Acid-Assisted Separation of Cathodic Material from Spent Electric Vehicle Batteries for Recycling
Abstract
:1. Introduction
2. Materials and Methods
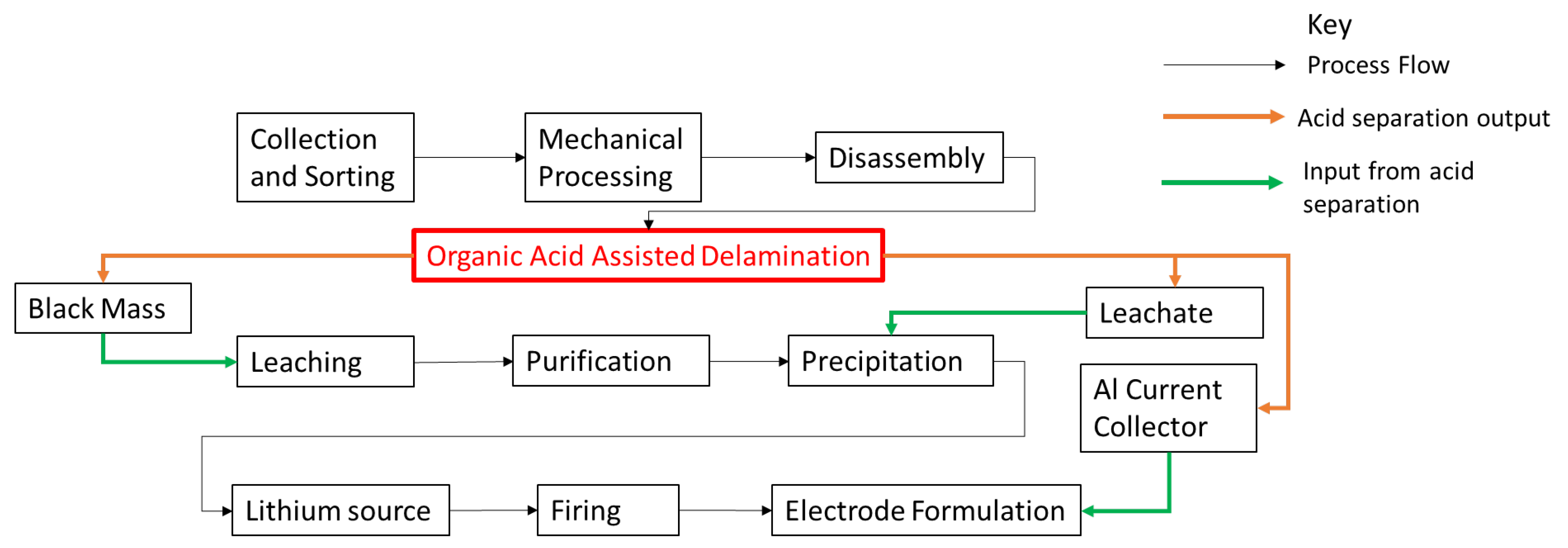
2.1. Separation Method
2.2. Material Characterisation
2.3. Electrochemical Testing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yoshino, A. The Birth of the Lithium-Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef]
- International Energy Agency. Global Electric Vehicle Outlook 2022; Global EV Outlook 2022; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Frith, J.T.; Lacey, M.J.; Ulissi, U. A Non-Academic Perspective on the Future of Lithium-Based Batteries. Nat. Commun. 2023, 14, 420. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Heelan, J.; Gratz, E.; Zheng, Z.; Wang, Q.; Chen, M.; Apelian, D.; Wang, Y. Current and Prospective Li-Ion Battery Recycling and Recovery Processes. JOM 2016, 68, 2632–2638. [Google Scholar] [CrossRef] [Green Version]
- Pagliaro, M.; Meneguzzo, F. Lithium Battery Reusing and Recycling: A Circular Economy Insight. Heliyon 2019, 5, e01866. [Google Scholar] [CrossRef] [Green Version]
- Gaines, L. The Future of Automotive Lithium-Ion Battery Recycling: Charting a Sustainable Course. Sustain. Mater. Technol. 2014, 1–2, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Ganter, M.J.; Landi, B.J.; Babbitt, C.W.; Anctil, A.; Gaustad, G. Cathode Refunctionalization as a Lithium Ion Battery Recycling Alternative. J. Power Sources 2014, 256, 274–280. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Gaines, L. To Recycle, or Not to Recycle, That Is the Question: Insights from Life-Cycle Analysis. MRS Bull. 2012, 37, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of Mixed Cathode Lithium-Ion Batteries for Electric Vehicles: Current Status and Future Outlook. Carbon Energy 2020, 2, 6–43. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.; Zhu, J.; Lyu, Z.; You, H.; Zhou, Y.; Gu, L.; Qu, J.; Xia, Z.; Zhang, Z.; Dai, H. Deep Learning Enhanced Lithium-Ion Battery Nonlinear Fading Prognosis. J. Energy Chem. 2023, 78, 565–573. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M. Cloud-Based in-Situ Battery Life Prediction and Classification Using Machine Learning. Energy Storage Mater. 2023, 57, 346–359. [Google Scholar] [CrossRef]
- Atalay, S.; Sheikh, M.; Mariani, A.; Merla, Y.; Bower, E.; Widanage, W.D. Theory of Battery Ageing in a Lithium-Ion Battery: Capacity Fade, Nonlinear Ageing and Lifetime Prediction. J. Power Sources 2020, 478, 229026. [Google Scholar] [CrossRef]
- Sommerville, R.; Shaw-Stewart, J.; Goodship, V.; Rowson, N.; Kendrick, E. A Review of Physical Processes Used in the Safe Recycling of Lithium Ion Batteries. Sustain. Mater. Technol. 2020, 25, e00197. [Google Scholar] [CrossRef]
- Sommerville, R.; Zhu, P.; Rajaeifar, M.A.; Heidrich, O.; Goodship, V.; Kendrick, E. A Qualitative Assessment of Lithium Ion Battery Recycling Processes. Resour. Conserv. Recycl. 2021, 165, 105219. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, X.; He, Y.; Wang, H.; Zhang, T.; Xie, W. Recent Advances in Pretreating Technology for Recycling Valuable Metals from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2021, 406, 124332. [Google Scholar] [CrossRef]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A Closed Loop Process for Recycling Spent Lithium Ion Batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Guan, Y.; Fan, E.; Wu, F.; Chen, R. Process for Recycling Mixed-Cathode Materials from Spent Lithium-Ion Batteries and Kinetics of Leaching. Waste Manag. 2018, 71, 362–371. [Google Scholar] [CrossRef]
