Wear Mechanism of Fe/Cu Self-Lubricating Composite Coatings Fabricated by Electro-Explosive Spraying under Dry Friction
Abstract
:1. Introduction
2. Preparation of the Composite Coating
2.1. Electro-Explosive Spraying Equipment
2.2. Materials and Parameters
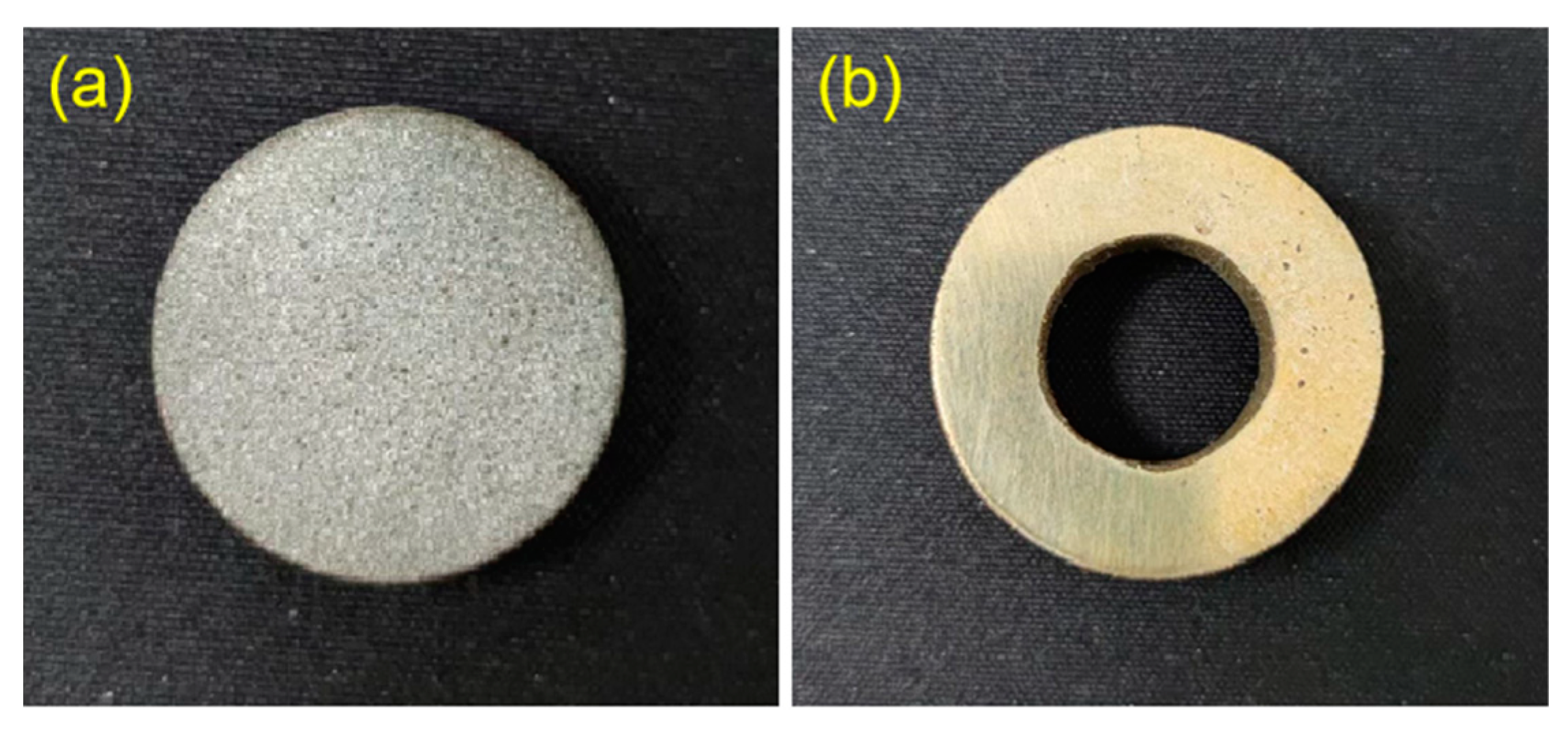
2.3. Design and Preparation of Composite Coatings
3. Characterization of Coatings
4. Results and Discussion
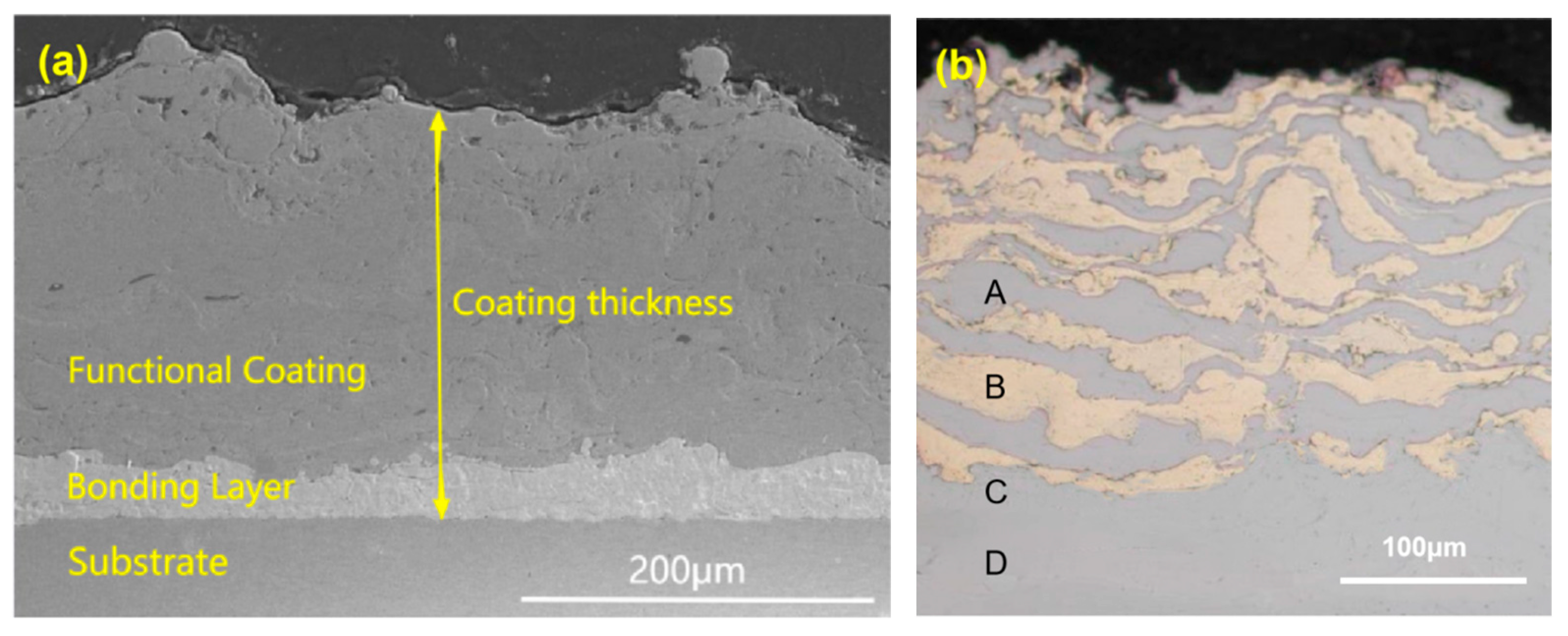
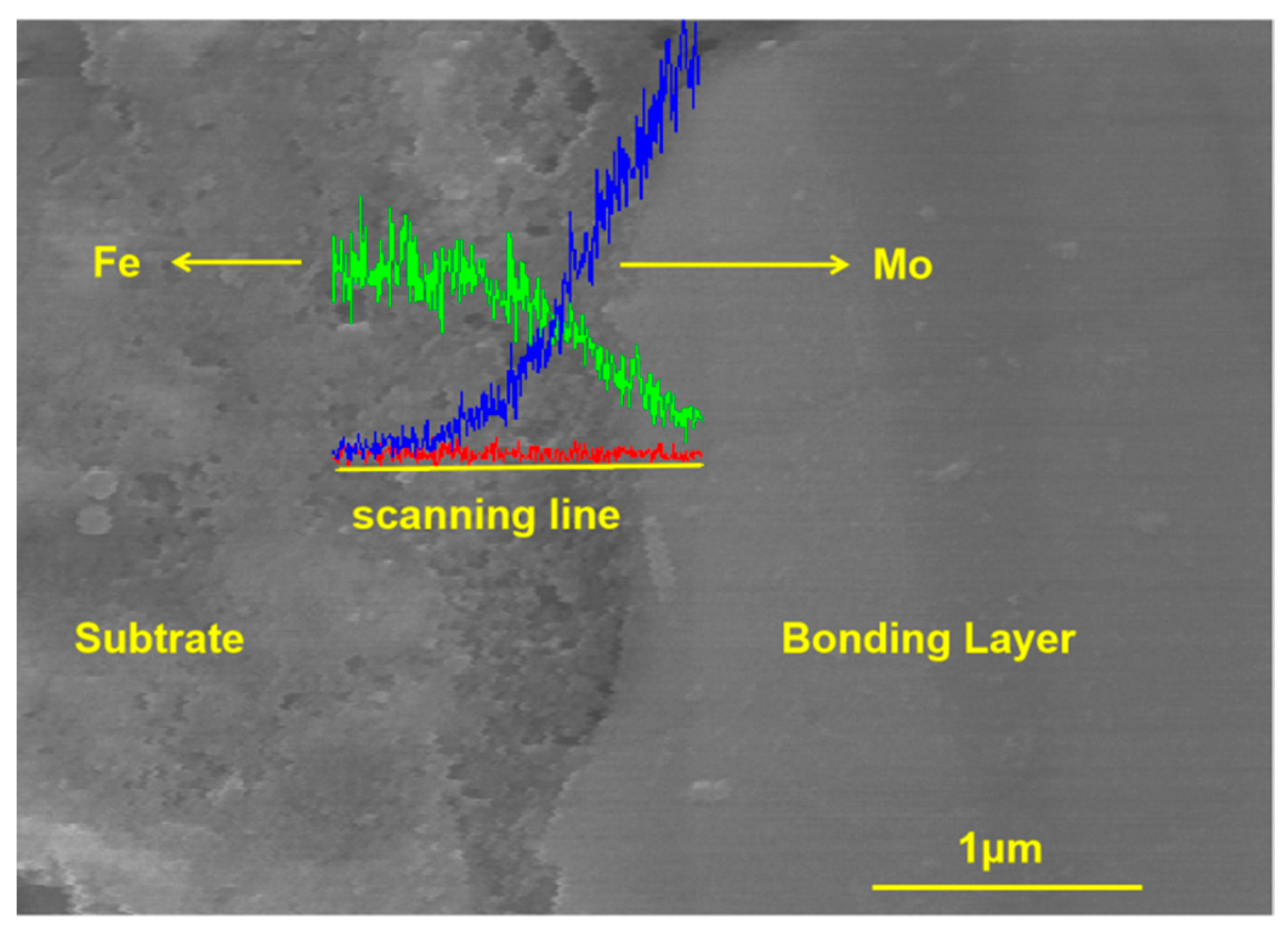
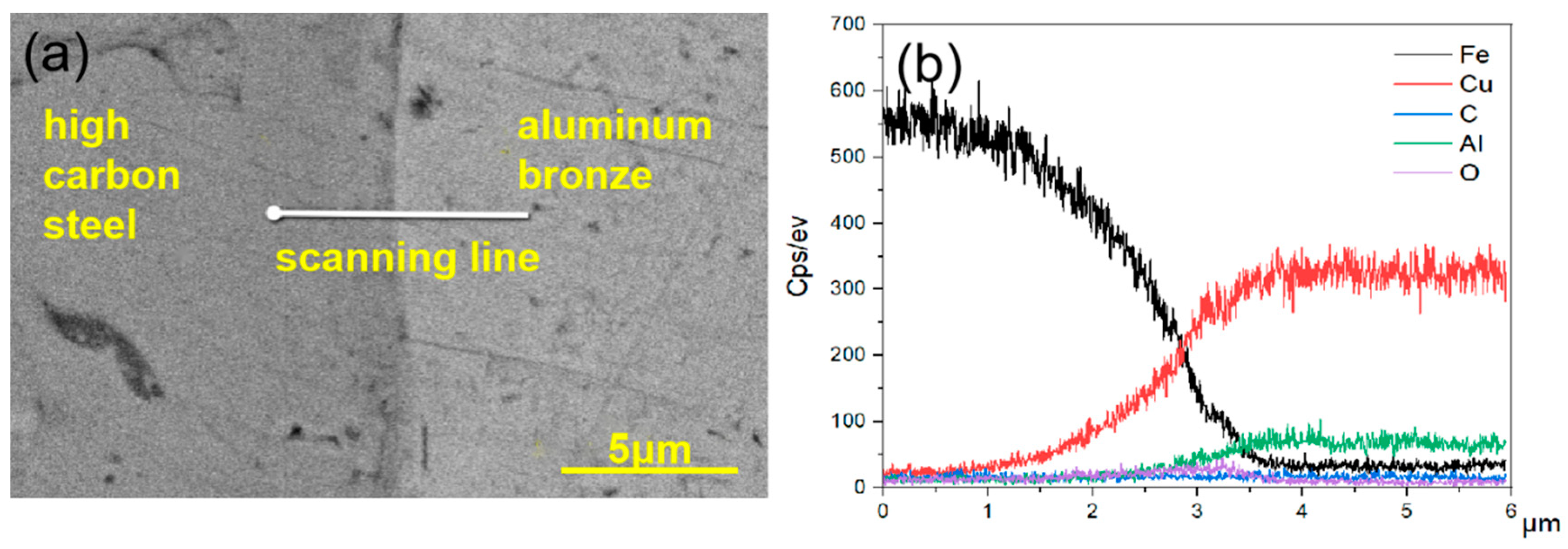
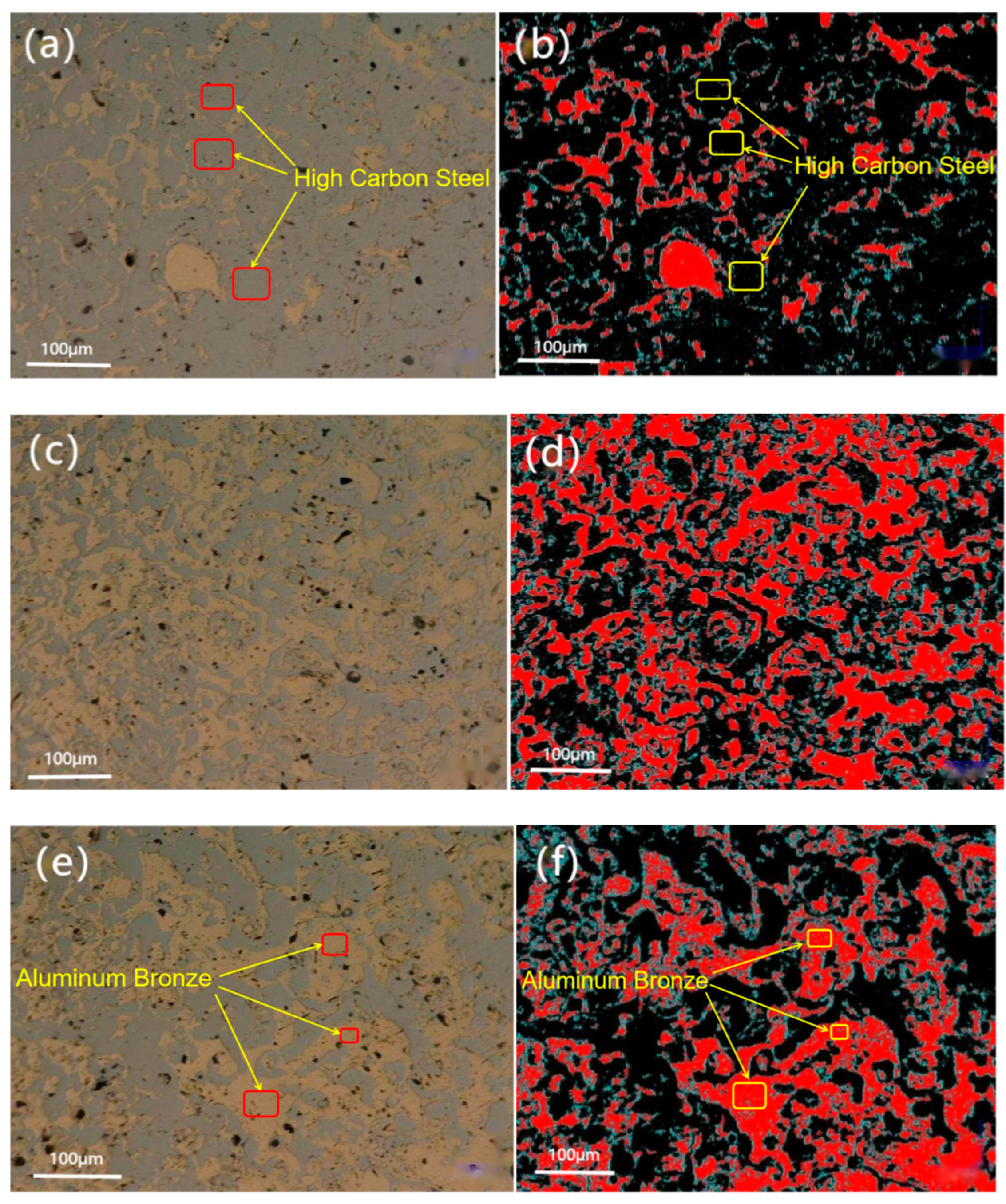
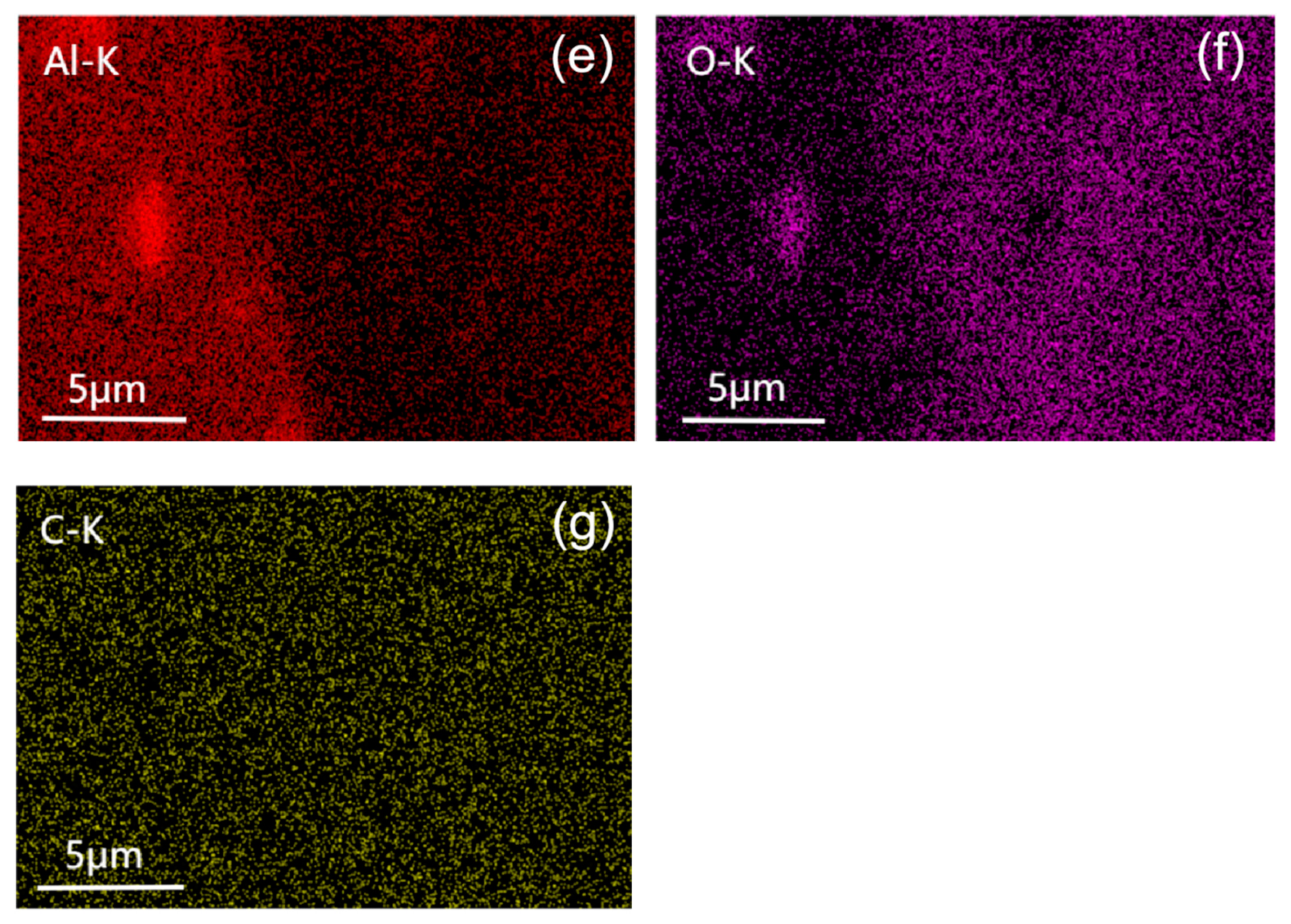
4.1. Cross-Sectional Morphology of the Coating
4.2. Surface Morphology of the Coating
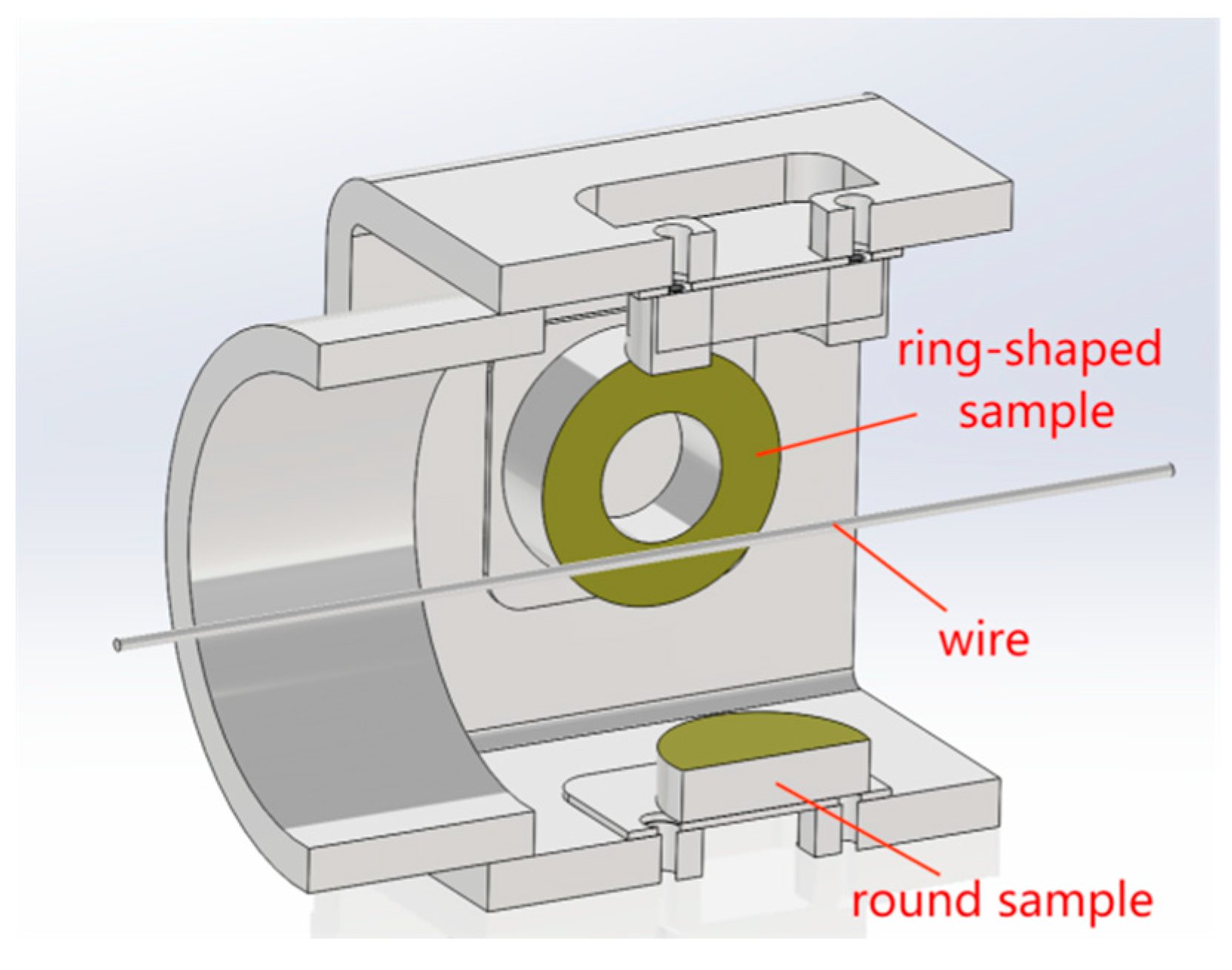
4.3. Bonding Strength of the Composite Coatings
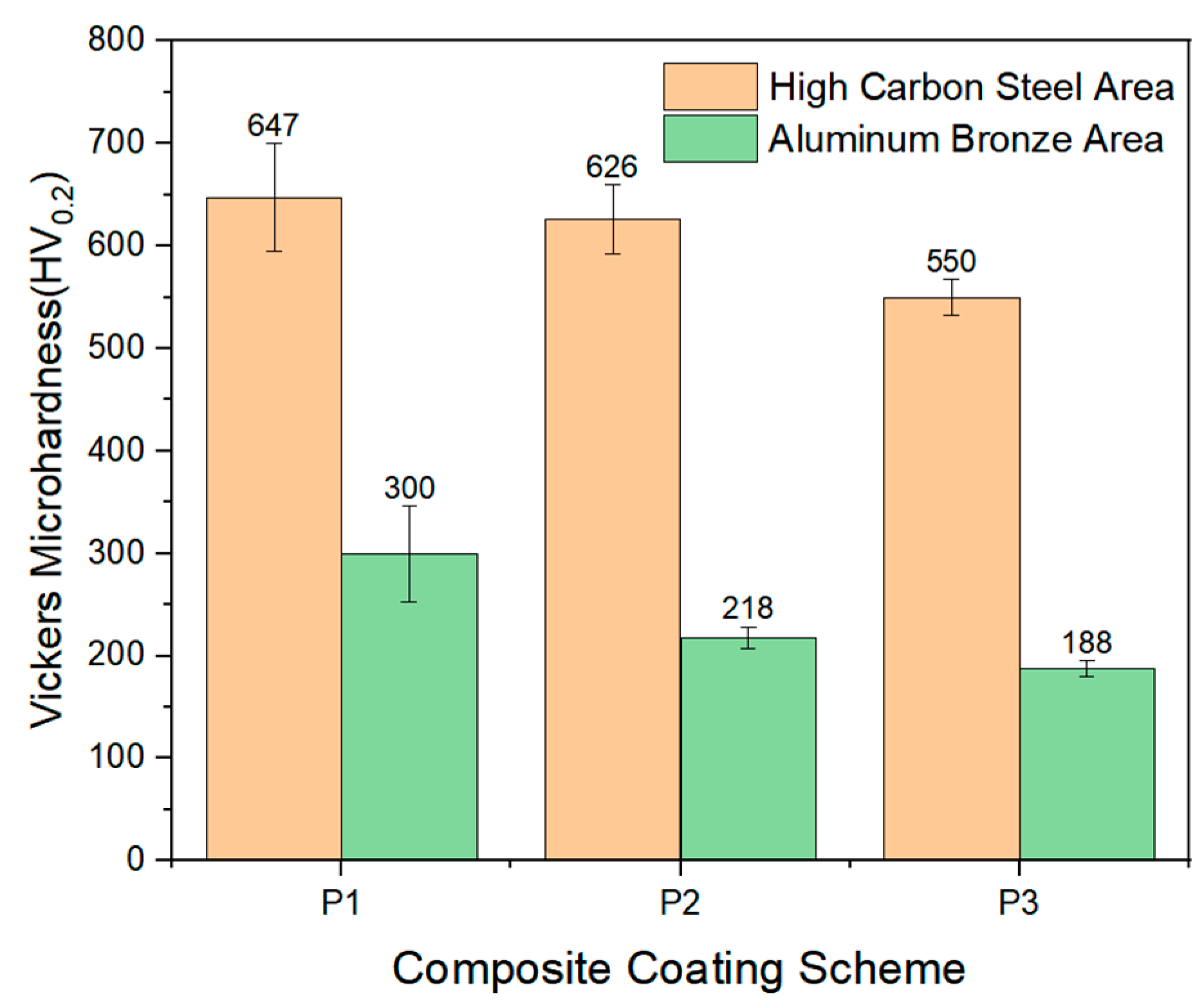
4.4. Hardness of the Composite Coatings
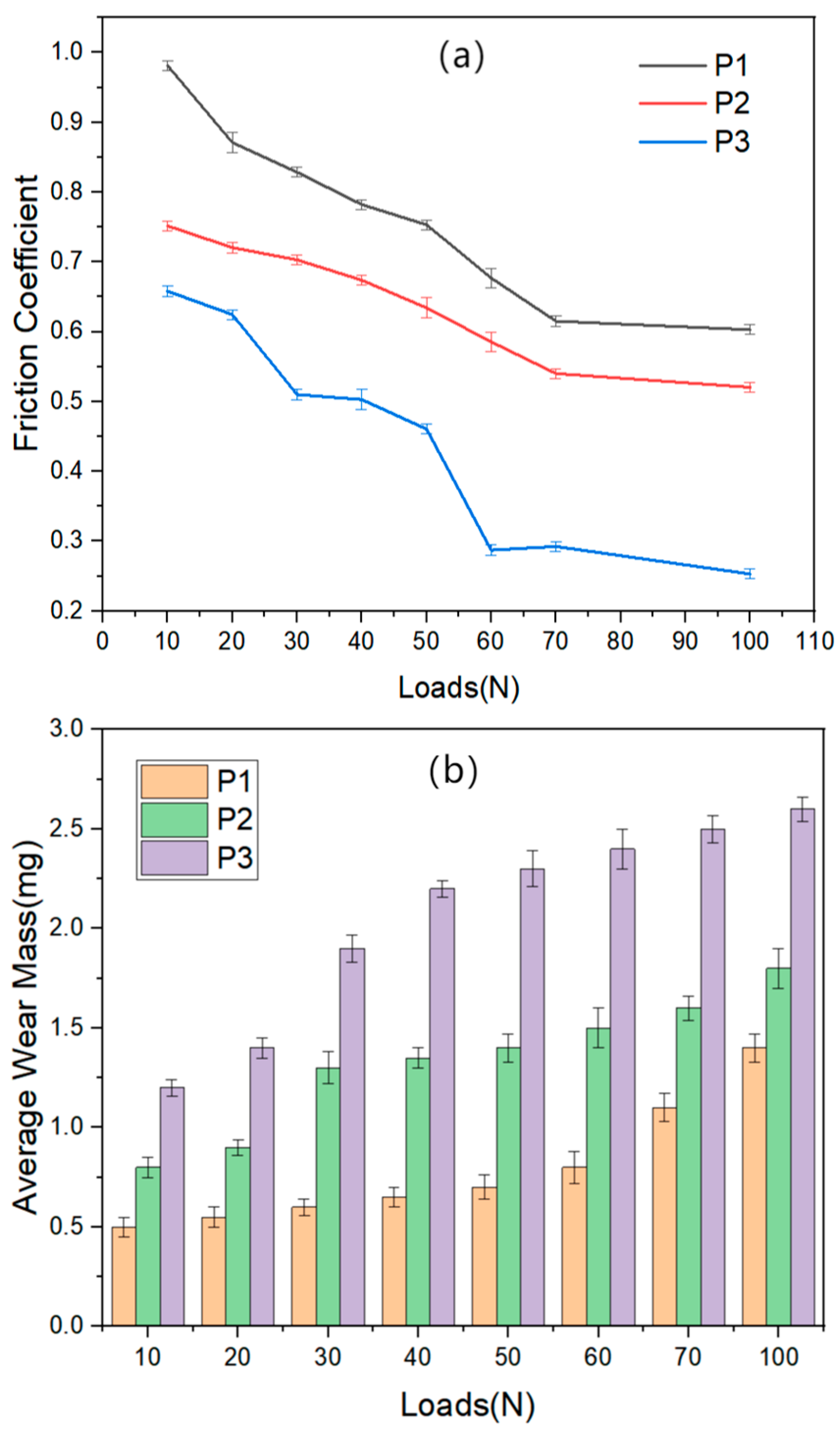
4.5. Friction and Wear Properties of the Composite Coatings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hui, W.; Wang, H. Fabrication of self-lubricating coating on aluminum and its frictional behavior. Appl. Surf. Sci. 2007, 253, 4386–4389. [Google Scholar]
- Caicedo, J.C.; Zambrano, O.A.; Aguilar, Y. Tribological study of the TiCN/TiNbNC system: From laboratory results to real industrial application. Surf. Coat. Technol. 2018, 342, 146–158. [Google Scholar] [CrossRef]
