Evaluation of the Influence Exerted by Increased Silicon Contents on the Leaching Behavior of NMC-Based Black Mass
Abstract
:1. Introduction
- By forming insoluble compounds with certain metals, such as aluminum and iron, which can decrease the recovery efficiency of these metals.
- By acting as a reducing agent, which can reduce the oxidation state of certain metals, making them less accessible to the recovery process.
- By precipitating as silica, which can clog the process equipment and decrease the overall efficiency of the recovery process.
- By complicating the purification and separation steps of the recovery process, as silicon compounds can be difficult to separate from those of the desired metal.
- By decreasing the selectivity of certain recovery agents, such as cyanide, which can lead to the recovery of unwanted metals in addition to the desired one.
- By increasing the reagent consumption due to formation of silicate and silica which need to be removed by using reagents such as caustic soda, lime, etc.
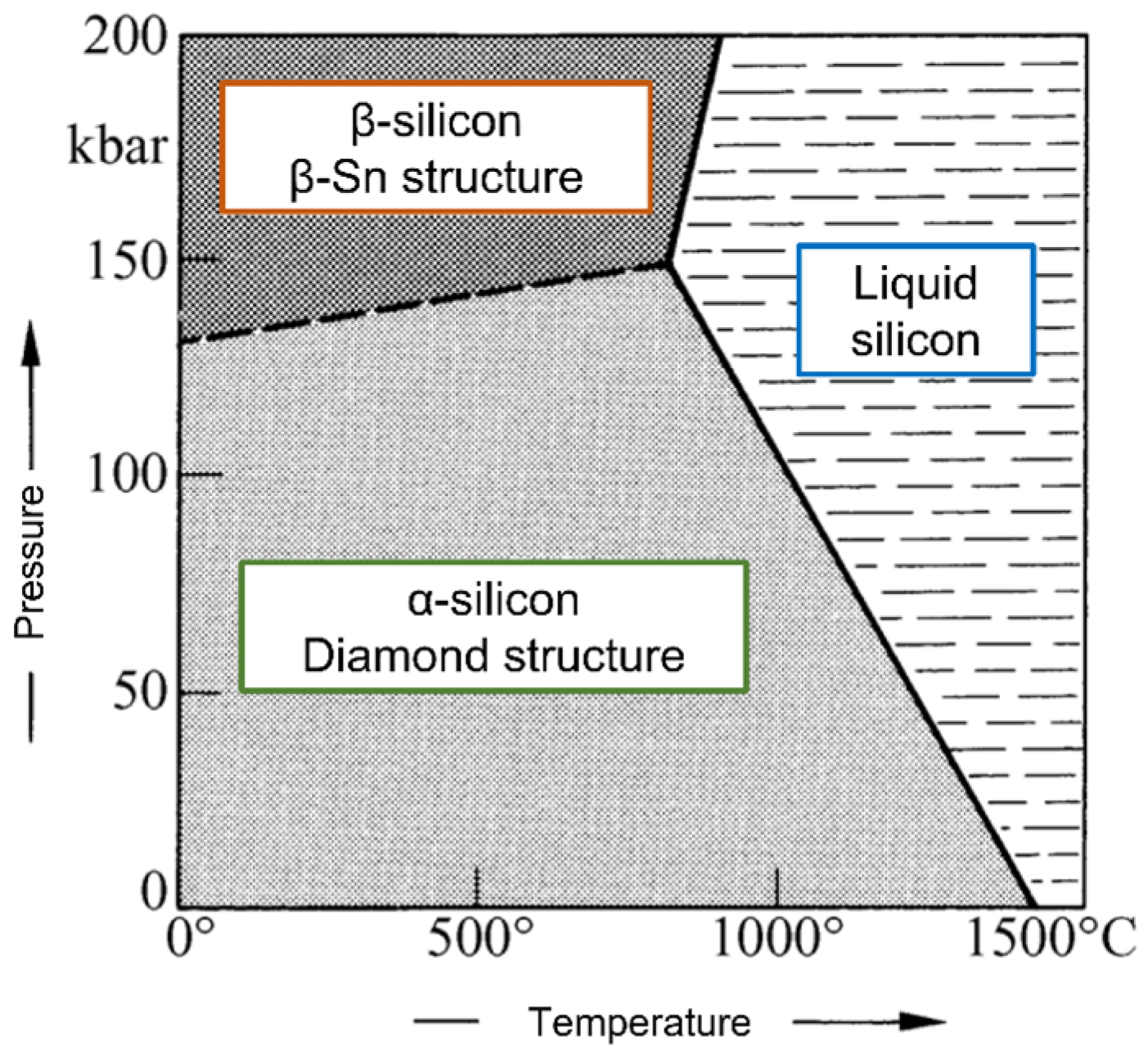
- Lithium silicate (Li2SiO3)
- Sodium silicate (Na2SiO3)
- Potassium silicate (K2SiO3)
- Calcium silicate (CaSiO3)
2. Materials and Methods
3. Results and Discussion