- Gaines, L. Lithium-Ion Battery Recycling Processes: Research towards a Sustainable Course. Sustain. Mater. Technol. 2018, 17, e00068. [Google Scholar] [CrossRef]
- Sloop, S.; Crandon, L.; Allen, M.; Koetje, K.; Reed, L.; Gaines, L.; Sirisaksoontorn, W.; Lerner, M. A Direct Recycling Case Study from a Lithium-Ion Battery Recall. Sustain. Mater. Technol. 2020, 25, e00152. [Google Scholar] [CrossRef]
- Zhang, G.; Du, Z.; He, Y.; Wang, H.; Xie, W.; Zhang, T. A Sustainable Process for the Recovery of Anode and Cathode Materials Derived from Spent Lithium-Ion Batteries. Sustainability 2019, 11, 2363. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhu, X. Pyrolysis-Ultrasonic-Assisted Flotation Technology for Recovering Graphite and LiCoO2 from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10896–10904. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, T.; He, Y.; Zhao, Y.; Wang, S.; Zhang, G.; Zhang, Y.; Feng, Y. Recovery of Valuable Materials from Spent Lithium-Ion Batteries by Mechanical Separation and Thermal Treatment. J. Clean. Prod. 2018, 185, 646–652. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z. Recovery of Lithium from Spent Lithium-Ion Batteries Using Precipitation and Electrodialysis Techniques. Sep. Purif. Technol. 2018, 206, 335–342. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Microbial Production of Specialty Organic Acids from Renewable and Waste Materials. Crit. Rev. Biotechnol. 2015, 35, 497–513. [Google Scholar] [CrossRef]
- Calvo, L.; Vallejo, D. Formation of Organic Acids during the Hydrolysis and Oxidation of Several Wastes in Sub- and Supercritical Water. Ind. Eng. Chem. Res. 2002, 41, 6503–6509. [Google Scholar] [CrossRef]
- Son, J.; Joo, J.C.; Baritugo, K.A.; Jeong, S.; Lee, J.Y.; Lim, H.J.; Lim, S.H.; Yoo, J.I.; Park, S.J. Consolidated Microbial Production of Four-, Five-, and Six-Carbon Organic Acids from Crop Residues: Current Status and Perspectives. Bioresour. Technol. 2022, 351, 127001. [Google Scholar] [CrossRef]
- Fujii, K.; Aoki, M.; Kitayama, K. Biodegradation of Low Molecular Weight Organic Acids in Rhizosphere Soils from a Tropical Montane Rain Forest. Soil Biol. Biochem. 2012, 47, 142–148. [Google Scholar] [CrossRef]
- Kaya, M.; Kursunoglu, S.; Hussaini, S.; Gül, E. Leaching of Turkish Oxidized Pb–Zn Flotation Tailings by Inorganic and Organic Acids. In PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Siegmund, A., Alam, S., Grogan, J., Kerney, U., Shibata, E., Eds.; Springer International Publishing: Cham, Switerland, 2020; pp. 447–468. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Weng, Y.; Xu, S.; Huang, G.; Jiang, C. Synthesis and Performance of Li[(Ni1/3Co1/3Mn1/3)1-Mg]O2 Prepared from Spent Lithium Ion Batteries. J. Hazard. Mater. 2013, 246–247, 163–172. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Removal of Organics by Pyrolysis for Enhancing Liberation and Flotation Behavior of Electrode Materials Derived from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 2205–2214. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, W.; Han, J.; Jiao, F.; Qin, W.; Liu, T.; Zhao, C. Pyrolysis and Physical Separation for the Recovery of Spent LiFePO4 Batteries. Waste Manag. 