- Sivakumar, G.; Dusane, R.; Shrikant, V. A novel approach to process phase pure α-Al2O3 coatings by solution precursor plasma spraying. J. Eur. Ceram. Soc. 2013, 33, 2823–2829. [Google Scholar] [CrossRef]
- Todor, V.; Talha, B.; Manuel, E.; Albano, C. Synthesis, Microstructural, and Mechano-Tribological Properties of Self-Lubricating W-S-C(H) Thin Films Deposited by Different RF Magnetron Sputtering Procedures. Coatings 2020, 10, 272. [Google Scholar]
- Datta, S.; Das, M.; Balla, V.K.; Bodhak, S.; Murugesan, V.K. Mechanical, wear, corrosion and biological properties of arc deposited titanium nitride coatings. Surf. Coat. Technol. 2018, 344, 214–222. [Google Scholar] [CrossRef]
- Tillmann, W.; Kokalj, D.; Stangier, D. Optimization of the deposition parameters of Ni-20Cr thin films on thermally sprayed Al2O3 for sensor application. Surf. Coat. Technol. 2018, 344, 223–232. [Google Scholar] [CrossRef]
- Zhao, G.; He, Y. Plasma electroplating Ni coating on pure copper sheet—The effects of H2SO4 concentration on the microstructure and mechanical properties. Surf. Coat. Technol. 2012, 206, 4411–4416. [Google Scholar] [CrossRef]
- Li, W.X.; Zhang, K.; Dai, J.F.; Liang, J.; Huo, X.D. Preparation and Tribological Properties of Self-Lubricating Al2O3/Graphite Composite Coating on TA2 Titanium. J. Aeronaut. Mater. 2013, 33, 46–52. [Google Scholar]