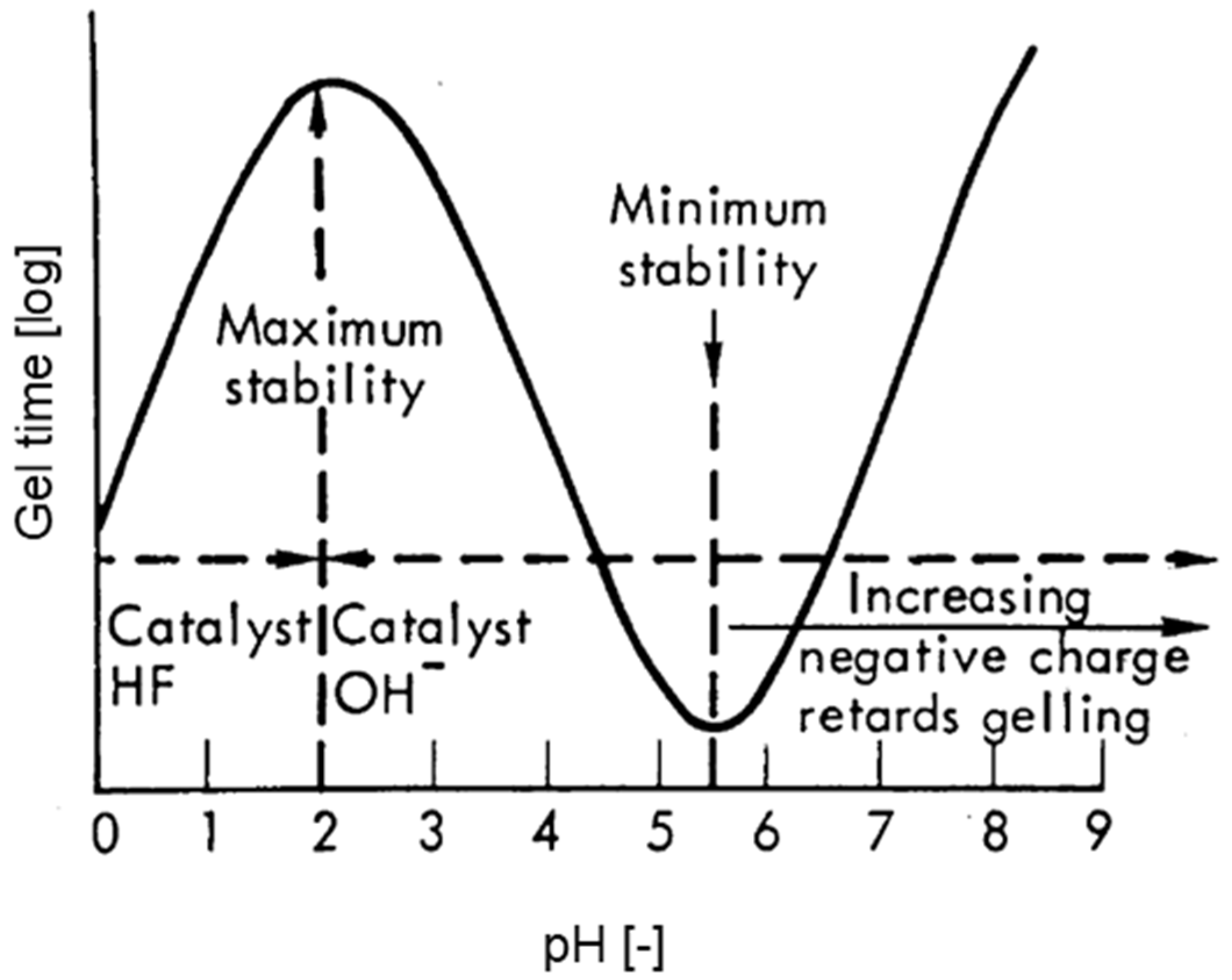
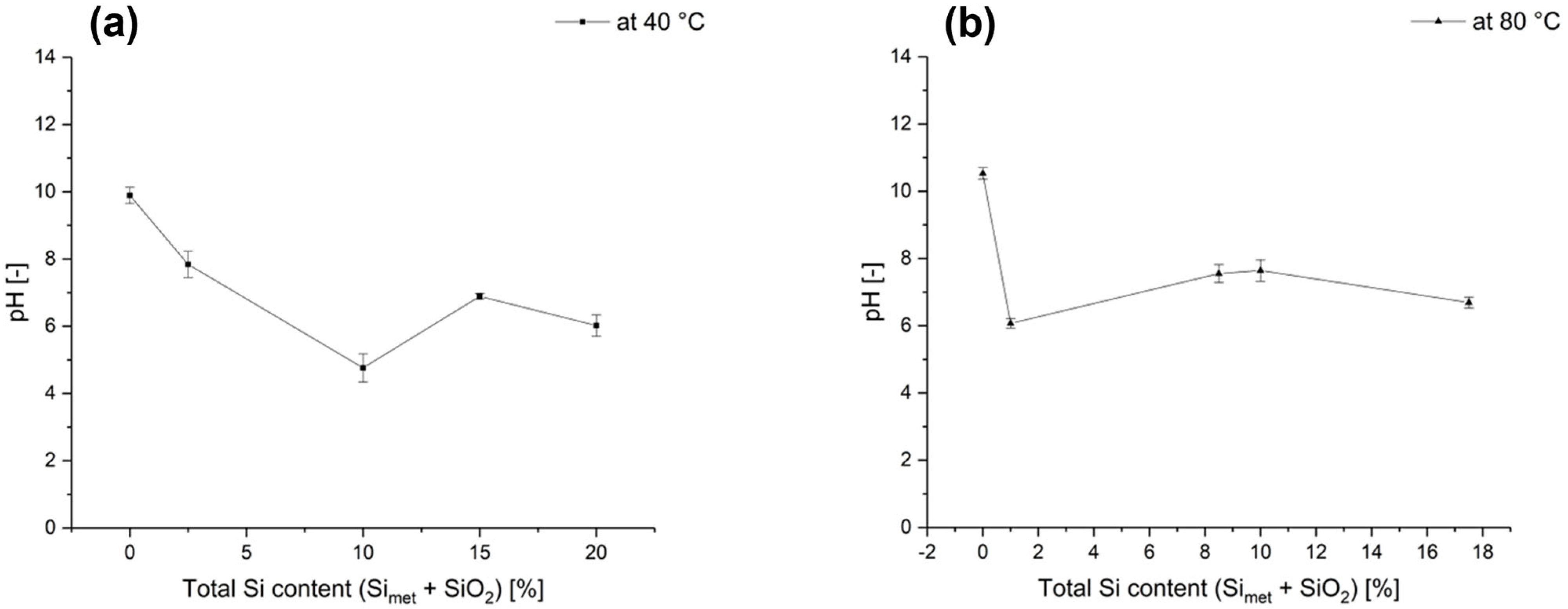
3.1. Reaction Mechanism
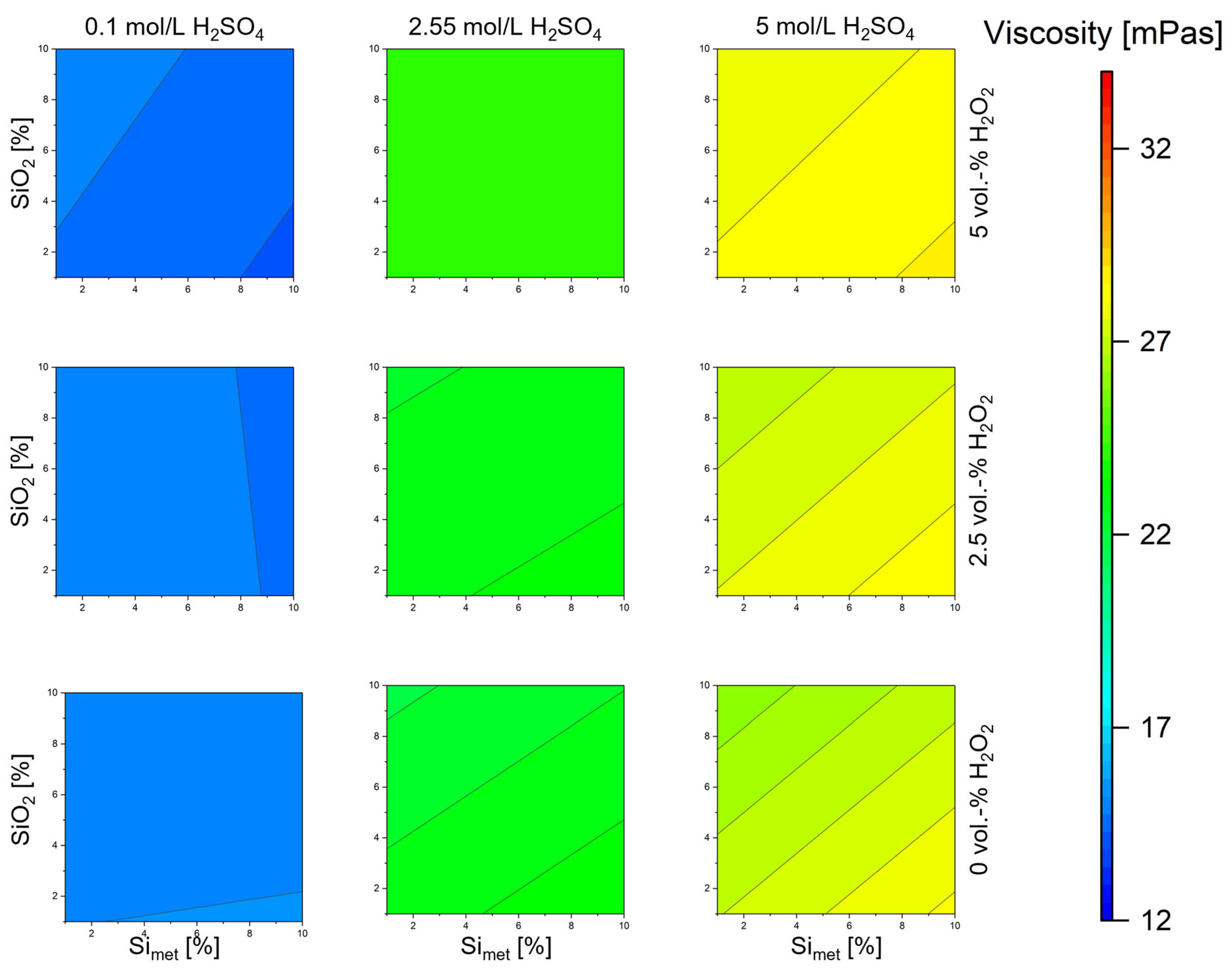
3.2. Influence on the Viscosity
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Queneau, P.B.; Berthold, C.E. Silica in Hydrometallurgy: An Overview. Can. Metall. Q. 1986, 25, 201–209. [Google Scholar] [CrossRef]
- Sjöberg, S. Silica in aqueous environments. J. Non-Cryst. Solids 1996, 196, 51–57. [Google Scholar] [CrossRef]
- Wiberg, N. Lehrbuch der Anorganischen Chemie; 102 Auflage; De Gruyter: Berlin, Germany, 2007. [Google Scholar]
- Okamoto, G.; Okura, T.; Goto, K. Properties of silica in water. Geochim. Cosmochim. Acta 1957, 12, 123–132. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica. Solubility, Polymerization, Colloid and Surface Properties and Biochemistry; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Gierada, M.; de Proft, F.; Sulpizi, M.; Tielens, F. Understanding the Acidic Properties of the Amorphous Hydroxylated Silica Surface. J. Phys. Chem. C 2019, 123, 17343–17352. [Google Scholar] [CrossRef]
