Transfer of Early-Stage Lithium Recovery from Laboratory-Scale Water Leaching to Upscale Challenges
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory-Scale Leaching
2.2. Upscale Leaching
3. Results
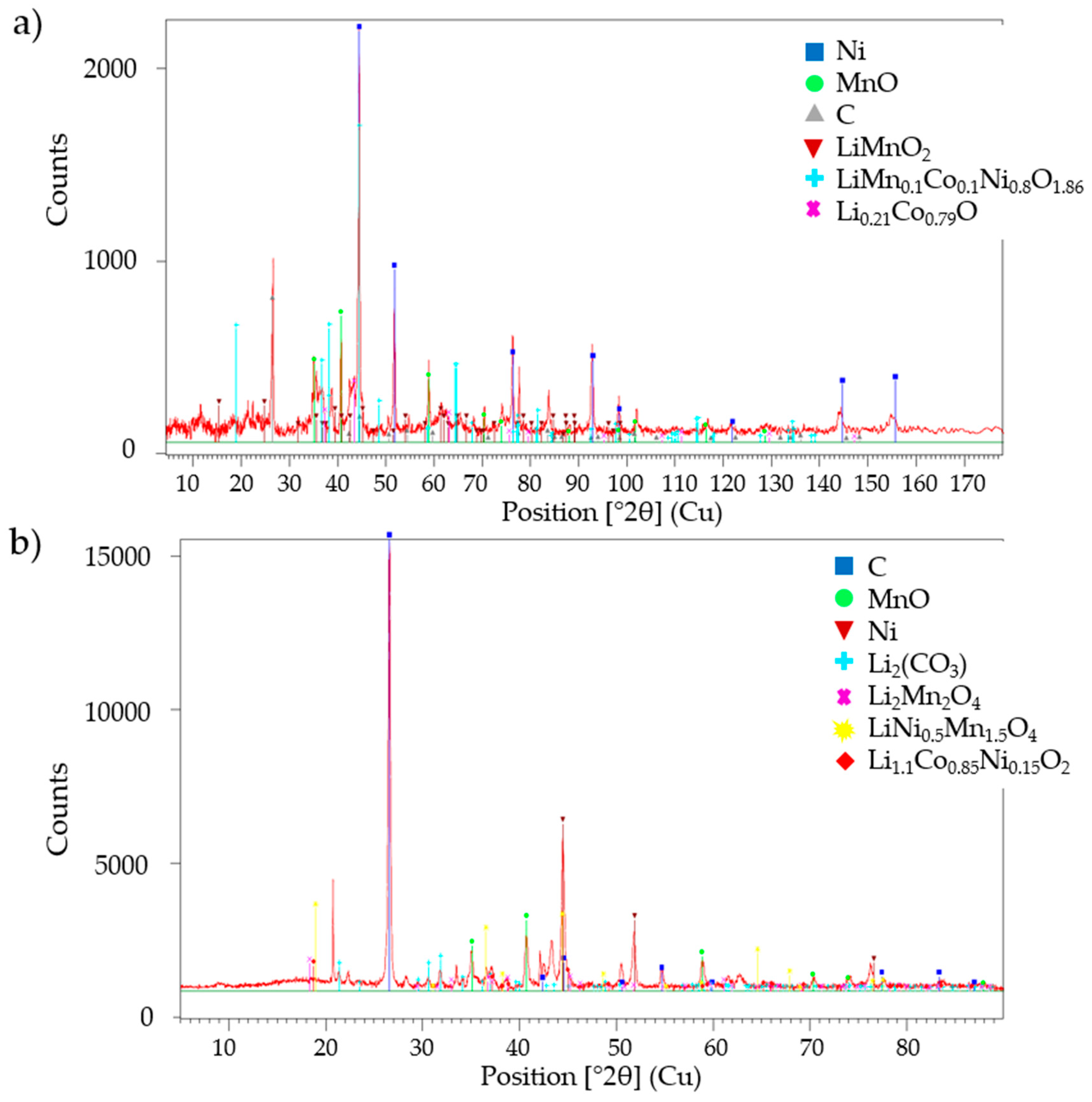