2019, 89, 83–93. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, F.S. Innovative Leaching of Cobalt and Lithium from Spent Lithium-Ion Batteries and Simultaneous Dechlorination of Polyvinyl Chloride in Subcritical Water. J. Hazard. Mater. 2016, 316, 19–25. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Sun, K.; Qian, C.; Bao, W. Emerging Green Technologies for Recovery and Reuse of Spent Lithium-Ion Batteries—A Review. J. Mater. Chem. A 2022, 10, 17053–17076. [Google Scholar] [CrossRef]
- Maske, T.; Anwani, S.; Methekar, R. Development of Environmentally and Economically Sustainable Delamination Process for Spent Lithium-Ion Batteries. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 2572–2586. [Google Scholar] [CrossRef]
- Shin, H.; Zhan, R.; Dhindsa, K.S.; Pan, L.; Han, T. Electrochemical Performance of Recycled Cathode Active Materials Using Froth Flotation-based Separation Process. J. Electrochem. Soc. 2020, 167, 020504. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Hu, T.; Bai, X.; Wang, S.; Xie, W.; Hao, J.; He, Y. Recovery of LiCoO2 and Graphite from Spent Lithium-Ion Batteries by Cryogenic Grinding and Froth Flotation. Miner. Eng. 2020, 148, 106223. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Bai, X.; Wang, S.; Yang, D.; Fu, Y.; He, Y. Separation of the Cathode Materials from the Al Foil in Spent Lithium-Ion Batteries by Cryogenic Grinding. Waste Manag. 2019, 91, 89–98. [Google Scholar] [CrossRef]
- Marshall, J.; Gastol, D.; Sommerville, R.; Middleton, B.; Goodship, V.; Kendrick, E. Disassembly of Li Ion Cells—Characterization and Safety Considerations of a Recycling Scheme. Metals 2020, 10, 773. [Google Scholar] [CrossRef]
- Zhu, P.; Driscoll, E.H.; Dong, B.; Sommerville, R.; Zorin, A.; Slater, P.R.; Kendrick, E. Direct Reuse of Aluminium and Copper Current Collectors from Spent Lithium-Ion Batteries. Green Chem. 2023, 25, 3503–3514. [Google Scholar] [CrossRef]
- Gastol, D.; Marshall, J.; Cooper, E.; Mitchell, C.; Burnett, D.; Song, T.; Sommerville, R.; Middleton, B.; Crozier, M.; Smith, R.; et al. Reclaimed and Up-Cycled Cathodes for Lithium-Ion Batteries. Glob. Chall. 2022, 6, 2200046. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, L.L.; Slater, P.R.; Anderson, P.A. Battery Recycling. WO2022084668A1, 28 April 2022. [Google Scholar]
- Peeters, N.; Binnemans, K.; Riaño, S. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem. 2020, 22, 4210–4221. [Google Scholar] [CrossRef]
- Higuchi, A.; Ankei, N.; Nishihama, S.; Yoshizuka, K. Selective Recovery of Lithium from Cathode Materials of Spent Lithium Ion Battery. JOM 2016, 68, 2624–2631. [Google Scholar] [CrossRef]
- Gerold, E.; Luidold, S.; Antrekowitsch, H. Selective Precipitation of Metal Oxalates from Lithium Ion Battery Leach Solutions. Metals 2020, 10, 1435. [Google Scholar] [CrossRef]
- Zang, D.; Zhu, R.; Zhang, W.; Wu, J.; Yu, X.; Zhang, Y. Stearic Acid Modified Aluminum Surfaces with Controlled Wetting Properties and Corrosion Resistance. Corros. Sci. 2014, 83, 86–93. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.; Zhang, Y.; Lv, Q.; Xu, T.; Liu, T. Super-Hydrophobic Surface Treatment as Corrosion Protection for Aluminum in Seawater. Corros. Sci. 2009, 51, 1757–1761. [Google Scholar] [CrossRef]
