A Detailed Kinetic Analysis of the Environmentally Friendly Leaching of Spent Lithium-Ion Batteries Using Monocarboxylic Acid
Abstract
:1. Introduction

2. Materials and Methods
2.1. Reagents
2.2. Analytical Method
2.3. Leaching Experiments
3. Results and Discussions
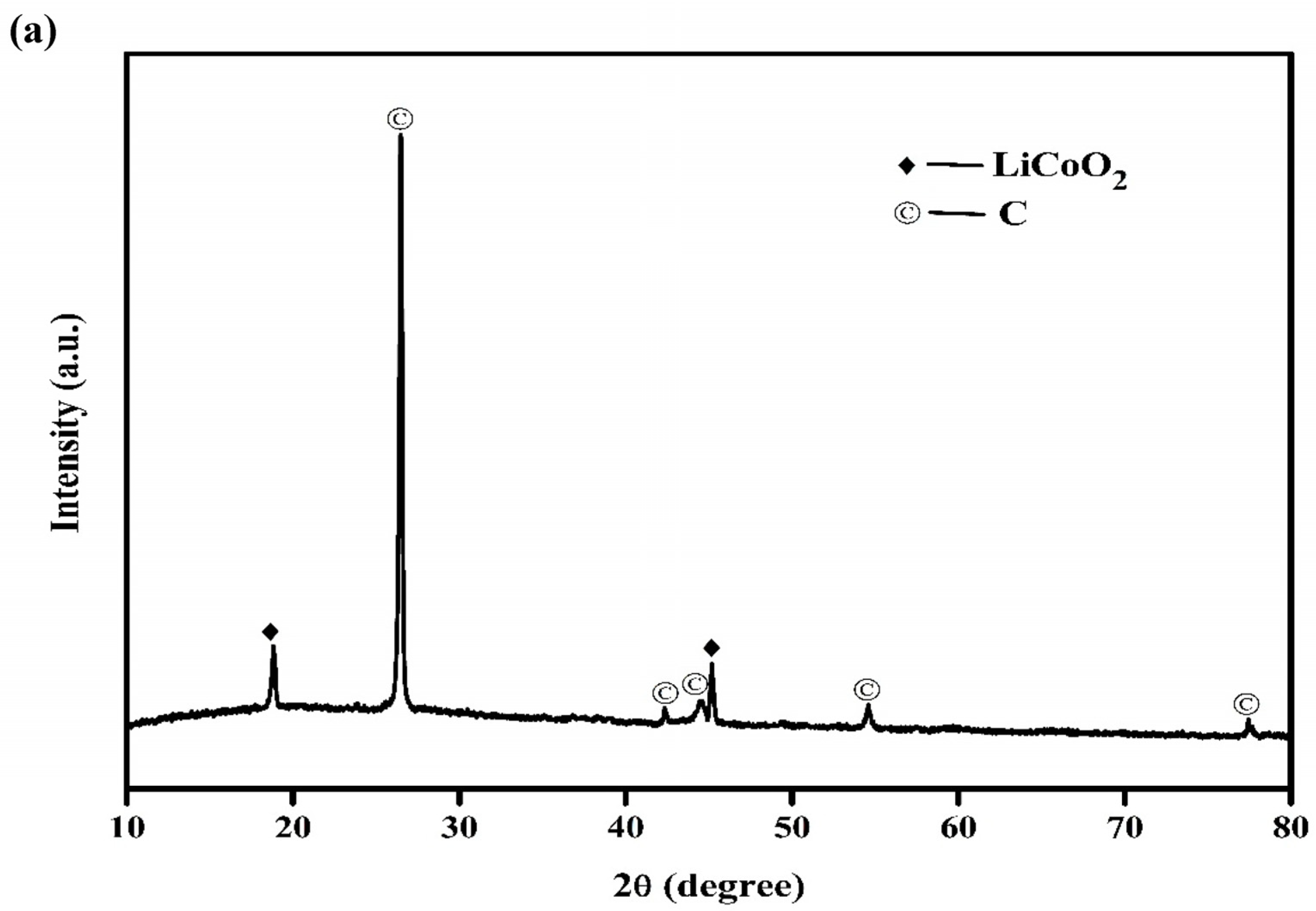
3.1. Characterization of the Spent LIBs
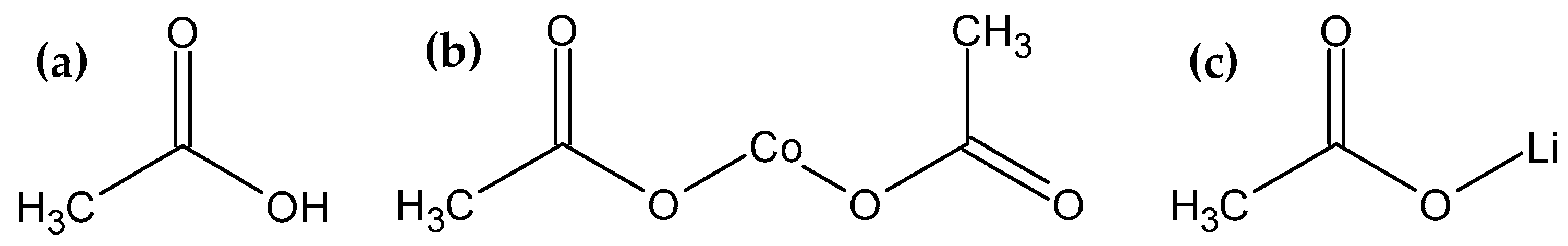
3.2. Leaching of the Metal Ions from the Spent LIBs
3.2.1. Impact of Leaching Time
3.2.2. Impact of Acetic Acid Concentration
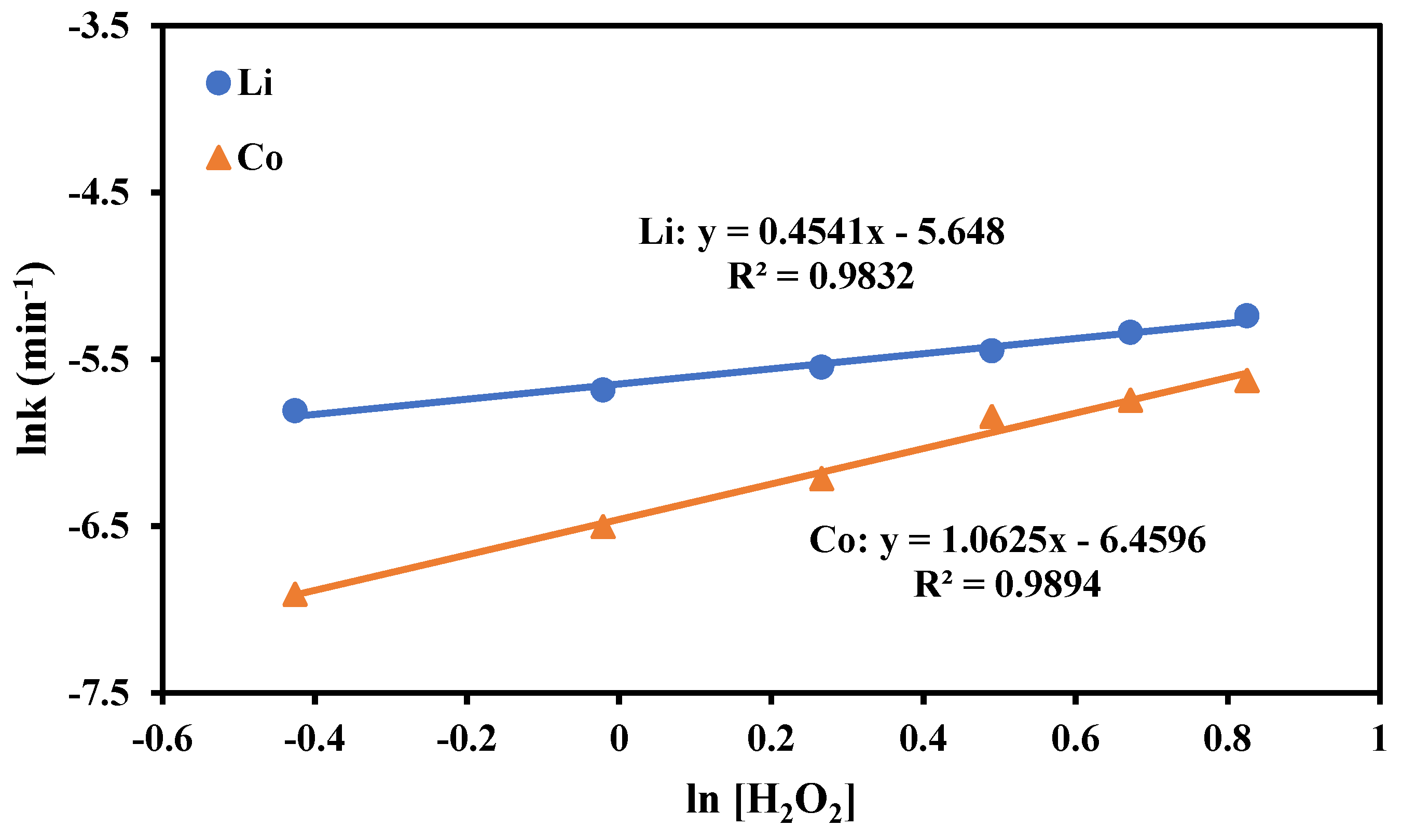
3.2.3. Impact of H2O2 Concentration
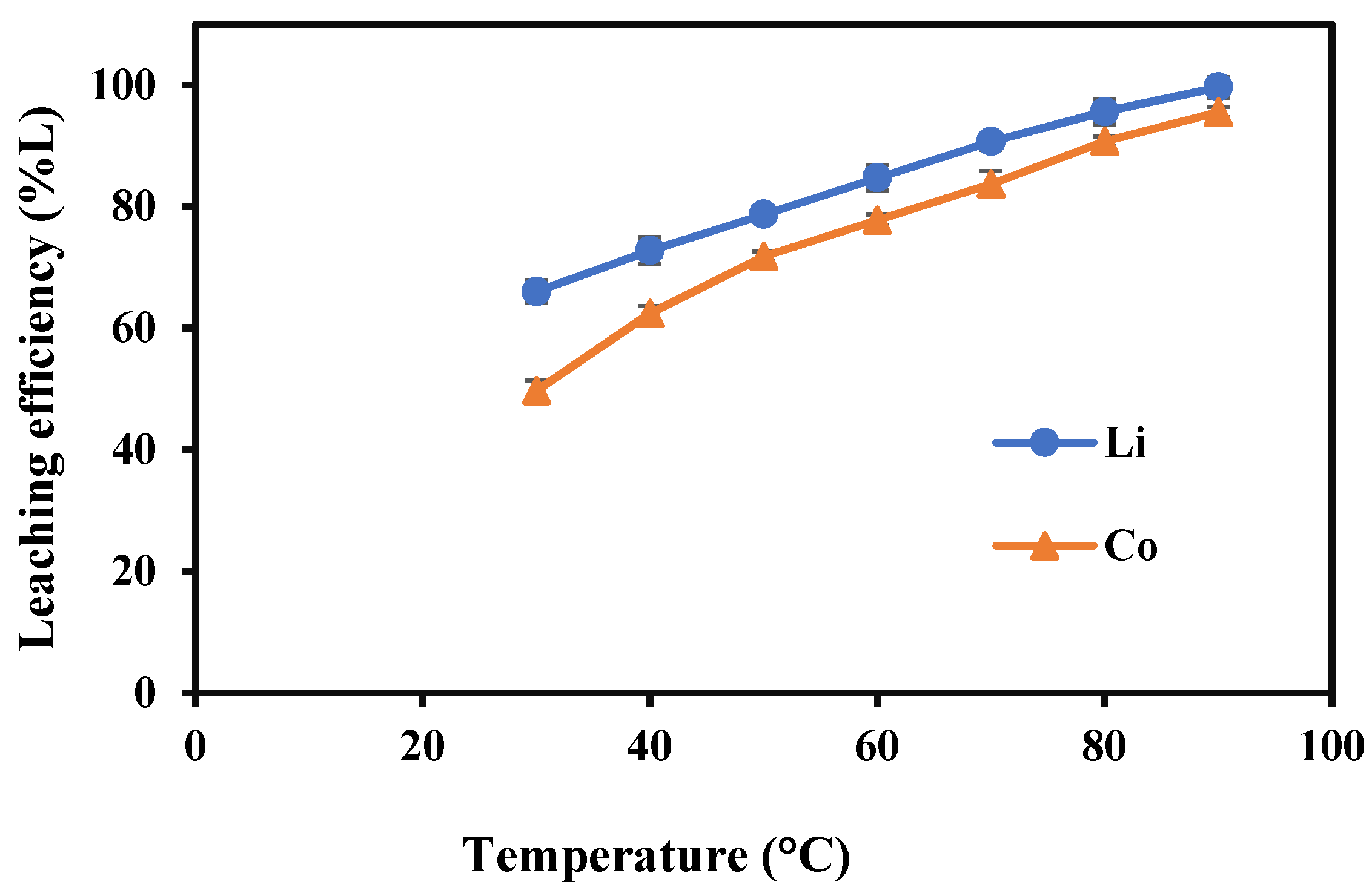
3.2.4. Impact of Temperature
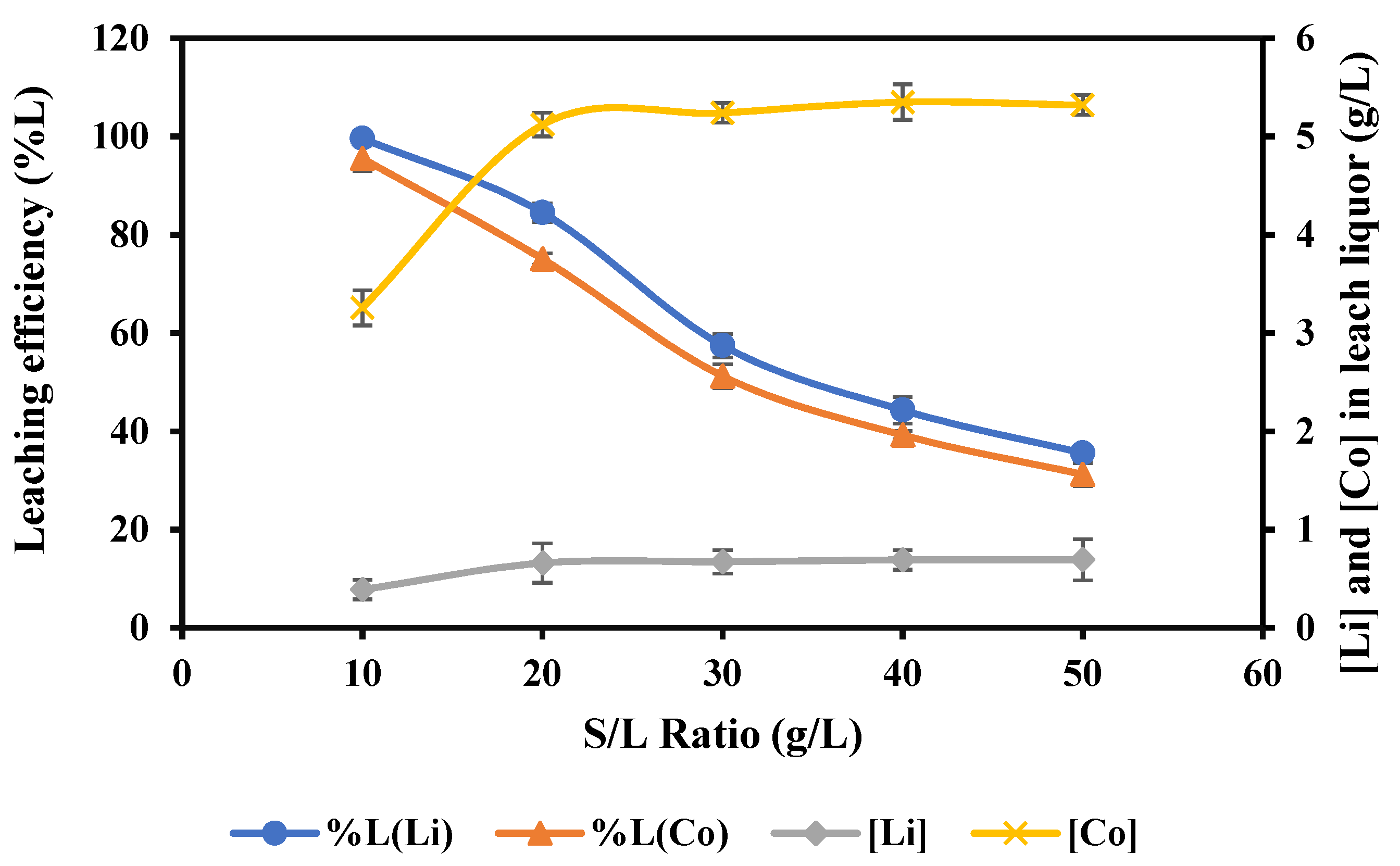
3.2.5. Impact of Solid to Liquid (S/L) Ratio
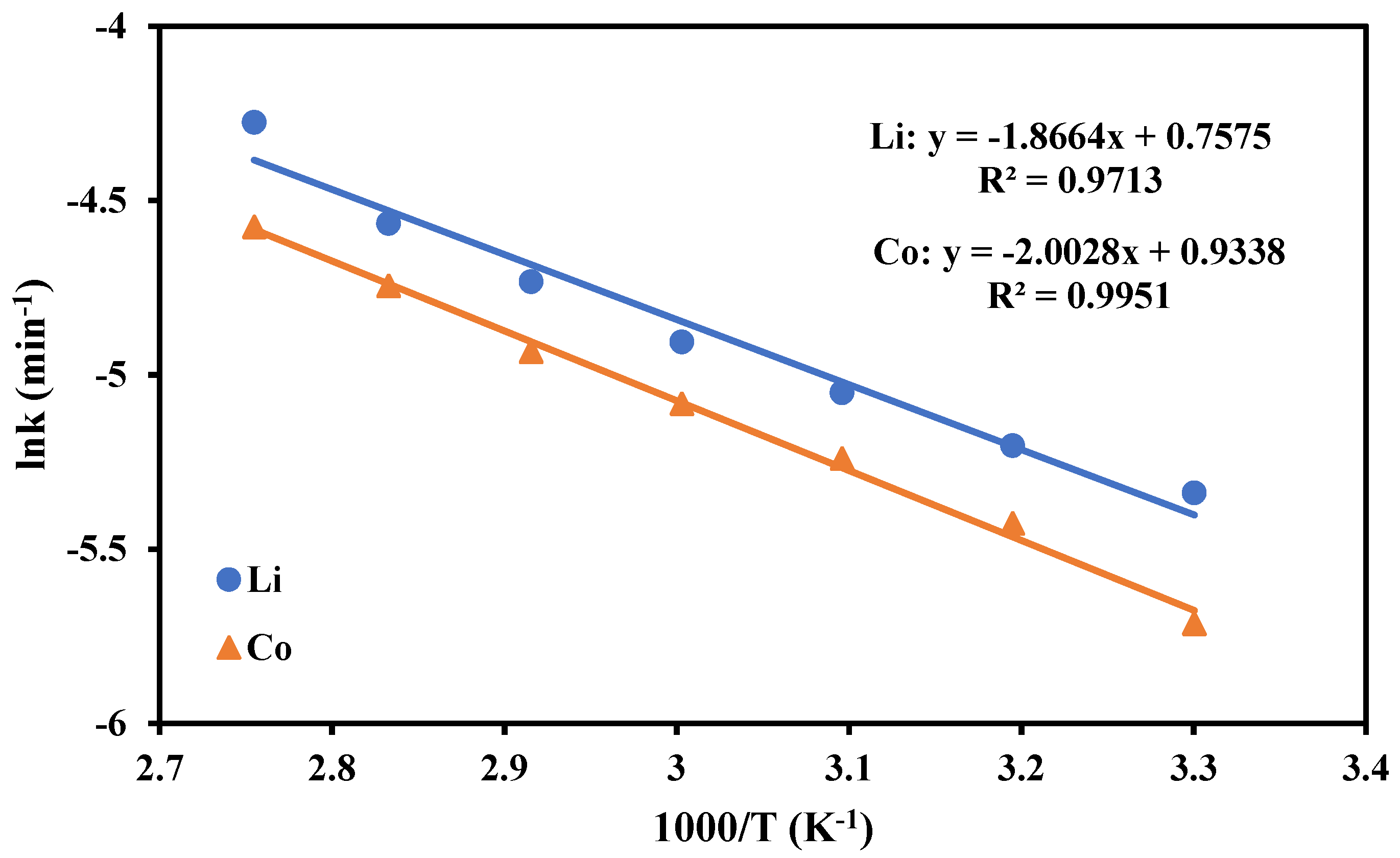
3.3. Kinetics Study
3.4. Economic Viability of the Current Leaching Process
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Liu, F.; Song, S.; Tang, H.; Ding, S.; Sun, W.; Lei, S.; Xu, S. Recovering Valuable Metals from the Leaching Liquor of Blended Cathode Material of Spent Lithium-Ion Battery. J. Environ. Chem. Eng. 2020, 8, 104358. [Google Scholar] [CrossRef]
- Chabhadiya, K.; Srivastava, R.R.; Pathak, P. Two-Step Leaching Process and Kinetics for an Eco-Friendly Recycling of Critical Metals from Spent Li-Ion Batteries. J. Environ. Chem. Eng. 2021, 9, 105232. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, J.; Wanaldi, R.; Zhou, X.; Wang, H.; Zhou, C.; Yang, J. A Promising Selective Recovery Process of Valuable Metals from Spent Lithium Ion Batteries via Reduction Roasting and Ammonia Leaching. J. Hazard. Mater. 