- Liu, Y.; Qu, W.; Su, Y. TiC Reinforcement Composite Coating Produced Using Graphite of the Cast Iron by Laser Cladding. Materials 2016, 9, 815. [Google Scholar] [CrossRef]
- Riquelme, A.; Rodrigo, P.; Escalera-Rodriguez, M.D.; Rams, J. Wear Resistance of Aluminum Matrix Composites’ Coatings Added on AA6082 Aluminum Alloy by Laser Cladding. Coatings 2022, 12, 41. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Cai, F.; Yang, Y.; Wang, Q.; Zhang, S. Characterization and corrosion behaviors of Ti N/Ti Al N multilayer coatings by ion source enhanced hybrid arc ion plating. Surf. Coat. Technol. 2019, 366, 355–365. [Google Scholar] [CrossRef]
- Kameneva, A.; Kichigin, V. Corrosion wear and friction behavior of a number of multilayer two-, three- and multicomponent nitride coatings on different substrates, depending on the phase and elemental composition gradient. Adv. Mater. Sci. Eng. 2019, 489, 165–174. [Google Scholar] [CrossRef]
- Vardelle, A.; Moreau, C. Nickolas, A Perspective on Plasma Spray Technology. Plasma Chem. Plasma 2015, 35, 491–509. [Google Scholar] [CrossRef]
- Xian, G.; Xiong, J.; Zhao, H.; Fan, H.; Li, Z.; Du, H. Evaluation of the structure and properties of the hard TiAlN-(TiAlN/CrAlSiN)-TiAlN multiple coatings deposited on different substrate materials. Int. J. Refract. Met. Hard Mater. 2019, 85, 105056. [Google Scholar] [CrossRef]