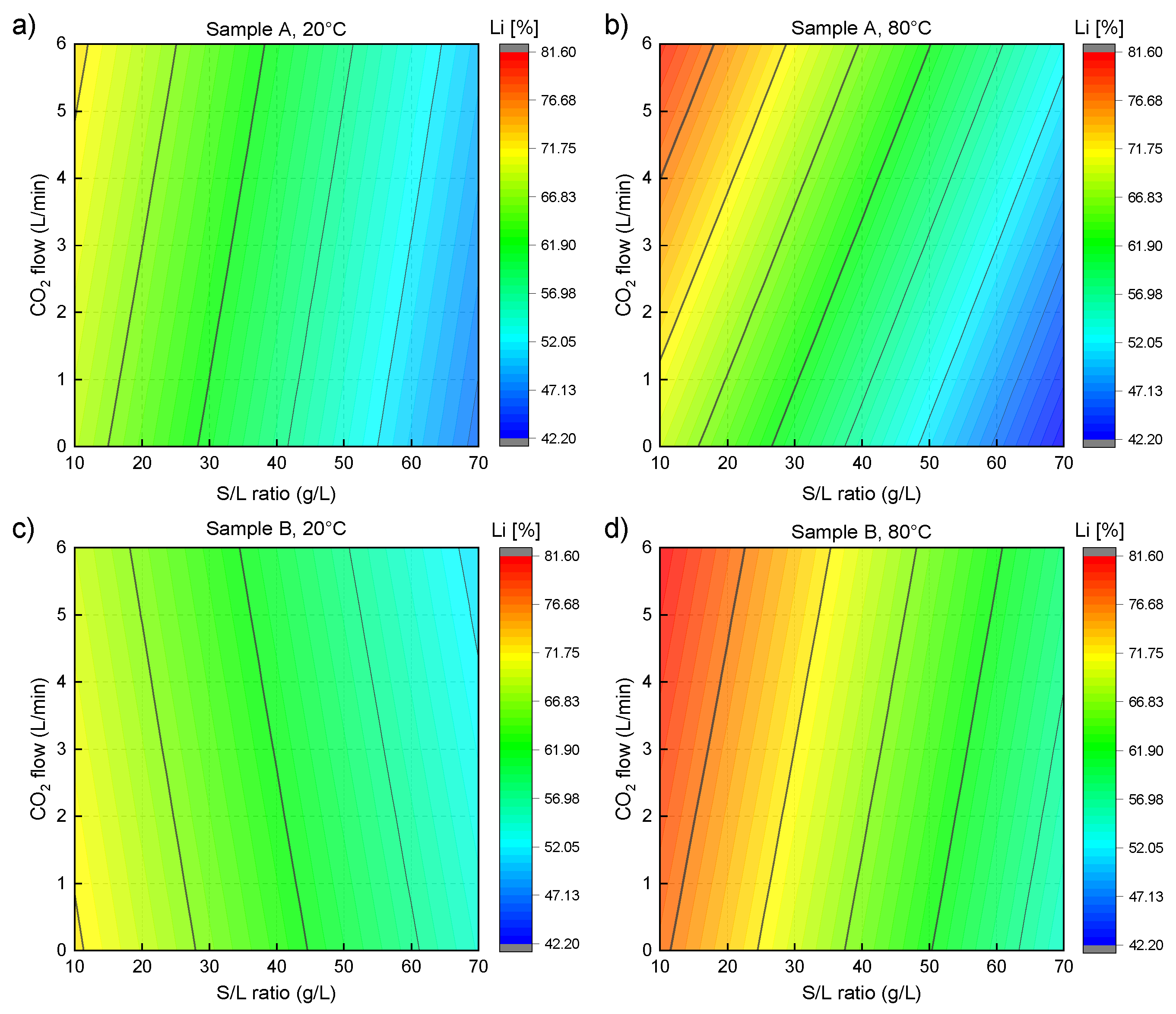
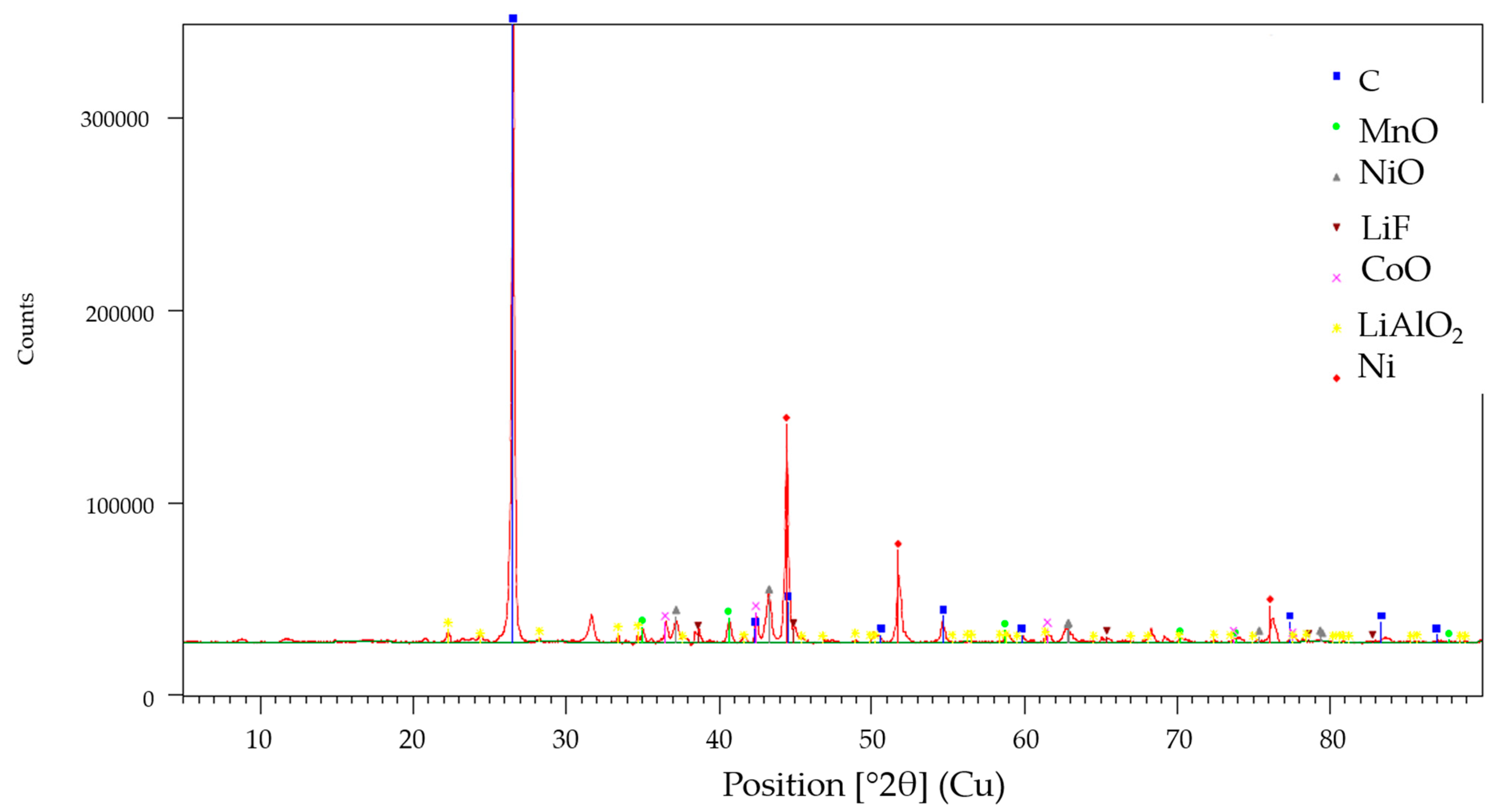
3.1. Laboratory-Scale Water Leaching Efficiency—Lithium
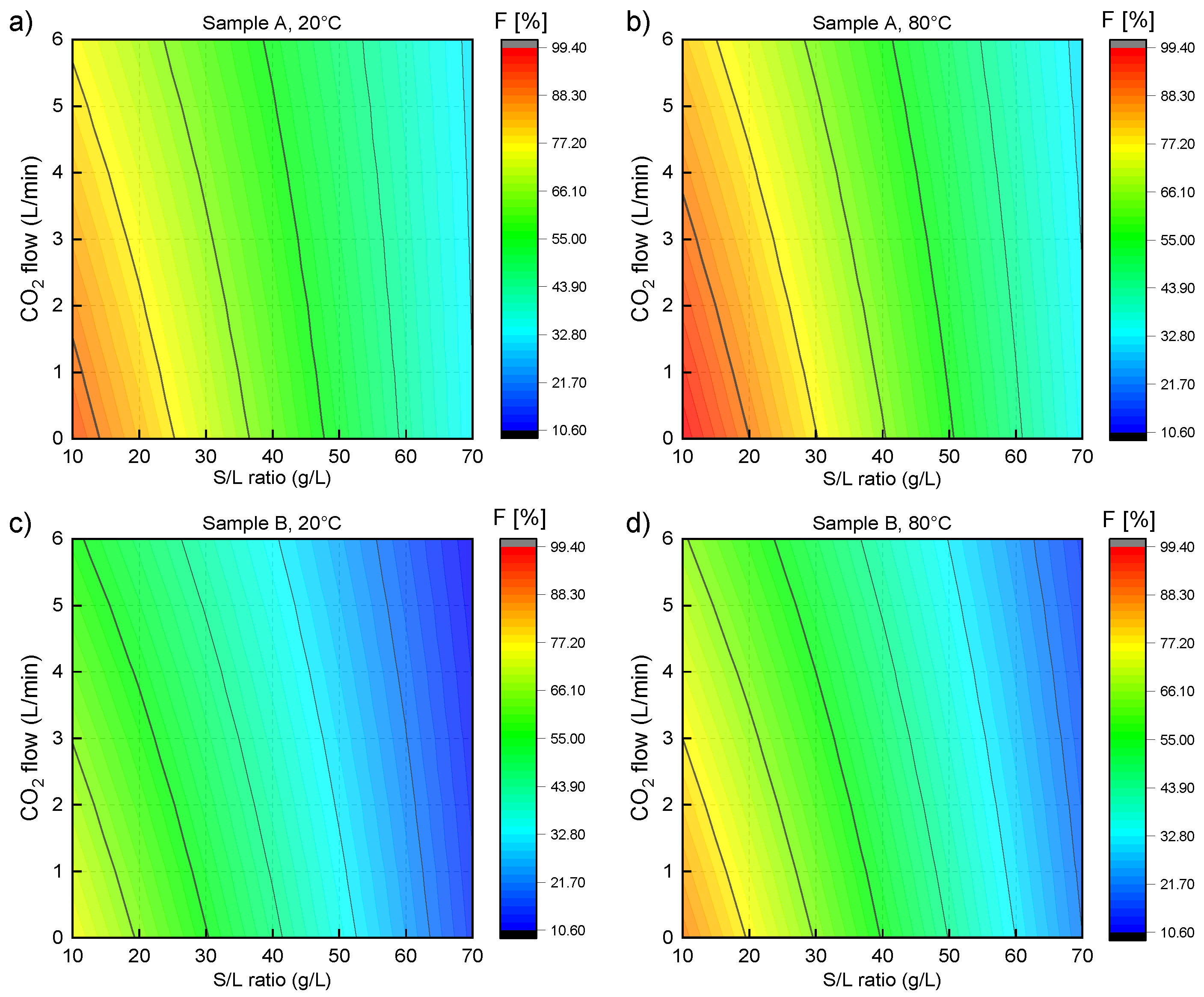
3.2. Laboratory-Scale Water Leaching Efficiency—Fluorine
3.3. pH Evaluation
3.4. Optimization and Upscale Water Leaching
- No upper limit was set for F leaching efficiency, and the target was set for 0% leaching efficiency;
- The lower limit set for Li leaching efficiency was 42.2%, which was the low value resulting from the laboratory-scale trials, and the target was set for 100%;
- The proportional weights of Li and F leaching efficiency in the optimization were set as equal, meaning that Li or F have the same importance for the result.