2021, 402, 123491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xiao, J.; Mao, Q.; Zhang, Z.; Tang, L.; Zhong, Q. Recycling of Waste Carbon Residue from Spent Lithium-Ion Batteries via Constant-Pressure Acid Leaching. Trans. Nonferrous Met. Soc. China 2022, 32, 1691–1704. [Google Scholar] [CrossRef]
- Ghassa, S.; Farzanegan, A.; Gharabaghi, M.; Abdollahi, H. Iron Scrap, a Sustainable Reducing Agent for Waste Lithium Ions Batteries Leaching: An Environmentally Friendly Method to Treating Waste with Waste. Resour. Conserv. Recycl. 2021, 166, 105348. [Google Scholar] [CrossRef]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating Organic Acids as Alternative Leaching Reagents for Metal Recovery from Lithium Ion Batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Lin, L.; Lu, Z.; Zhang, W. Recovery of Lithium and Cobalt from Spent Lithium- Ion Batteries Using Organic Aqua Regia (OAR): Assessment of Leaching Kinetics and Global Warming Potentials. Resour. Conserv. Recycl. 2021, 167, 105416. [Google Scholar] [CrossRef]
- Chen, M.; Wang, R.; Qi, Y.; Han, Y.; Wang, R.; Fu, J.; Meng, F.; Yi, X.; Huang, J.; Shu, J. Cobalt and Lithium Leaching from Waste Lithium Ion Batteries by Glycine. J. Power Sources 2021, 482, 228942. [Google Scholar] [CrossRef]
- Chen, H.; Gu, S.; Guo, Y.; Dai, X.; Zeng, L.; Wang, K.; He, C.; Dodbiba, G.; Wei, Y.; Fujita, T. Leaching of Cathode Materials from Spent Lithium-Ion Batteries by Using a Mixture of Ascorbic Acid and HNO3. Hydrometallurgy 2021, 205, 105746. [Google Scholar] [CrossRef]