- Shan, L.; Wang, Y.; Li, J.; Chen, J. Effect of N2 flow rate on microstructure and mechanical properties of PVD CrNx coatings for tribological application in seawater. Surf. Coat. Technol. 2014, 242, 74–82. [Google Scholar] [CrossRef]
- Zhao, S.; Meng, F.; Fan, B.; Dong, Y.; Wang, J.; Qi, X. Evaluation of wear mechanism between TC4 titanium alloys and self-lubricating fabrics. Wear 2023, 512, 204532. [Google Scholar] [CrossRef]
- Wang, J.; Peng, C.; Dai, H.; Lu, F.; Zhao, Z. Synthesis of Zirconium Dioxide Nanoparticles by Electrical Explosion of Zirconium Wire and Characteristics. Rare Metal Mater. Eng. 2019, 48, 2118–2121. [Google Scholar]
- Tamura, H.; Ogura, T.; Nagahama, M.; Tanabe, Y.; Sawaoka, A.B. Spraying of the brittle ceramic zirconium diboride by a wire explosion technique. J. Appl. Phys. 1994, 75, 1789. [Google Scholar] [CrossRef]
- Jin, G.; Xu, B.S.; Wang, H.D.; Li, Q.F.; Wei, S.C. Tribological properties of molybdenum coatings sprayed by electro-thermal explosion directional spraying. Surf. Coat. Technol. 2007, 201, 6678–6680. [Google Scholar] [CrossRef]
- Hou, S.X.; Liu, D.Y.; Li, B.R.; Zhang, N.Q. Microstructure and oxidation resistance of Mo–Si and Mo–Si–Al alloy coatings prepared by electro-thermal explosion ultrahigh speed spraying. Mater. Sci. Eng. A 2009, 518, 108–117. [Google Scholar] [CrossRef]