- Belton, D.J.; Deschaume, O.; Perry, C.C. An overview of the fundamentals of the chemistry of silica with relevance to biosilicification and technological advances. FEBS J. 2012, 279, 1710–1720. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. The Twelve Principles of Circular Hydrometallurgy. J. Sustain. Metall. 2022, 9, 1–25. [Google Scholar] [CrossRef]
- Lunevich, L. Aqueous Silica and Silica Polymerisation. In Desalination—Challenges and Opportunities; Mohammad, H.D.A.F., Vatanpour, V., Taheri, A.H., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Shenggong, H.; Shimin, H.; Shaofeng, W.; Isao, M.; Xiang, L.; Xianhua, H. Considering Critical Factors of Silicon/Graphite Anode Materials for Practical High-Energy Lithium-Ion Battery Applications. Energy Fuels 2021, 35, 944–964. [Google Scholar]
- Zhang, M.; Li, J.; Sun, C.; Wang, Z.; Li, Y.; Zhang, D. Durable flexible dual-layer and free-standing silicon/carbon composite anode for lithium-ion batteries. J. Alloys Compd. 2023, 932, 167687. [Google Scholar] [CrossRef]
- Zhao, P.-Y.; Gonzalez, A.R.; Li, B.; Choy, K.-L. Eco-friendly aerosol multicoated silicon anodes in lithium-ion batteries. Mater. Lett. 2022, 324, 132677. [Google Scholar] [CrossRef]
- Christoph, N.; Thomas, S.; Axel, T. Recycling von Lithium-Ionen-Batterien: Chancen und Heraus Forderungen für den Maschinen- und Anlagenbau; Fraunhofer-ISI: Karlsruhe, Germany, 2021. [Google Scholar]