- Sahu, S.; Mohapatra, M.; Devi, N. Effective Hydrometallurgical Route for Recovery of Energy Critical Elements from E-Wastes and Future Aspects. Mater. Today Proc. 2022, 67, 1016–1023. [Google Scholar] [CrossRef]
- Li, G.; Rao, M.; Jiang, T.; Huang, Q.; Peng, Z. Leaching of Limonitic Laterite Ore by Acidic Thiosulfate Solution. Miner. Eng. 2011, 24, 859–863. [Google Scholar] [CrossRef]
- Wang, R.-C.; Lin, Y.-C.; Wu, S.-H. A Novel Recovery Process of Metal Values from the Cathode Active Materials of the Lithium-Ion Secondary Batteries. Hydrometallurgy 2009, 99, 194–201. [Google Scholar] [CrossRef]
- Medić, D.V.; Sokić, M.D.; Nujkić, M.M.; Đorđievski, S.S.; Milić, S.M.; Alagić, S.Č.; Antonijević, M.M. Cobalt Extraction from Spent Lithium-Ion Battery Cathode Material Using a Sulfuric Acid Solution Containing SO2. J. Mater. Cycles Waste Manag. 2023, 25, 1008–1018. [Google Scholar] [CrossRef]
- Chen, X.; Ma, H.; Luo, C.; Zhou, T. Recovery of Valuable Metals from Waste Cathode Materials of Spent Lithium-Ion Batteries Using Mild Phosphoric Acid. J. Hazard. Mater. 2017, 326, 77–86. [Google Scholar] [CrossRef]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic Acid-Based Leaching System: A Sustainable Process for Recovery of Valuable Metals from Spent Li-Ion Batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Sahu, S.; Devi, N. Effective Leaching of Spent Lithium-Ion Batteries Using DL-Lactic Acid as Lixiviant and Selective Separation of Metals through Precipitation and Solvent Extraction. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- de Oliveira Demarco, J.; Stefanello Cadore, J.; da Silveira de Oliveira, F.; Hiromitsu Tanabe, E.; Assumpção Bertuol, D. Recovery of Metals from Spent Lithium-Ion Batteries Using Organic Acids. Hydrometallurgy 2019, 190, 105169. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-Acid-Assisted Recovery of Cobalt and Lithium from Spent Li-Ion Batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Verma, A.; Johnson, G.H.; Corbin, D.R.; Shiflett, M.B. Separation of Lithium and Cobalt from LiCoO2: A Unique Critical Metals Recovery Process Utilizing Oxalate Chemistry. ACS Sustain. Chem. Eng. 2020, 8, 6100–6108. [Google Scholar] [CrossRef]
- Chen, X.; Fan, B.; Xu, L.; Zhou, T.; Kong, J. An Atom-Economic Process for the Recovery of High Value-Added Metals from Spent Lithium-Ion Batteries. J. Clean. Prod. 2016, 112, 3562–3570. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.d.M.; Villen-Guzman, M.; Acedo-Bueno, L.F.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Hydrometallurgical Extraction of Li and Co from LiCoO2 Particles–Experimental and Modeling. Appl. Sci. 2020, 10, 6375. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.M.; Villen-Guzman, M.; Vereda-Alonso, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Acid Leaching of LiCoO2 Enhanced by Reducing Agent. Model Formulation and Validation. Chemosphere 2022, 287, 132020. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Shi, P.; Zhang, X.; Lin, J.; Wu, F.; Li, L.; Chen, R. Glucose Oxidase-Based Biocatalytic Acid-Leaching Process for Recovering Valuable Metals from Spent Lithium-Ion Batteries. Waste Manag. 