- Romanov, D.; Moskovskii, S.; Konovalov, S.; Sosnin, K.; Gromov, V.; Ivanov, Y. Improvement of copper alloy properties in electro-explosive spraying of ZnO-Ag coatings resistant to electrical erosion. J. Mater. Res. Technol. 2019, 8, 5515–5523. [Google Scholar] [CrossRef]
- Romanov, D.A. Improving die tooling properties by spraying TiC-Ti-Al and TiB2-Ti-Al electro-explosive coatings. Mater. Res. Express 2020, 7, 045010. [Google Scholar] [CrossRef]
- Huang, K.; Song, Q.; Chen, P.; Liu, Y. Preparation and Performance of Mo/Cu/Fe MultiLayer Composite Coating with Staggered Spatial Structure by Electro-Explosive Spraying Technology. Materials 2022, 15, 3552. [Google Scholar] [CrossRef]
- Yan, W.; Chen, W.; Li, J. Quality Control of High Carbon Steel for Steel Wires. Materials 2019, 12, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Song, Q.Z.; Wang, J.Z.; Duo, Y.X. Effect of charging energy on droplet diameters and properties of high-carbon steel coatings sprayed by wire explosion spraying. Surf. Coat. Technol. 2011, 206, 202–207. [Google Scholar] [CrossRef]
- Liu, J.J.; Liu, Z.D. An experimental study on synthesizing TiC–TiB2–Ni composite coating using electro-thermal explosion ultra-high speed spraying method. Mater. Lett. 2010, 64, 684–687. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, Y.M.; Tan, Y.H.; Huang, B.Y. Apparent Morphologies and Nature of Packet Martensite in High Carbon Steels. J. Iron Steel Res. Int. 2006, 13, 40–46. [Google Scholar] [CrossRef]