- Windisch-Kern, S.; Gerold, E.; Nigl, T.; Jandric, A.; Altendorfer, M.; Rutrecht, B.; Scherhaufer, S.; Raupenstrauch, H.; Pomberger, R.; Antrekowitsch, H.; et al. Recycling chains for lithium-ion batteries: A critical examination of current challenges, opportunities and process dependencies. Waste Manag. 2022, 138, 125–139. [Google Scholar] [CrossRef]
- Mohanty, A.; Sahu, S.; Sukla, L.B.; Devi, N. Application of various processes to recycle lithium-ion batteries (LIBs): A brief review. Mater. Today Proc. 2021, 47, 1203–1212. [Google Scholar] [CrossRef]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Mossali, E.; Picone, N.; Gentilini, L.; Rodrìguez, O.; Pérez, J.M.; Colledani, M. Lithium-ion batteries towards circular economy: A literature review of opportunities and issues of recycling treatments. J. Environ. Manag. 2020, 264, 110500. [Google Scholar] [CrossRef]
- Chandran, V.; Ghosh, A.; Patil, C.K.; Mohanavel, V.; Priya, A.K.; Rahim, R.; Madavan, R.; Muthuraman, U.; Karthick, A. Comprehensive review on recycling of spent lithium-ion batteries. Mater. Today Proc. 2021, 47, 167–180. [Google Scholar] [CrossRef]