2020, 114, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, B.; Xie, H.; Qu, J.; Qu, X.; Xing, P.; Yin, H. Hydrometallurgical Recovery of Spent Cobalt-Based Lithium-Ion Battery Cathodes Using Ethanol as the Reducing Agent. Environ. Res. 2020, 181, 108803. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo-Gonzalez, M.M.; Villen-Guzman, M.; Vereda-Alonso, C.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Recovery of Li and Co from LiCoO2 via Hydrometallurgical–Electrodialytic Treatment. Appl. Sci. 2020, 10, 2367. [Google Scholar] [CrossRef]
- Liu, X.; Li, K.; Li, X. The Electrochemical Performance and Applications of Several Popular Lithium-Ion Batteries for Electric Vehicles—A Review. Commun. Comput. Inf. Sci. 2018, 925, 201–213. [Google Scholar] [CrossRef]
- Meng, F.; Liu, Q.; Kim, R.; Wang, J.; Liu, G.; Ghahreman, A. Selective Recovery of Valuable Metals from Industrial Waste Lithium-Ion Batteries Using Citric Acid under Reductive Conditions: Leaching Optimization and Kinetic Analysis. Hydrometallurgy 2020, 191, 105160. [Google Scholar] [CrossRef]
- Sahu, S.; Devi, N. Two-Step Leaching of Spent Lithium-Ion Batteries and Effective Regeneration of Critical Metals and Graphitic Carbon Employing Hexuronic Acid. RSC Adv. 2023, 13, 7193–7205. [Google Scholar] [CrossRef]
- Gu, K.; Zheng, W.; Ding, B.; Han, J.; Qin, W. Comprehensive Extraction of Valuable Metals from Waste Ternary Lithium Batteries via Roasting and Leaching: Thermodynamic and Kinetic Studies. Miner. Eng. 2022, 186, 107736. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Xu, J.; Thomas, H.R.; Francis, R.W.; Lum, K.R.; Wang, J.; Liang, B. A Review of Processes and Technologies for the Recycling of Lithium-Ion Secondary Batteries. J. Power Sources 2008, 177, 512–527. [Google Scholar] [CrossRef]