- Shi, B.; Huang, S.; Zhu, P.; Xu, C.; Zhang, T. Microstructure and Wear Behaviorof In-Situ NbC Reinforced Composite Coatings. Materials 2020, 13, 3459. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.H.; Li, X.B.; Huang, G.S.; Li, C.X.; Li, Y. Microstructure an Mechanical Properties of Zn-Ni-Al2O3 Composite Coatings. Materials 2018, 11, 853. [Google Scholar] [CrossRef]
- Jia, K.; Fischer, T.E. Microstructure, Mechanical Properties and Wear Resistance of WC/Co Nanocomposites. MRS Proc. 1996, 457, 303. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Liu, Z.; Xue, Z.; Yang, G. Surface modifications of Al-based amorphous composite coatings on 7075 Al plate prepared by high-speed electrothermal explosion. Procedia Eng. 2012, 27, 1042–1047. [Google Scholar] [CrossRef]
- Morimoto, T.; Mukaihara, T.; Kusumoto, Y.; Oda, M.; Takeshima, K.; Yatoh, H. Development of Fine-Grained High-Carbon Steel in High Reduction Low Temperature Rolling. Steel Res. Int. 2011, 82, 155–163. [Google Scholar] [CrossRef]
- Zhang, S.L.; Sun, X.J.; Dong, H. Mechanism of Austenite Evolution During Deformation of Ultra-High Carbon Steel. J. Iron Steel Res. Int. 2008, 15, 42–46. [Google Scholar] [CrossRef]
- Cai, F.; Zhang, J.; Wang, J.; Zheng, J.; Wang, Q.; Zhang, S. Improved adhesion and erosion wear performance of Cr Si N/Cr multi-layer coatings on Ti alloy by inserting ductile Cr layers. Tribol. Int. 2021, 153, 106657. [Google Scholar] [CrossRef]