- The upper limit set for F leaching efficiency was 50%, and the target was the lowest achieved leaching efficiency of 10.6%;
- The lower limit set for Li leaching efficiency was 60%, and the target was the highest achieved leaching efficiency of 81.6%;
- The proportional weight of Li leaching efficiency in the optimization was higher than F leaching efficiency, meaning that it is more important to maximize Li leaching efficiency than to minimize F leaching efficiency.
4. Discussion
- The geometric similarity states that the ratio between the dimensions of the reactors should be proportional on both scales, which was not fully respected in this work, since, for example, the laboratory-scale reactor contained no baffles on the walls, while the upscale reactor contained four.
- The impeller, which is another construction parameter that influences the solution agitation and enhances leaching efficiencies in general, in this work was also different in size and type, not achieving the proportional similarity. It was also hypothesized that although the stirring rate of 150 rpm provided great turbulence, it was not enough, which caused the settlement of the particles on the bottom, in the cupped region of the reactor, consequently hindering the dissolution. A higher stirring speed was tested, but spilling was observed since the liquid surface was only 10 cm away from the boarder of the reactor.
- In Sample B, which has particle sizes < 1000 μm, the settlement velocity is probably higher, what contributes to the concentration of particles on the bottom of the reactor. However, lower particle sizes are unfeasible in industry application due to mechanical crushing being a costly process.
- Another aspect considered to contribute to the lower Li leaching efficiency in the upscale was the dwell time of 1 h, which might not be enough when a much higher amount of BM was used, and combined with the mentioned settlement, it affects the kinetics.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouquette, L.M.; Lemaître, T.; Vieceli, N.; Petranikova, M. Intensification of lithium carbonation in the thermal treatment of spent EV Li-ion batteries via waste utilization and selective recovery by water leaching. Resour. Conserv. Recycl. Adv. 2023, 17, 200125. [Google Scholar] [CrossRef]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Hu, X.; Mousa, E.; Ye, G. Recovery of Co, Ni, Mn, and Li from Li-ion batteries by smelting reduction—Part II: A pilot-scale demonstration. J. Power Sources 2021, 483, 229089. [Google Scholar] [CrossRef]
- Dobó, Z.; Dinh, T.; Kulcsár, T. A review on recycling of spent lithium-ion batteries. Energy Rep. 2023, 9, 6362–6395. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Lithium Carbonate Recovery from Cathode Scrap of Spent Lithium-Ion Battery: A Closed-Loop Process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Sattar, R.; Ilyas, S.; Bhatti, H.N.; Ghaffar, A. Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep. Purif. Technol. 2019, 209, 725–733. [Google Scholar] [CrossRef]
- Schwich, L.; Schubert, T.; Friedrich, B. Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation. Metals 2021, 11, 177. [Google Scholar] [CrossRef]
- Liu, F.; Peng, C.; Ma, Q.; Wang, J.; Zhou, S.; Chen, Z.; Wilson, B.P.; Lundström, M. Selective lithium recovery and integrated preparation of high-purity lithium hydroxide products from spent lithium-ion batteries. Sep. Purif. Technol. 2021, 259, 118181. [Google Scholar] [CrossRef]