- Asadi Dalini, E.; Karimi, G.; Zandevakili, S.; Goodarzi, M. A Review on Environmental, Economic and Hydrometallurgical Processes of Recycling Spent Lithium-ion Batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 451–472. [Google Scholar] [CrossRef]
- Jia, T.; Zhong, G.; Lv, Y.; Li, N.; Liu, Y.; Yu, X.; Zou, J.; Chen, Z.; Peng, L.; Kang, F.; et al. Prelithiation strategies for silicon-based anode in high energy density lithium-ion battery. Green Energy Environ. 2022, in press. [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Q.; Zhang, H.; Cheng, J.; Li, Y.; Zeng, Z.; Zhang, S.; Xu, X.; Ji, F.; Li, D.; et al. The application road of silicon-based anode in lithium-ion batteries: From liquid electrolyte to solid-state electrolyte. Energy Storage Mater. 2023, 55, 244–263. [Google Scholar] [CrossRef]
- Naveenkumar, P.; Maniyazagan, M.; Yang, H.-W.; Kang, W.S.; Kim, S.-J. Nitrogen-doped graphene/silicon-oxycarbide nanosphere as composite anode for high-performance lithium-ion batteries. J. Energy Storage 2023, 59, 106572. [Google Scholar] [CrossRef]
- Li, P.; Zhao, G.; Zheng, X.; Xu, X.; Yao, C.; Sun, W.; Dou, S.X. Recent progress on silicon-based anode materials for practical lithium-ion battery applications. Energy Storage Mater. 2018, 15, 422–446. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Han, J.; Bai, S.; Tan, J.; Liu, J.; Li, F. Challenges and Recent Progress on Silicon-Based Anode Materials for Next-Generation Lithium-Ion Batteries. Small Struct. 2021, 2, 2100009. [Google Scholar] [CrossRef]
- Luo, W.; Chen, X.; Xia, Y.; Chen, M.; Wang, L.; Wang, Q.; Li, W.; Yang, J. Surface and Interface Engineering of Silicon-Based Anode Materials for Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1701083. [Google Scholar] [CrossRef]
- Gerold, E.; Antrekowitsch, H. Potenzialabschätzung von Synergieeffekten zur simultanen Rückgewinnung von Wertmetallen aus komplexen, metallhaltigen Reststoffen. Osterr. Wasser Abfallwirtsch. 2022, 74, 22–31. [Google Scholar] [CrossRef]




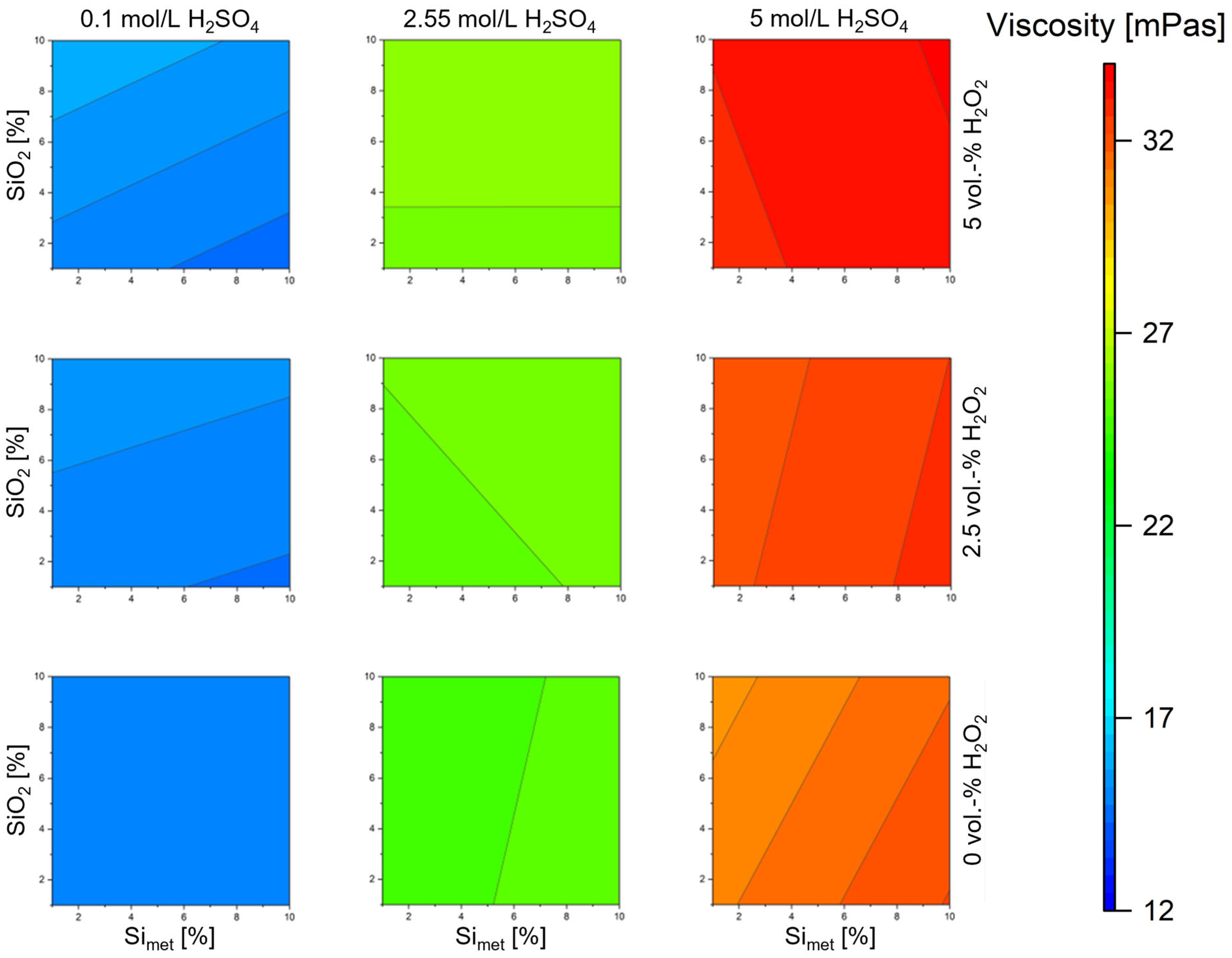

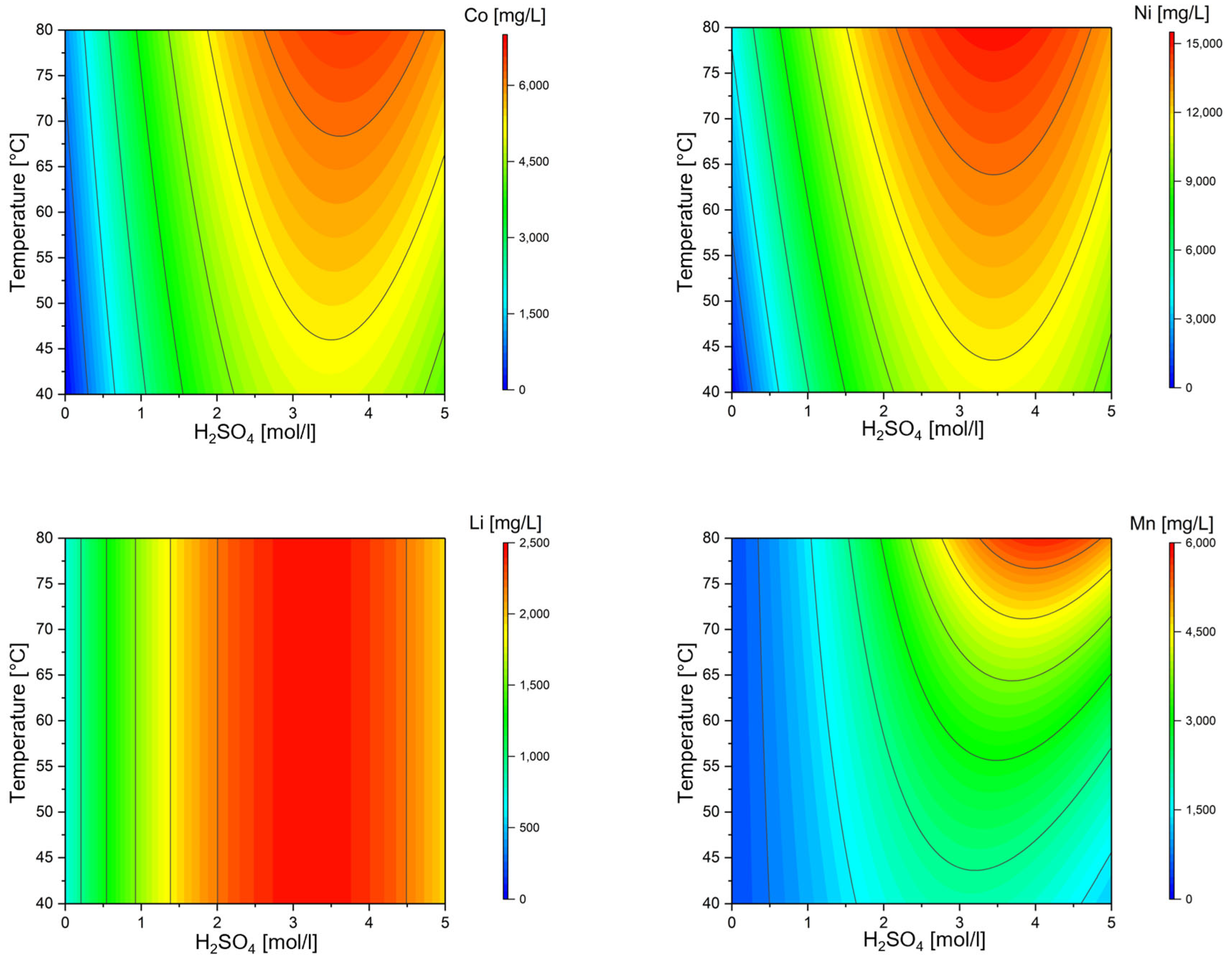
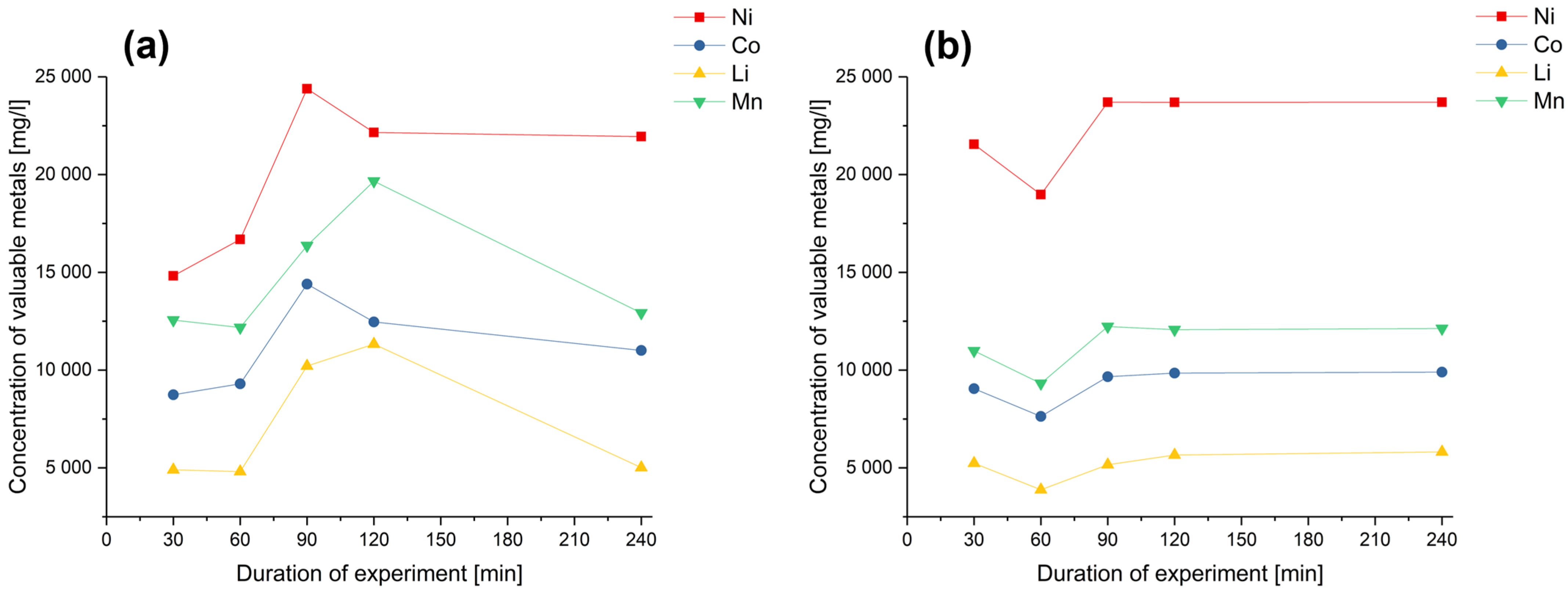
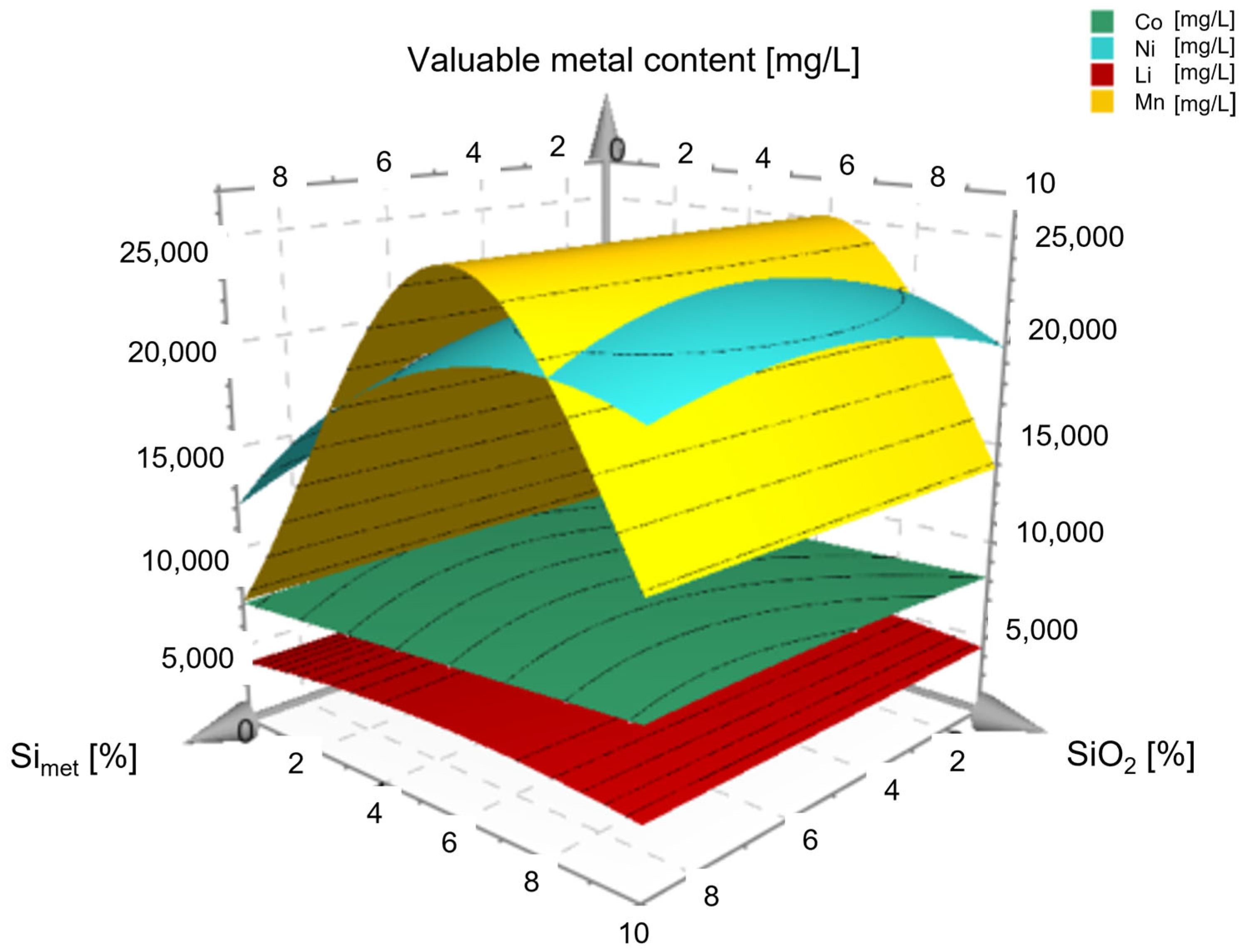
| Exp. Name | Run Order | Conc. H2SO4 | Temperature | S/L | Conc. H2O2 | Si | SiO2 |
|---|---|---|---|---|---|---|---|
| [i] | [-] | [mol/L] | [°C] | [g/L] | [vol.%] | [wt.%] | [wt.%] |
| Si-1 | 12 | 5 | 80 | 100 | 0 | 0 | 0 |
| Si-2 | 24 | 0.1 | 40 | 83.3 | 0 | 0 | 0 |
| Si-3 | 15 | 0.1 | 66.7 | 100 | 5 | 0 | 0 |
| Si-4 | 9 | 5 | 40 | 66.7 | 5 | 0 | 0 |