- Hou, S.X.; Liu, D.Y.; Zhao, L.P.; Li, B.; Liu, J.J. An experimental study on synthesizing submicron MoSi2-based coatings using electrothermal explosion ultra-high speed spraying method. Surf. Coat. Technol. 2008, 202, 2917–2921. [Google Scholar]
- Yonekura, D.; Fujita, J.; Miki, K. Fatigue and wear properties of Ti–6Al–4V alloy with Cr/CrN multilayer coating. Surf. Coat. Technol. 2015, 275, 232–238. [Google Scholar] [CrossRef]
- Thankachan, T.; Prakash, K.S. Microstructural, mechanical and tribological behavior of aluminum nitride reinforced copper surface composites fabricated through friction stir processing route. Mater. Sci. Eng. A 2017, 688, 301–308. [Google Scholar] [CrossRef]
- Kong, J.Z.; Li, C.; Sun, X.Y.; Xuan, Y.; Zhai, H.F.; Li, A.D.; Wang, Q.Z.; Zhou, F. Improved tribological properties and corrosion protection of CrN coating by ultrathin composite oxide interlayer. Appl. Surf. Sci. 2021, 541, 148606. [Google Scholar] [CrossRef]









| Materials | Mo | 80# | S215 |
|---|---|---|---|
| Voltages (kV) | 17.5 | 14.5 | 15 |
| Composite Coating Schemes | Bonding Layer | Functional Coating | Total Spraying | ||
|---|---|---|---|---|---|
| Mo | 80# | S215 | Groups | ||
| P1 | 5 | 3 | 1 | 7 | 33 |
| P2 | 2 | 2 | 7 | 33 | |
| P3 | 1 | 3 | 7 | 33 | |
| Coating | 1 | 2 | 3 | 4 | 5 | Average Thickness |
|---|---|---|---|---|---|---|