- Di, C.; Yongming, C.; Yan, X.; Cong, C.; Yafei, J.; Fang, H. Selective Recovery of Lithium from Ternary Spent Lithium-Ion Batteries Using Sulfate Roasting-Water Leaching Process. In Energy Technology 2020: Recycling, Carbon Dioxide Management, and Other Technologies; Chen, X., Zhong, Y., Zhang, L., Howarter, J.A., Baba, A.A., Wang, C., Sun, Z., Zhang, M., Olivetti, E., Luo, A., et al., Eds.; Springer International Publishing; Springer: Cham, Switzerland, 2020; pp. 387–395. [Google Scholar]
- Ma, Y.; Tang, J.; Wanaldi, R.; Zhou, X.; Wang, H.; Zhou, C.; Yang, J. A promising selective recovery process of valuable metals from spent lithium ion batteries via reduction roasting and ammonia leaching. J. Hazard. Mater. 2021, 402, 123491. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, L.; Tang, Y.; Chen, Y.; Ye, L.; Zhu, Y. Study on the reduction roasting of spent LiNixCoyMnzO2 lithium-ion battery cathode materials. J. Therm. Anal. Calorim 2019, 136, 1323–1332. [Google Scholar] [CrossRef]
- Kuzuhara, S.; Ota, M.; Tsugita, F.; Kasuya, R. Recovering Lithium from the Cathode Active Material in Lithium-Ion Batteries via Thermal Decomposition. Metals 2020, 10, 433. [Google Scholar] [CrossRef]
- Tao, R.; Xing, P.; Li, H.; Sun, Z.; Wu, Y. Recovery of spent LiCoO2 lithium-ion battery via environmentally friendly pyrolysis and hydrometallurgical leaching. Resour. Conserv. Recycl. 2022, 176, 105921. [Google Scholar] [CrossRef]
- Yan, Z.; Sattar, A.; Li, Z. Priority Lithium recovery from spent Li-ion batteries via carbothermal reduction with water leaching. Resour. Conserv. Recycl. 2023, 192, 106937. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, X.; Tay, C.Y.; He, Y.; Wang, H.; Duan, C. Selective recycling of lithium from spent lithium-ion batteries by carbothermal reduction combined with multistage leaching. Sep. Purif. Technol. 2023, 314, 123555. [Google Scholar] [CrossRef]
- Chordia, M.; Nordelöf, A.; Ellingsen, L.A.-W. Environmental life cycle implications of upscaling lithium-ion battery production. Int. J. Life Cycle Assess. 2021, 26, 2024–2039. [Google Scholar] [CrossRef]
- Sommerville, R.; Zhu, P.; Rajaeifar, M.A.; Heidrich, O.; Goodship, V.; Kendrick, E. A qualitative assessment of lithium ion battery recycling processes. Resour. Conserv. Recycl. 2021, 165, 105219. [Google Scholar] [CrossRef]
- Saju, D.; Ebenezer, J.; Chandran, N.; Chandrasekaran, N. Recycling of Lithium Iron Phosphate Cathode Materials from Spent Lithium-Ion Batteries: A Mini-Review. Ind. Eng. Chem. Res. 2023, 62, 11768–11783. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001; ISBN 0471316490. [Google Scholar]
- Stallmeister, C.; Friedrich, B. Influence of Flow-Gas Composition on Reaction Products of Thermally Treated NMC Battery Black Mass. Metals 2023, 13, 923. [Google Scholar] [CrossRef]
- Balachandran, S.; Forsberg, K.; Lemaître, T.; Vieceli, N.; Lombardo, G.; Petranikova, M. Comparative Study for Selective Lithium Recovery via Chemical Transformations during Incineration and Dynamic Pyrolysis of EV Li-Ion Batteries. Metals 2021, 11, 1240. [Google Scholar] [CrossRef]
- Mishra, G.; Jha, R.; Meshram, A.; Singh, K.K. A review on recycling of lithium-ion batteries to recover critical metals. J. Environ. Chem. Eng. 2022, 10, 108534. [Google Scholar] [CrossRef]