| Si-5 | 19 | 3.4 | 80 | 50 | 5 | 0 | 0 |
| Si-6 | 5 | 5 | 40 | 50 | 1.7 | 2.5 | 0 |
| Si-7 | 29 | 3.4 | 40 | 100 | 5 | 2.5 | 0 |
| Si-8 | 22 | 0.1 | 53.3 | 50 | 0 | 5 | 0 |
| Si-9 | 21 | 5 | 80 | 50 | 0 | 10 | 0 |
| Si-10 | 33 | 5 | 40 | 100 | 0 | 10 | 0 |
| Si-11 | 3 | 0.1 | 80 | 100 | 0 | 10 | 0 |
| Si-12 | 4 | 0.1 | 40 | 50 | 5 | 10 | 0 |
| Si-13 | 31 | 5 | 80 | 100 | 5 | 10 | 0 |
| Si-14 | 1 | 2.6 | 60 | 75 | 2.5 | 10 | 0 |
| Si-15 | 26 | 0.1 | 80 | 50 | 1.7 | 0 | 1 |
| Si-16 | 32 | 0.1 | 80 | 83.3 | 5 | 7.5 | 1 |
| Si-17 | 6 | 0.1 | 40 | 100 | 3.3 | 0 | 2.5 |
| Si-18 | 14 | 5 | 66.7 | 50 | 5 | 2.5 | 2.5 |
| Si-19 | 28 | 3.4 | 40 | 50 | 0 | 7.5 | 2.5 |
| Si-20 | 13 | 1.7 | 40 | 50 | 0 | 0 | 7.5 |
| Si-21 | 25 | 0.1 | 80 | 66.7 | 5 | 2.5 | 7.5 |
| Si-22 | 30 | 0.1 | 40 | 100 | 1.7 | 7.5 | 7.5 |
| Si-23 | 11 | 0.1 | 80 | 50 | 0 | 10 | 7.5 |
| Si-24 | 35 | 5 | 40 | 83.3 | 5 | 10 | 7.5 |
| Si-25 | 36 | 5 | 80 | 50 | 0 | 0 | 10 |
| Si-26 | 7 | 5 | 40 | 100 | 0 | 0 | 10 |
| Si-27 | 23 | 0.1 | 80 | 100 | 0 | 0 | 10 |
| Si-28 | 27 | 0.1 | 40 | 50 | 5 | 0 | 10 |
| Si-29 | 38 | 5 | 80 | 100 | 5 | 0 | 10 |
| Si-30 | 16 | 1.7 | 40 | 100 | 5 | 5 | 10 |
| Si-31 | 37 | 0.1 | 80 | 50 | 3.3 | 7.5 | 10 |
| Si-32 | 18 | 5 | 80 | 100 | 0 | 10 | 10 |