- Li, J.; Wei, H.; Zhao, K.; Wang, M.; Chen, D.; Chen, M. Effect of Anodizing Temperature and Organic Acid Addition on the Structure and Corrosion Resistance of Anodic Aluminum Oxide Films. Thin Solid Film. 2020, 713, 138359. [Google Scholar] [CrossRef]
- Gao, W.; Song, J.; Cao, H.; Lin, X.; Zhang, X.; Zheng, X.; Zhang, Y.; Sun, Z. Selective Recovery of Valuable Metals from Spent Lithium-Ion Batteries—Process Development and Kinetics Evaluation. J. Clean. Prod. 2018, 178, 833–845. [Google Scholar] [CrossRef]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable Recovery of Cathode Materials from Spent Lithium-Ion Batteries Using Lactic Acid Leaching System. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Mu, Y.Y.; Song, X.F.; Yu, J.G. Recovery of Lithium, Nickel, Cobalt, and Manganese from Spent Lithium-Ion Batteries Using L-Tartaric Acid as a Leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic Acid-Based Leaching System: A Sustainable Process for Recovery of Valuable Metals from Spent Li-ion Batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of Cobalt and Lithium from Spent Lithium Ion Batteries Using Organic Citric Acid as Leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating Organic Acids as Alternative Leaching Reagents for Metal Recovery from Lithium Ion Batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Harper, G.D.J.; Kendrick, E.; Anderson, P.A.; Mrozik, W.; Christensen, P.; Lambert, S.; Greenwood, D.; Das, P.K.; Ahmeid, M.; Milojevic, Z.; et al. Roadmap for a Sustainable Circular Economy in Lithium-Ion and Future Battery Technologies. J. Phys. Energy 2023, 5, 021501. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Gratz, E.; Apelian, D.; Wang, Y. A Novel Method to Recycle Mixed Cathode Materials for Lithium Ion Batteries. Green Chem. 2013, 15, 1183. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Zhang, L.; Xu, S. Cleaner Recycling of Cathode Material by In-Situ Thermite Reduction. J. Clean. Prod. 2020, 249, 119340. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Deng, X.; Bohu, T.; Zea, L.; Khaleque, H.N.; Gumulya, Y.; Boxall, N.J.; Morris, C.; Cheng, K.Y. Prospective Directions for Biohydrometallurgy. Hydrometallurgy 2020, 195, 105376. [Google Scholar] [CrossRef]
- Wittkowski, A.; Schirmer, T.; Qiu, H.; Goldmann, D.; Fittschen, U.E.A. Speciation of Manganese in a Synthetic Recycling Slag Relevant for Lithium Recycling from Lithium-Ion Batteries. Metals 2021, 11, 188. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a Low-Carbon Society: A Review of Lithium Resource Availability, Challenges and Innovations in Mining, Extraction and Recycling, and Future Perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]



| Method | Advantage | Disadvantage |
|---|---|---|
| Mineral Acid Delamination [33] | Acids are readily available. | Acids may only be used once. Acids may be highly hazardous. Large amounts of waste generated. Inappropriate for direct recycling due to leaching. |
| Organic Acid Delamination [27,28,29] | Acids can be biologically derived and so can be renewable/green. Similar process to mineral acid delamination. | Acids may only be used once. Large amounts of waste generated. |
| Thermal Treatment [34,35] | Simple technology required. May be tailored to specific chemistries. | Release of toxic compounds. May not be chemistry agnostic |
