterra, C.; Low, M. Spectroscopic studies of carbons. XVII. Pyrolysis of polyvinylidene fluoride. Mater. Chem. Phys. 1990, 26, 193–209. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Freiberg, A.T.; Sicklinger, J.; Solchenbach, S.; Gasteiger, H.A. Li2CO3 decomposition in Li-ion batteries induced by the electrochemical oxidation of the electrolyte and of electrolyte impurities. Electrochim. Acta 2020, 346, 136271. [Google Scholar] [CrossRef]
- Leisegang, T.; Meutzner, F.; Zschornak, M.; Münchgesang, W.; Schmid, R.; Nestler, T.; Eremin, R.A.; Kabanov, A.A.; Blatov, V.A.; Meyer, D.C. The Aluminum-Ion Battery: A Sustainable and Seminal Concept? Front. Chem. 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed]
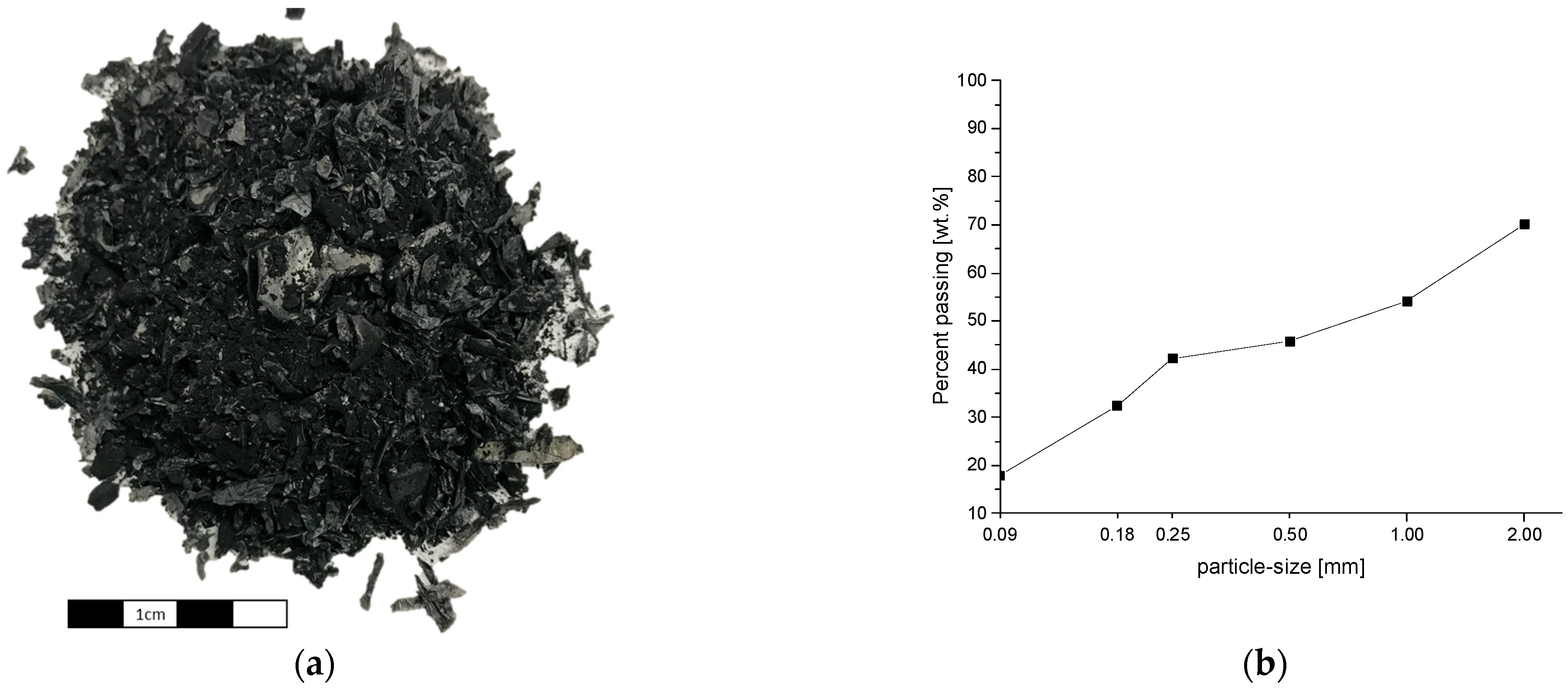

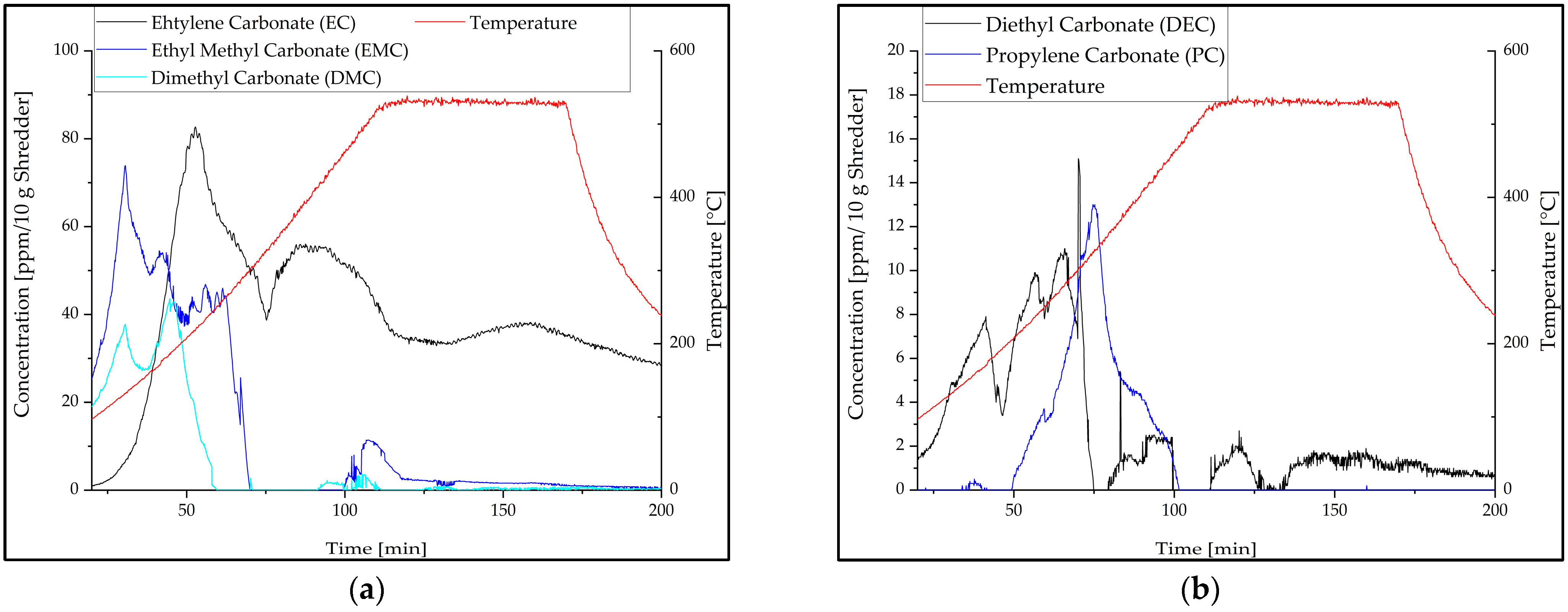



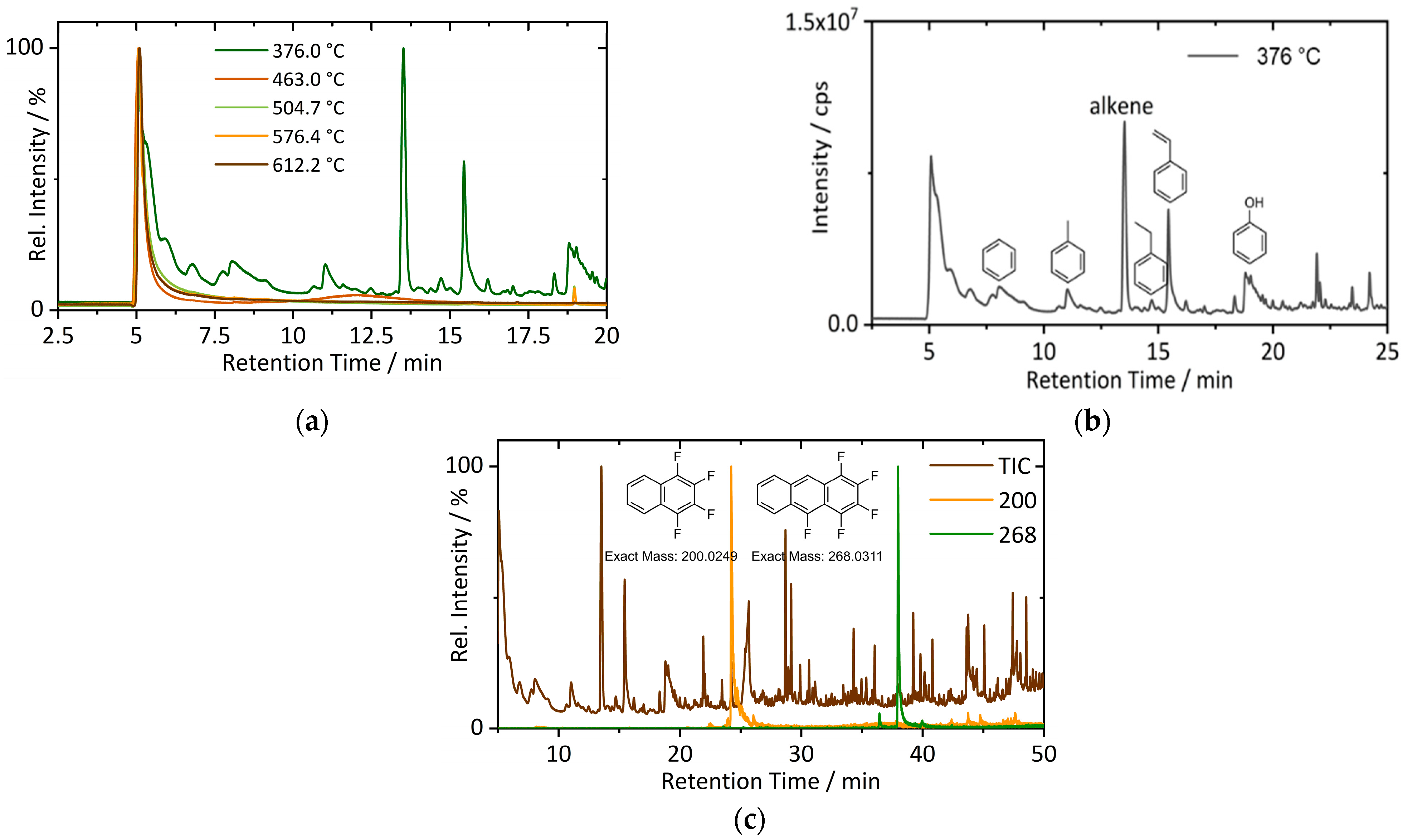
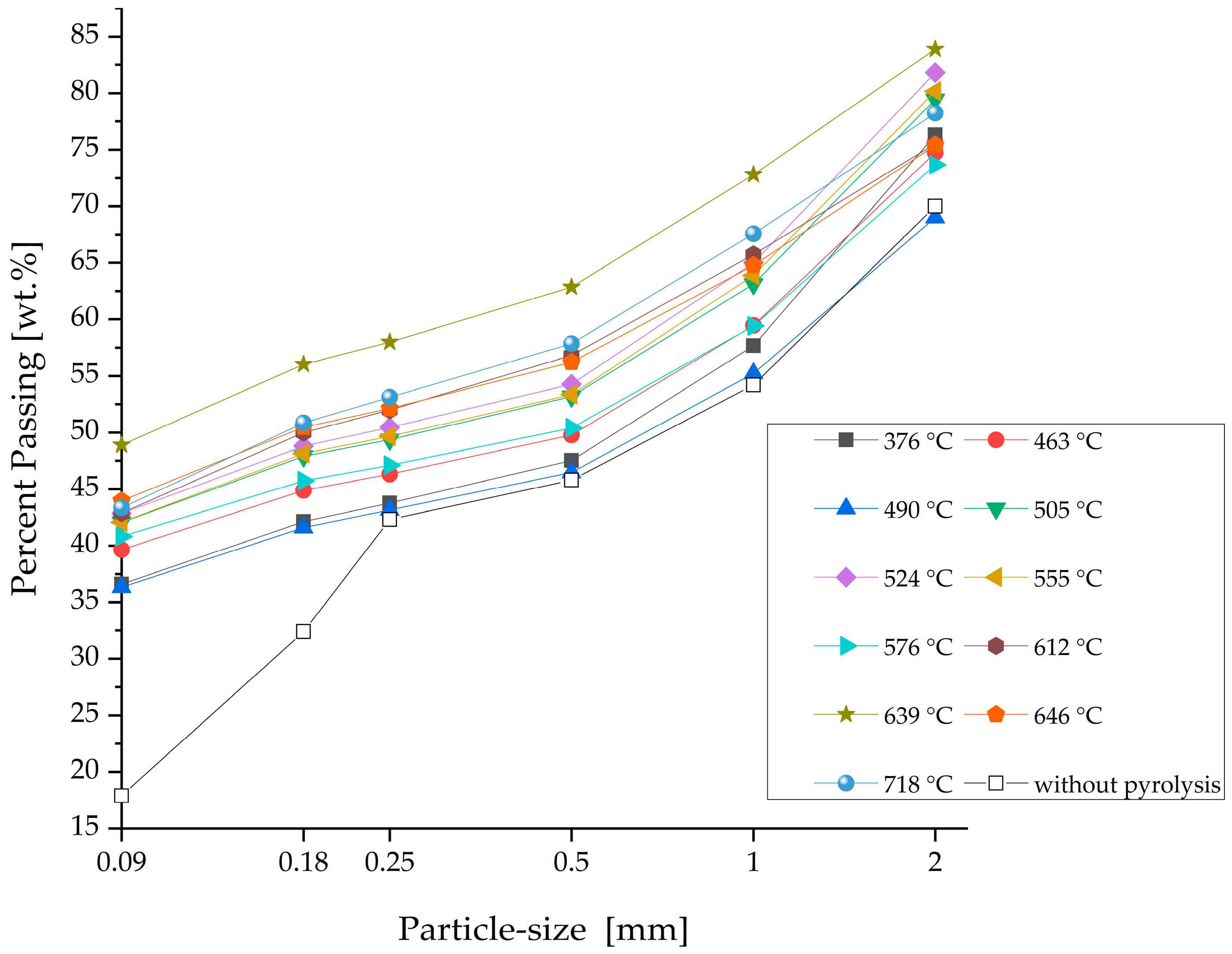

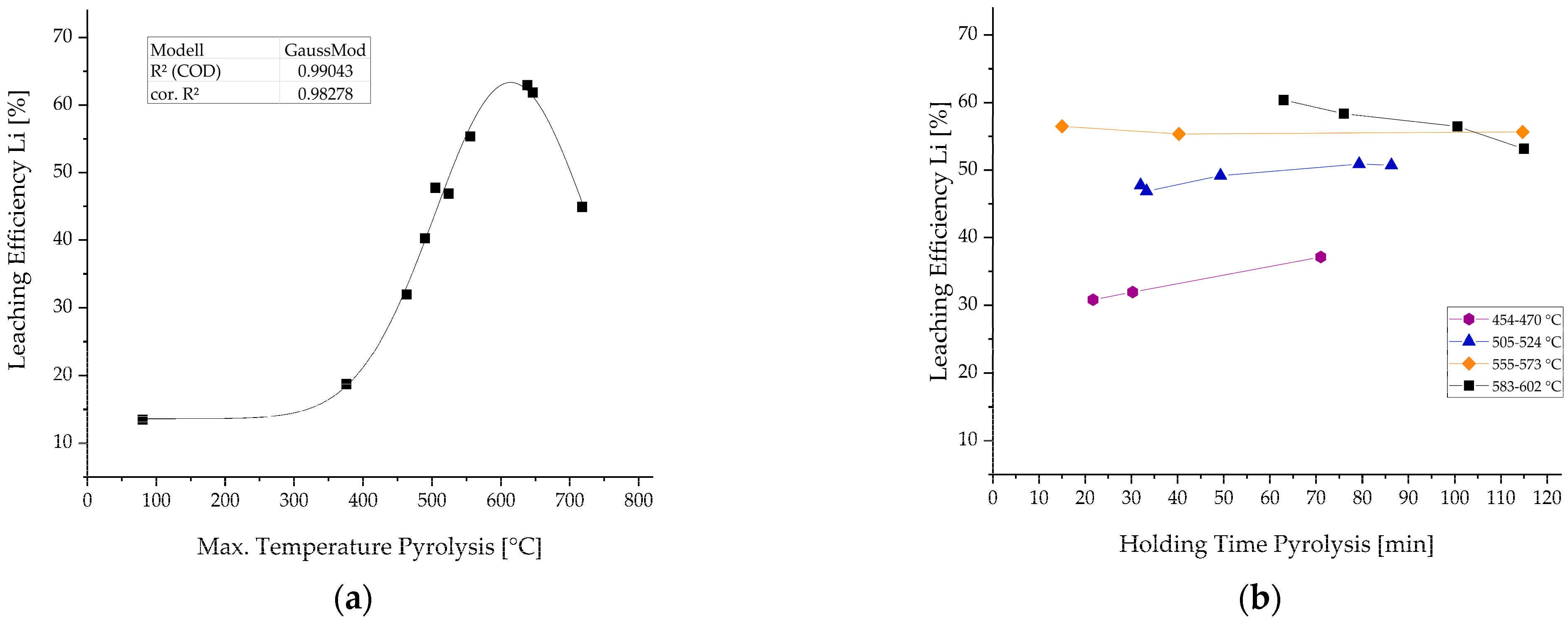
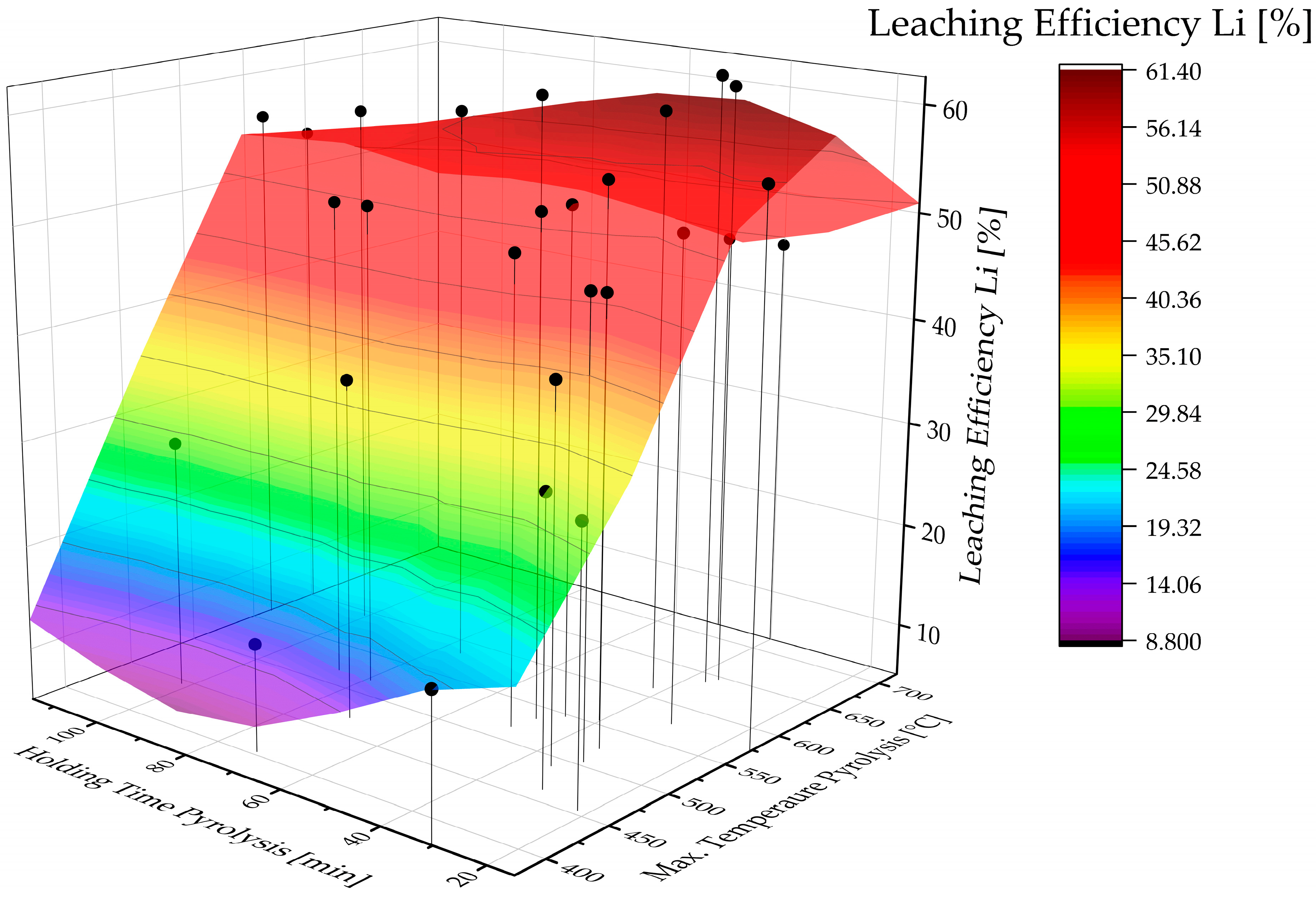



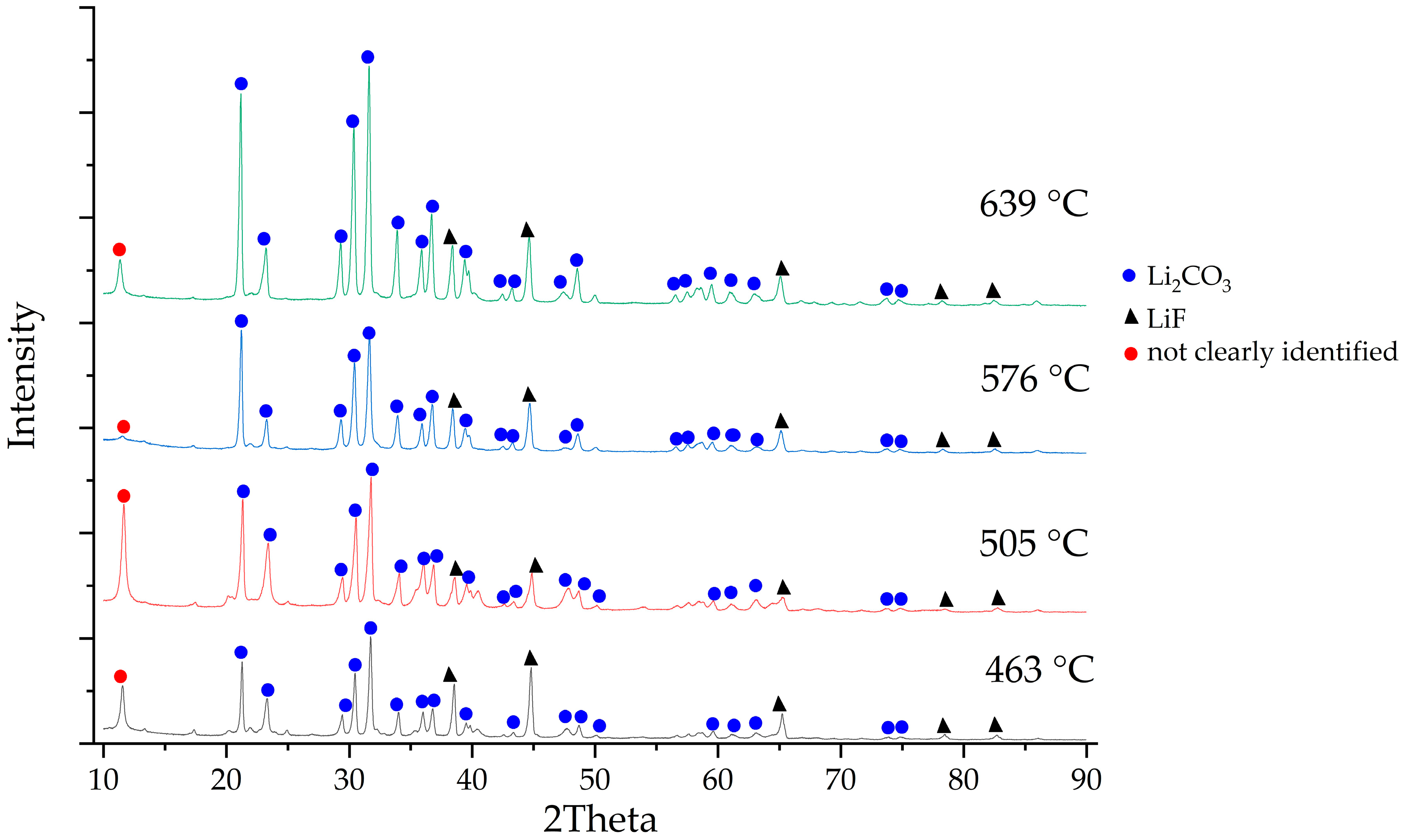
| Input Material | Experimental Parameters | Results | Source |
|---|---|---|---|
| Spent NMC cathode active material | Heating and cooling under Ar atmosphere, holding at 800 °C under CO2 atmosphere for 2 h | 88.97% Li recovered | [22] |
| Water leaching: 33.33 g/L, 5 h | |||