- He, L.-P.; Sun, S.-Y.; Mu, Y.-Y.; Song, X.-F.; Yu, J.-G. Recovery of Lithium, Nickel, Cobalt, and Manganese from Spent Lithium-Ion Batteries Using l-Tartaric Acid as a Leachant. ACS Sustain. Chem. Eng. 2016, 5, 714–721. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Z.; Xue, F.; Yang, B.; Guo, G.; Qiu, J. A More Simple and Efficient Process for Recovery of Cobalt and Lithium from Spent Lithium-Ion Batteries with Citric Acid. Sep. Purif. Technol. 2019, 215, 398–402. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Yang, Y.; Qu, L.; Li, J.; Zhou, R. Microwave Reduction Enhanced Leaching of Valuable Metals from Spent Lithium-Ion Batteries. J. Alloys Compd. 2020, 832, 154920. [Google Scholar] [CrossRef]
- Bu, X.; Danstan, J.K.; Hassanzadeh, A.; Behrad Vakylabad, A.; Chelgani, S.C. Metal Extraction from Ores and Waste Materials by Ultrasound-Assisted Leaching -an Overview. Miner. Process. Extr. Metall. Rev. 2022, 1–18. [Google Scholar] [CrossRef]
- Lie, J.; Liu, J.-C. Closed-Vessel Microwave Leaching of Valuable Metals from Spent Lithium-Ion Batteries (LIBs) Using Dual-Function Leaching Agent: Ascorbic Acid. Sep. Purif. Technol. 2021, 266, 118458. [Google Scholar] [CrossRef]
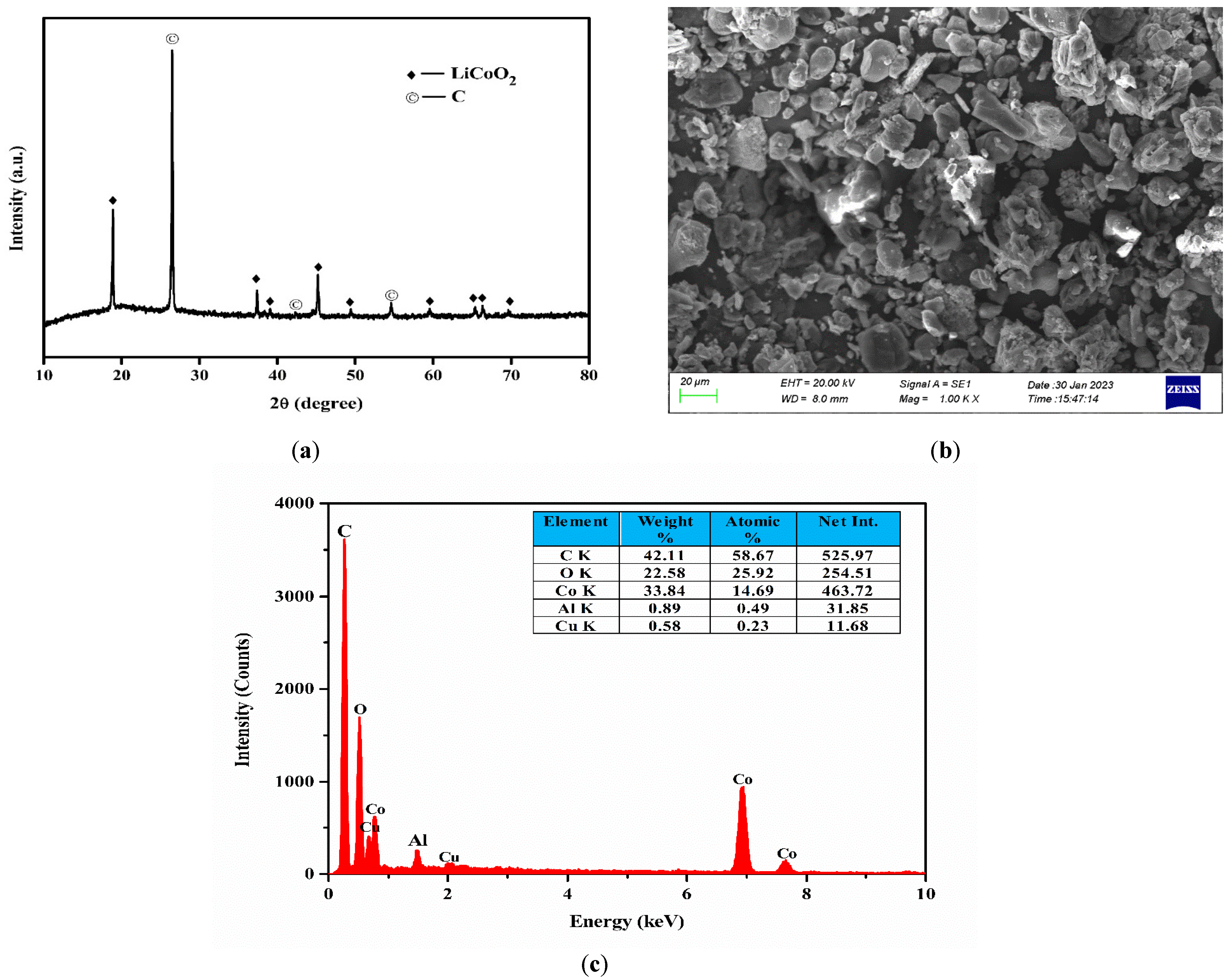





| Metals | Co | Li | Cu | Al |
|---|---|---|---|---|
| Wt.% | 3.9 | 34.12 | 0.31 | 0.27 |
| Kinetic Model | Integrated Form of the Equation | Parameters |
|---|---|---|