- Porvali, A.; Mäkelä, T.; Bachér, J. Observations on the Leaching of Milled Black Mass with Additives. J. Sustain. Metall. 2023, 9, 816–825. [Google Scholar] [CrossRef]
- Nylén, P.; Wigren, N.; Joppien, G. Einführung in die Stöchiometrie: Kurzes Lehrbuch der Allgemeinen und Physikalischen Chemie; mit 516 Aufgaben und Lösungen, 18., Vollst. Überarb. Aufl.; Steinkopff: Darmstadt, Germany, 1991; ISBN 3798508038. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2017; ISBN 9781498754286. [Google Scholar]
- Stubblefield, C.B.; Bach, R.O. Solubility of lithium fluoride in water. J. Chem. Eng. Data 1972, 17, 491–492. [Google Scholar] [CrossRef]
- Yi, W.-T.; Yan, C.-Y.; Ma, P.-H. Kinetic study on carbonation of crude Li2CO3 with CO2-water solutions in a slurry bubble column reactor. Korean J. Chem. Eng. 2011, 28, 703–709. [Google Scholar] [CrossRef]
- Plakhotnyk, A.V.; Ernst, L.; Schmutzler, R. Hydrolysis in the system LiPF6—Propylene carbonate—Dimethyl carbonate—H2O. J. Fluor. Chem. 2005, 126, 27–31. [Google Scholar] [CrossRef]
- Ende, D.J. Chemical Engineering in the Pharmaceutical Industry: R&D to Manufacturing; Scale-Up of Mixing Processes: A Primer; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 0470882220. [Google Scholar]
- Coker, A.K. Modeling of Chemical Kinetics and Reactor Design; Scale-Up in Reactor Design; Gulf Professional Pub: Boston, MA, USA, 2001; ISBN 978-0-88415-481-5. [Google Scholar]

| Parameter/Levels | Low | High |
|---|---|---|
| Black mass batch | Sample A—63 µm | Sample B—1000 µm |
| CO2 gas flow (L/min) | 0 | 6 |
| Temperature (°C) | 20 | 80 |
| S/L ratio (g/L) | 10 | 70 |
| Li | F | Ni | Co | Mn | Fe | Al | Cu | P | C | |
|---|---|---|---|---|---|---|---|---|---|---|
| % | ||||||||||
| Sample A | 2.64 | 2.44 | 5.20 | 4.83 | 5.77 | 2.66 | 3.06 | 3.30 | 0.69 | 47.0 |
| Sample B | 4.17 | 2.96 | 12.6 | 11.0 | 9.20 | 1.19 | 4.57 | 3.38 | 0.73 | 25.8 |
| pH | ||||
|---|---|---|---|---|
| Time (min) | No CO2 Gas S/L Ratio 10 g/L | No CO2 Gas S/L Ratio 70 g/L | CO2 Gas 6 L/min S/L Ratio 10 g/L | CO2 Gas 6 L/min S/L Ratio 70 g/L |
| 0 (initial) | 6.91 | 6.72 | 4.39 | 4.60 |
| 60 | 10.12 | 10.60 | 6.49 | 7.30 |
| Solution | Sample | Temperature (°C) | CO2 Flow (L/min) | S/L Ratio (g/L) | F Fit (%) | Li Fit (%) | Composite Desirability a |
|---|---|---|---|---|---|---|---|
| Scenario 1 | B | 80 | 6 | 30 | 49.87 | 73.75 | 0.027 |
| Scenario 2 | B | 80 | 6 | 41.51 | 40.08 | 69.26 | 0.529 |
| Li Leaching Efficiency (%) | F Leaching Efficiency (%) | |
|---|---|---|
| Laboratory Scale | 70.52 ± 2.11 | 34.54 ± 4.47 |
| Upscale | 60.95 ± 3.00 | 52.93 ± 3.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munchen, D.D.; Milicevic Neumann, K.; Öner, I.E.; Friedrich, B. Transfer of Early-Stage Lithium Recovery from Laboratory-Scale Water Leaching to Upscale Challenges. Metals 2024, 14, 67. https://doi.org/10.3390/met14010067
Munchen DD, Milicevic Neumann K, Öner IE, Friedrich B. Transfer of Early-Stage Lithium Recovery from Laboratory-Scale Water Leaching to Upscale Challenges. Metals. 2024; 14(1):67. https://doi.org/10.3390/met14010067
Chicago/Turabian StyleMunchen, Daniel Dotto, Ksenija Milicevic Neumann, Ilayda Elif Öner, and Bernd Friedrich. 2024. "Transfer of Early-Stage Lithium Recovery from Laboratory-Scale Water Leaching to Upscale Challenges" Metals 14, no. 1: 67. https://doi.org/10.3390/met14010067