| Spent NMC cathode active material, addition of lignite | 500–900 °C under Ar atmosphere for 3 h, Addition of lignite: 25.2%, Water leaching: 20 g/L, 2 h, | LE (Li)500 °C: 74.6% LE (Li)650 °C: 89.4% LE (Li)900 °C: 55.3% | [13] |
| Pure LiCoO2 + graphite (4:3) | Oxygen-free roasting at 1000 °C for 30 min under N2 Wet magnetic separation in H2O, 5 g/L, 48 h; 20 °C | LE (Li)1000 °C: 98.93% | [31] |
| Spent LIBs (not specified) | Oxygen-free roasting at 1000 °C for 30 min under N2 Wet magnetic separation in H2O: 5 g/L, 48 h; 20 °C | LE (Li)1000 °C: 70.43% | [31] |
| active mass < 53 µm of shredded LCO LIBs (organics burned at 450 °C for 30 min) | Atmospheric heat treatment 500–900 °C for 5–45 min under air, Water leaching: 50 g/L, 30 min | Best reduction at 900 °C, 15 min (XRD), 6 g Li2CO3 out of 100 g LIBs, no LE or recovery rate given | [23] |
| Active mass from spent and shredded LMO LIBs | Oxygen-free roasting at 800 °C, for 45 min, Enclosed vacuum Water leaching: 10 g/L, 20–30 min | Li recovery in form of Li2CO3: 91.3% | [24] |
| NMC battery cells | Thermal treatment of battery cells at
parameters of active mass fraction < 1 mm | Higher Li-yields for higher temperature in thermal treatment, Best result: Li-Yield (Carbonate)603 °C: 64% (33 g/L, 120 min, washed filter cake) | [3] |