| Si-33 | 8 | 5 | 80 | 50 | 5 | 10 | 10 |
| Si-34 | 34 | 0.1 | 40 | 66.7 | 0 | 10 | 10 |
| Si-35 | 10 | 0.1 | 66.7 | 100 | 5 | 10 | 10 |
| Si-36 | 2 | 5 | 40 | 50 | 3.3 | 10 | 10 |
| Si-37 | 17 | 2.6 | 60 | 75 | 2.5 | 5 | 5 |
| Si-38 | 39 | 2.6 | 60 | 75 | 2.5 | 5 | 5 |
| Si-39 | 20 | 2.6 | 60 | 75 | 2.5 | 5 | 5 |
| Black Mass | Li | Al | Mn | Fe | Co | Ni | Cu |
|---|---|---|---|---|---|---|---|
| [g/100 g] | 3.40 | 5.06 | 7.30 | 0.54 | 5.80 | 22.20 | 6.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerold, E.; Lerchbammer, R.; Antrekowitsch, H. Evaluation of the Influence Exerted by Increased Silicon Contents on the Leaching Behavior of NMC-Based Black Mass. Metals 2023, 13, 785. https://doi.org/10.3390/met13040785
Gerold E, Lerchbammer R, Antrekowitsch H. Evaluation of the Influence Exerted by Increased Silicon Contents on the Leaching Behavior of NMC-Based Black Mass. Metals. 2023; 13(4):785. https://doi.org/10.3390/met13040785
Chicago/Turabian StyleGerold, Eva, Reinhard Lerchbammer, and Helmut Antrekowitsch. 2023. "Evaluation of the Influence Exerted by Increased Silicon Contents on the Leaching Behavior of NMC-Based Black Mass" Metals 13, no. 4: 785. https://doi.org/10.3390/met13040785