| Solvent Dissolution [36,37,38] | Solvent can be recovered and reused. High recover efficiency. | Solvents may be toxic and flammable. Possible expense. |
| Mechanical Grinding [15,34,39] | Well-studied technology. Cheap. | Contaminated output requiring further separation. |
| Cryogenic Grinding [15,40,41] | Good peeling efficiency. Prevent surface chemical change. | Liquid nitrogen is expensive. Depending on size, aluminium may be difficult to recover. |
| Acid | Mass of Electrode Sample (g) | Mass of Separated Black Mass (g) | Al Mass (g) |
|---|---|---|---|
| Oxalic acid | 7.513 | 6.897 | 1.157 |
| Citric acid | 7.485 | 3.552 | 1.476 |
| Malic acid | 7.510 | 5.000 | 1.614 |
| Acetic acid | 7.517 | 5.563 | 1.494 |
| Lactic acid | 7.487 | 4.724 | 1.416 |
| Succinic acid | 7.488 | 5.085 | 1.742 |
| Pimelic acid | 7.519 | 5.000 | 1.786 |
| Element | Oxalic | Citric | Malic | Acetic | Lactic | Succinic | Pimelic |
|---|---|---|---|---|---|---|---|
| Al | 10.5 | 12.8 | 11.9 | 12.0 | 11.7 | 11.8 | 11.3 |
| Co | 56.2 | 65.1 | 59.7 | 54.9 | 55.5 | 55.1 | 54.5 |
| Mn | 747.2 | 436.2 | 679.7 | 806.6 | 858.7 | 862.1 | 857.0 |
| Ni | 401.7 | 250.6 | 369.7 | 430.8 | 457.1 | 458.6 | 455.7 |
| Li | 57.5 | 60.4 | 58.7 | 66.8 | 61.3 | 62.6 | 64.4 |
| Element | Oxalic | Citric | Malic | Acetic | Lactic | Succinic | Pimelic |
|---|---|---|---|---|---|---|---|
| Al | 820.3 | 588.8 | 475.0 | 372.4 | 417.5 | 313.8 | 294.6 |
| Co | 1.6 | 87.1 | 100.6 | 28.7 | 60.0 | 43.7 | 24.4 |
| Mn | 12.3 | 131.0 | - | 57.8 | 172.4 | 106.9 | 58.6 |
| Ni | 22.8 | 104.0 | 196.3 | 169.7 | 254.0 | 201.5 | 132.3 |
| Li | 1007.7 | 1389.6 | 942.1 | 388.7 | 657.3 | 522.5 | 409.2 |
| Acid | Phase 1 Wt.Frac. | Phase 2 Wt.Frac. | Ratio Wt. Frac. 1/2 | Rwp % | Rp % | Chi2 |
|---|---|---|---|---|---|---|
| Oxalic acid | 0.75264 | 0.24736 | 3.04 | 5.02 | 3.21 | 10.15 |
| Citric acid | 0.75918 | 0.24082 | 3.15 | 5.90 | 4.08 | 4.577 |
| Malic acid | 0.76608 | 0.23392 | 3.27 | 6.21 | 3.48 | 15.63 |
| Acetic acid | 0.76843 | 0.23157 | 3.32 | 3.64 | 2.54 | 4.777 |
| Lactic acid | 0.81942 | 0.18058 | 4.54 | 5.72 | 3.27 | 11.82 |
| Succinic acid | 0.80778 | 0.19222 | 4.20 | 4.12 | 2.91 | 1.07 |
| Pimelic acid | 0.82649 | 0.17351 | 4.76 | 4.27 | 2.97 | 1.23 |
| Processing Acid | First-Cycle Capacity (mAh/g) | Peeling Efficiency (%) | Li+ Content (ppm) |
|---|---|---|---|
| Citric acid | 57.32 | 99.5 | 60.44 |
| Acetic Acid | 84.51 | 100.0 | 66.76 |
| Malic Acid | 54.23 | 86.4 | 58.66 |
| Oxalic Acid | 69.16 | 96.53 | 57.49 |
| Pimelic Acid | 67.26 | 100 | 64.41 |
| Succinic Acid | 60.15 | 100 | 62.57 |
| Lactic Acid | 66.04 | 96.50 | 61.26 |
| EoL Unprocessed | 107.62 | N/A | 71.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorin, A.; Song, T.; Gastol, D.; Kendrick, E. Acid-Assisted Separation of Cathodic Material from Spent Electric Vehicle Batteries for Recycling. Metals 2023, 13, 1276. https://doi.org/10.3390/met13071276
Zorin A, Song T, Gastol D, Kendrick E. Acid-Assisted Separation of Cathodic Material from Spent Electric Vehicle Batteries for Recycling. Metals. 2023; 13(7):1276. https://doi.org/10.3390/met13071276
Chicago/Turabian StyleZorin, Anton, Tengfei Song, Dominika Gastol, and Emma Kendrick. 2023. "Acid-Assisted Separation of Cathodic Material from Spent Electric Vehicle Batteries for Recycling" Metals 13, no. 7: 1276. https://doi.org/10.3390/met13071276