| Cathode, anode and separator foil from NMC 111 cells | Thermal treatment: pyrolysis, incineration, pyrolysis + coke at different temperatures from 400 to 700 °C for 30–90 min Water leaching: 100 g/L and 200 g/L, for 3 h, at RT and 80 °C | Higher LE for higher temperature and pyrolysis, no effect of coke Best result: LE (Li)700 °C pyrolysis for 60 min; 200 g/L at 80 °C 3 h: 60% | [20] |
| Different spent NMC cathode material + coke | Thermal treatment 500–650 °C, for 15–120 min under Ar, Coke addition 7.5–20% Water leaching: 33.33 g/L for 1 h at RT | Best Li-yields for roasting at 650 °C for 30 min and coke addition of 10% LE(Li)650 °C, 30 min, 10% coke: 93.67% | [14] |
| Spent NMC battery cells, manually dismantled, with and without separator | Thermal treatment 400–700 °C for 30–90 min under Ar and air Water leaching: 20 g/L for 1 min to 3 h at RT | Best Li-yields for pyrolysis with separator, at 700 °C for 60 min LE (Li)700 °C, 60 min, with separator: 62% | [21] |
| Spent batteries of type LMO, LCO, NMC | Vacuum pyrolysis of each separated active mass at 600–1000 °C for 15–90 min, Water leaching 50 and 25 g/L | Best Li-yields with 50 g/L LE (Li)700 °C, 30 min, LMO: 81.9% LE (Li)700 °C, 30 min, LCO: 82.7% LE (Li)700 °C, 30 min, NMC: 66.3% | [25] |
| Compound | Li | Ti | Mn | S | Co | Ni | Cu | Al | Si | P | Zr | F | C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | ICP-OES | IC | Comb. | ||||||||||