| Surface chemical reaction control | X is the leaching rate of a metal; kc is the chemical reaction rate constant | |
| Diffusion control | kd is the diffusion rate constant | |
| Avrami kinetic model | kr is the reaction rate constant |
| T (°C) | Li | Co | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface Chemical Reaction | Diffusion Control | Avrami Equation | Surface Chemical Reaction | Diffusion Control | Avrami Equation | |||||||
| kc × 103 (min−1) | R2 | kd × 103 (min−1) | R2 | R2 | kc × 103 (min−1) | R2 | kd × 103 (min−1) | R2 | R2 | |||
| 30 | 4.8 | 0.990 | 1.0 | 0.857 | −4.240 | 0.977 | 3.3 | 0.975 | 0.5 | 0.831 | −5.607 | 0.983 |
| 40 | 5.5 | 0.987 | 1.2 | 0.845 | −3.895 | 0.965 | 4.4 | 0.983 | 0.8 | 0.818 | −4.629 | 0.978 |
| 50 | 6.4 | 0.987 | 1.6 | 0.875 | −3.498 | 0.967 | 5.3 | 0.986 | 1.2 | 0.827 | −4.464 | 0.979 |
| 60 | 7.4 | 0.986 | 2.0 | 0.889 | −3.389 | 0.969 | 6.2 | 0.990 | 1.5 | 0.850 | −4.099 | 0.978 |
| 70 | 8.8 | 0.988 | 2.6 | 0.896 | −3.394 | 0.960 | 7.2 | 0.987 | 1.9 | 0.861 | −3.711 | 0.965 |
| 80 | 10.4 | 0.989 | 3.3 | 0.928 | −3.301 | 0.964 | 8.7 | 0.990 | 2.6 | 0.884 | −3.658 | 0.967 |
| 90 | 13.9 | 0.988 | 4.9 | 0.983 | −3.189 | 0.953 | 10.3 | 0.994 | 3.3 | 0.918 | −3.600 | 0.974 |
| Acetic Acid Concentration | Surface Chemical Reaction | |||
|---|---|---|---|---|
| Li | Co | |||
| kc × 103 (min−1) | R2 | kc × 103 (min−1) | R2 | |
| 0.1 | 0.4 | 0.995 | 0.05 | 0.996 |
| 0.3 | 0.7 | 0.980 | 0.18 | 0.978 |
| 0.5 | 1.2 | 0.982 | 0.21 | 0.980 |
| 0.8 | 1.8 | 0.981 | 0.31 | 0.985 |
| 1.0 | 2.3 | 0.984 | 0.5 | 0.987 |
| 1.2 | 2.9 | 0.983 | 0.7 | 0.983 |