| P1 | 215 | 230 | 235 | 227 | 219 | 225.2 |
| P2 | 218 | 225 | 232 | 237 | 227 | 227.8 |
| P3 | 222 | 220 | 238 | 231 | 219 | 226 |
| Coatings | HCS Area (mm2) | Pore Area (mm2) | Total Area (mm2) | Ratio of HCS | Porosity (%) |
|---|---|---|---|---|---|
| P1 | 643,448.9 | 6673 | 953,257.5 | 67.5 | 0.7 |
| P2 | 474,278.4 | 7626 | 953,257.5 | 49.8 | 0.8 |
| P3 | 215,436.2 | 8579 | 953,257.5 | 22.6 | 0.9 |
| Coatings | Bonding Strength (MPa) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Average Value | |
| P1 | 53.4 | 55.3 | 58.2 | 54.1 | 55.3 |
| P2 | 52.1 | 53.8 | 56.2 | 53.1 | 53.8 |
| P3 | 42.5 | 43.7 | 45.0 | 43.5 | 43.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Song, Q.; Chen, P.; Liu, Y.; Jing, Y. Wear Mechanism of Fe/Cu Self-Lubricating Composite Coatings Fabricated by Electro-Explosive Spraying under Dry Friction. Metals 2023, 13, 390. https://doi.org/10.3390/met13020390
Huang K, Song Q, Chen P, Liu Y, Jing Y. Wear Mechanism of Fe/Cu Self-Lubricating Composite Coatings Fabricated by Electro-Explosive Spraying under Dry Friction. Metals. 2023; 13(2):390. https://doi.org/10.3390/met13020390
Chicago/Turabian StyleHuang, Kun, Qiuzhi Song, Pengwan Chen, Ye Liu, and Yinping Jing. 2023. "Wear Mechanism of Fe/Cu Self-Lubricating Composite Coatings Fabricated by Electro-Explosive Spraying under Dry Friction" Metals 13, no. 2: 390. https://doi.org/10.3390/met13020390