| Mean | 2.51 | 0.01 | 4.26 | 0.19 | 4.56 | 14.20 | 4.51 | 5.04 | 0.11 | 0.57 | 0.15 | 3.51 | 34.55 |
| Max. Temperature Pyrolysis [°C] | Li | Al | F | S | Na | P |
|---|---|---|---|---|---|---|
| 463 | 16.09 | 4.36 | 19.01 | 1.19 | 0.61 | 0.27 |
| 505 | 13.82 | 6.62 | 6.01 | 0.86 | 0.45 | 0.16 |
| 576 | 17.31 | 2.30 | 12.94 | 0.70 | 0.43 | 0.00 |
| 639 | 16.78 | 2.32 | 8.40 | 0.44 | 0.34 | 0.00 |
| 646 | 17.04 | 1.99 | 6.89 | 0.51 | 0.40 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stallmeister, C.; Friedrich, B. Holistic Investigation of the Inert Thermal Treatment of Industrially Shredded NMC 622 Lithium-Ion Batteries and Its Influence on Selective Lithium Recovery by Water Leaching. Metals 2023, 13, 2000. https://doi.org/10.3390/met13122000
Stallmeister C, Friedrich B. Holistic Investigation of the Inert Thermal Treatment of Industrially Shredded NMC 622 Lithium-Ion Batteries and Its Influence on Selective Lithium Recovery by Water Leaching. Metals. 2023; 13(12):2000. https://doi.org/10.3390/met13122000
Chicago/Turabian StyleStallmeister, Christin, and Bernd Friedrich. 2023. "Holistic Investigation of the Inert Thermal Treatment of Industrially Shredded NMC 622 Lithium-Ion Batteries and Its Influence on Selective Lithium Recovery by Water Leaching" Metals 13, no. 12: 2000. https://doi.org/10.3390/met13122000