| H2O2 Concentration | Surface Chemical Reaction | |||
|---|---|---|---|---|
| Li | Co | |||
| kc × 103 (min−1) | R2 | kc × 103 (min−1) | R2 | |
| 2% | 0.31 | 0.997 | 0.1 | 0.996 |
| 3% | 0.34 | 0.992 | 0.15 | 0.984 |
| 4% | 0.39 | 0.984 | 0.2 | 0.982 |
| 5% | 0.43 | 0.980 | 0.29 | 0.988 |
| 6% | 0.48 | 0.981 | 0.32 | 0.981 |
| 7% | 0.53 | 0.981 | 0.36 | 0.980 |
| SL No. | Leaching Agent | Acid Strength (mol/L) | Reductant (H2O2) (vol%) | Cost of Acid/USD | Cost of Reductant/USD | Total Cost/USD | References |
|---|---|---|---|---|---|---|---|
| 1. | Acetic Acid | 1.2 | 7% | 0.09 | 0.189 | 0.279 | Present Work |
| 2. | Succinic acid | 1.5 | 4% | 0.73 | 0.108 | 0.838 | [15] |
| 3. | L-Aspartic acid | 1.5 | 4% | 0.63 | 0.108 | 0.738 | [31] |
| 4. | L-Tartaric acid | 2.0 | 4% | 8.80 | 0.108 | 8.908 | [32] |
| 5. | Citric acid | 1.0 | 8% | 1.80 | 0.216 | 2.016 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahu, S.; Pati, S.; Devi, N. A Detailed Kinetic Analysis of the Environmentally Friendly Leaching of Spent Lithium-Ion Batteries Using Monocarboxylic Acid. Metals 2023, 13, 947. https://doi.org/10.3390/met13050947
Sahu S, Pati S, Devi N. A Detailed Kinetic Analysis of the Environmentally Friendly Leaching of Spent Lithium-Ion Batteries Using Monocarboxylic Acid. Metals. 2023; 13(5):947. https://doi.org/10.3390/met13050947
Chicago/Turabian StyleSahu, Sibananda, Subhankar Pati, and Niharbala Devi. 2023. "A Detailed Kinetic Analysis of the Environmentally Friendly Leaching of Spent Lithium-Ion Batteries Using Monocarboxylic Acid" Metals 13, no. 5: 947. https://doi.org/10.3390/met13050947






