Gluconic Acid Leaching of Spent Lithium-Ion Batteries as an Environmentally Friendly Approach to Achieve High Leaching Efficiencies in the Recycling of NMC Active Material
Abstract
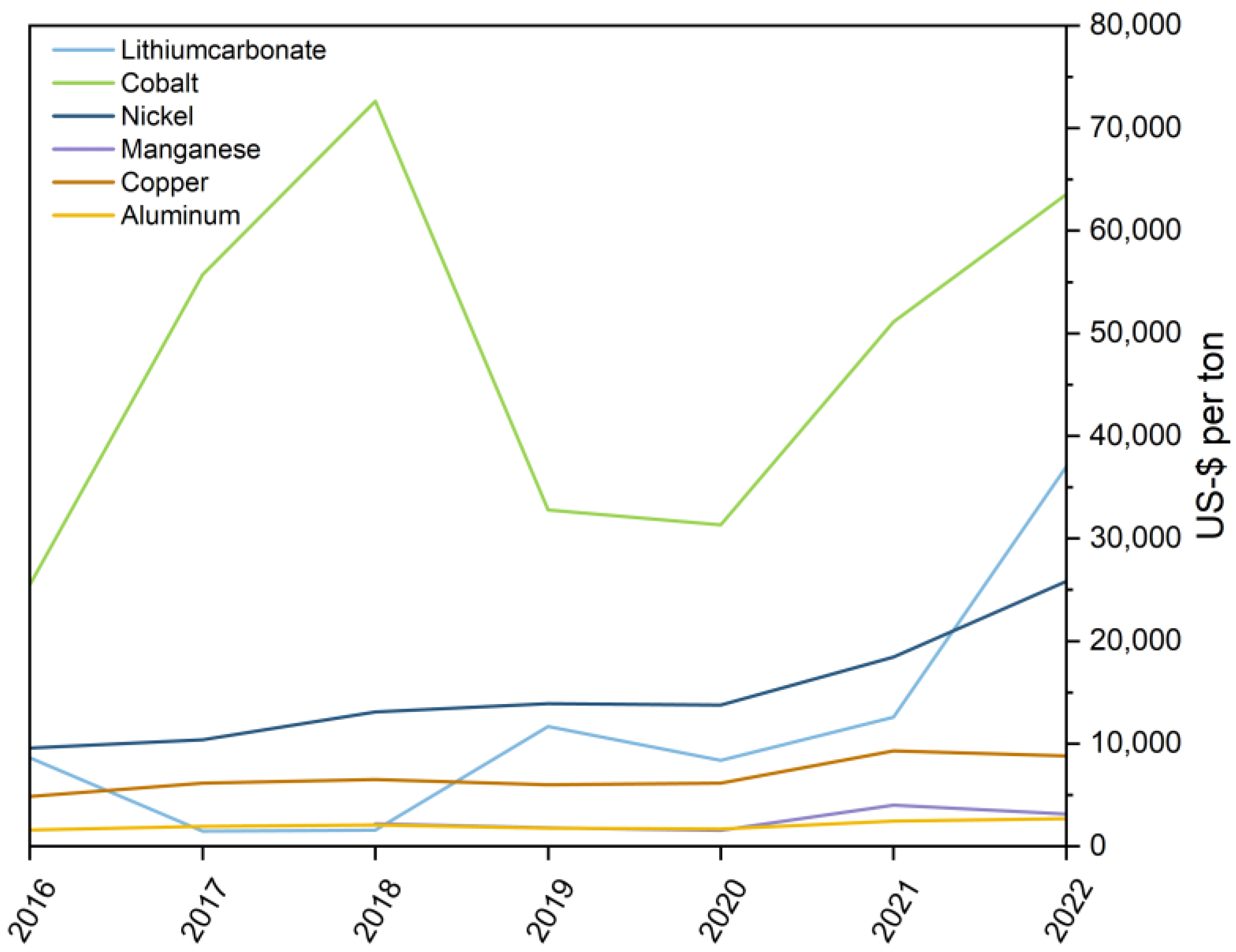
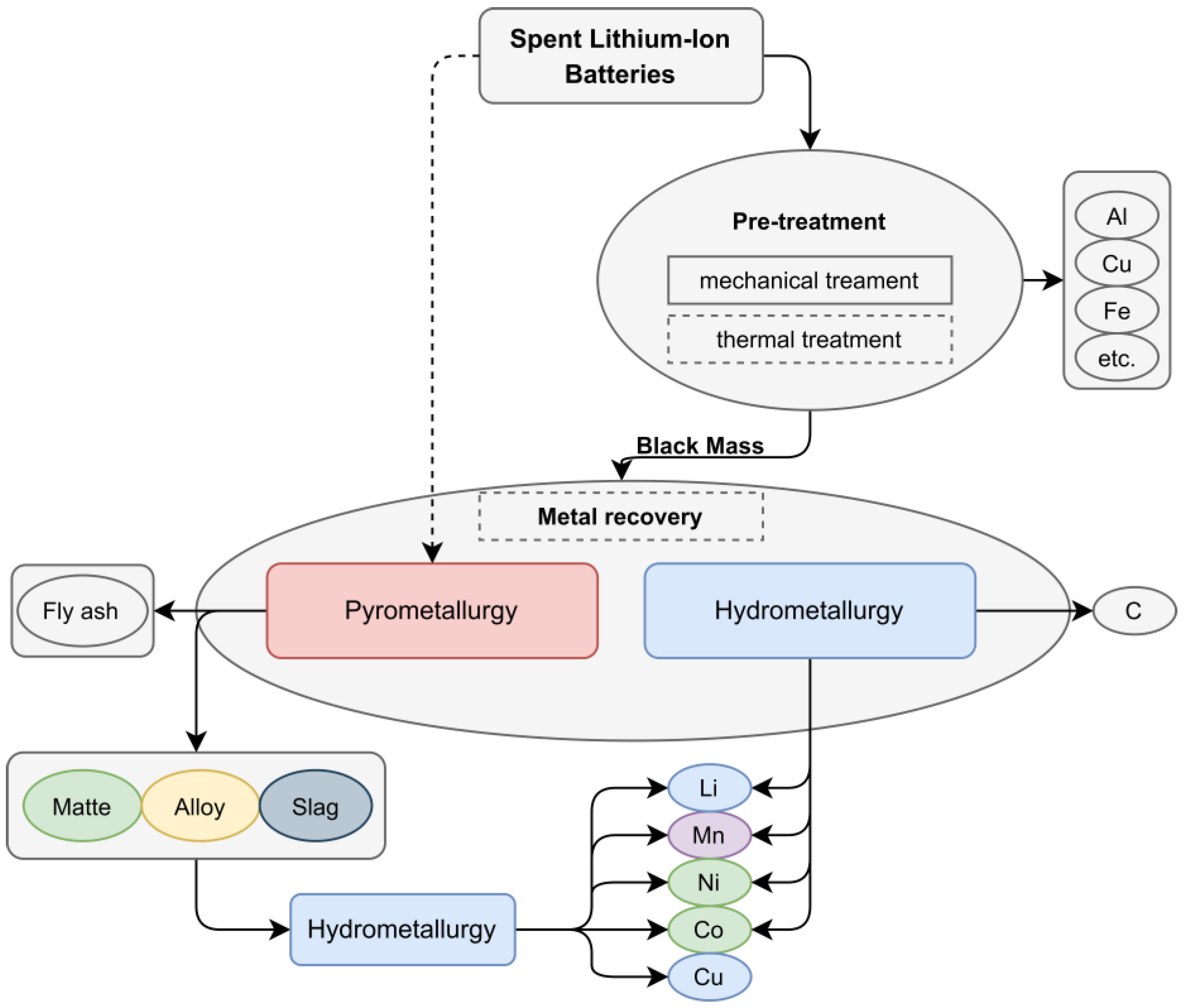
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental
2.3. Analytical Methods
3. Results
3.1. Leaching Behavior of Valuable Metals
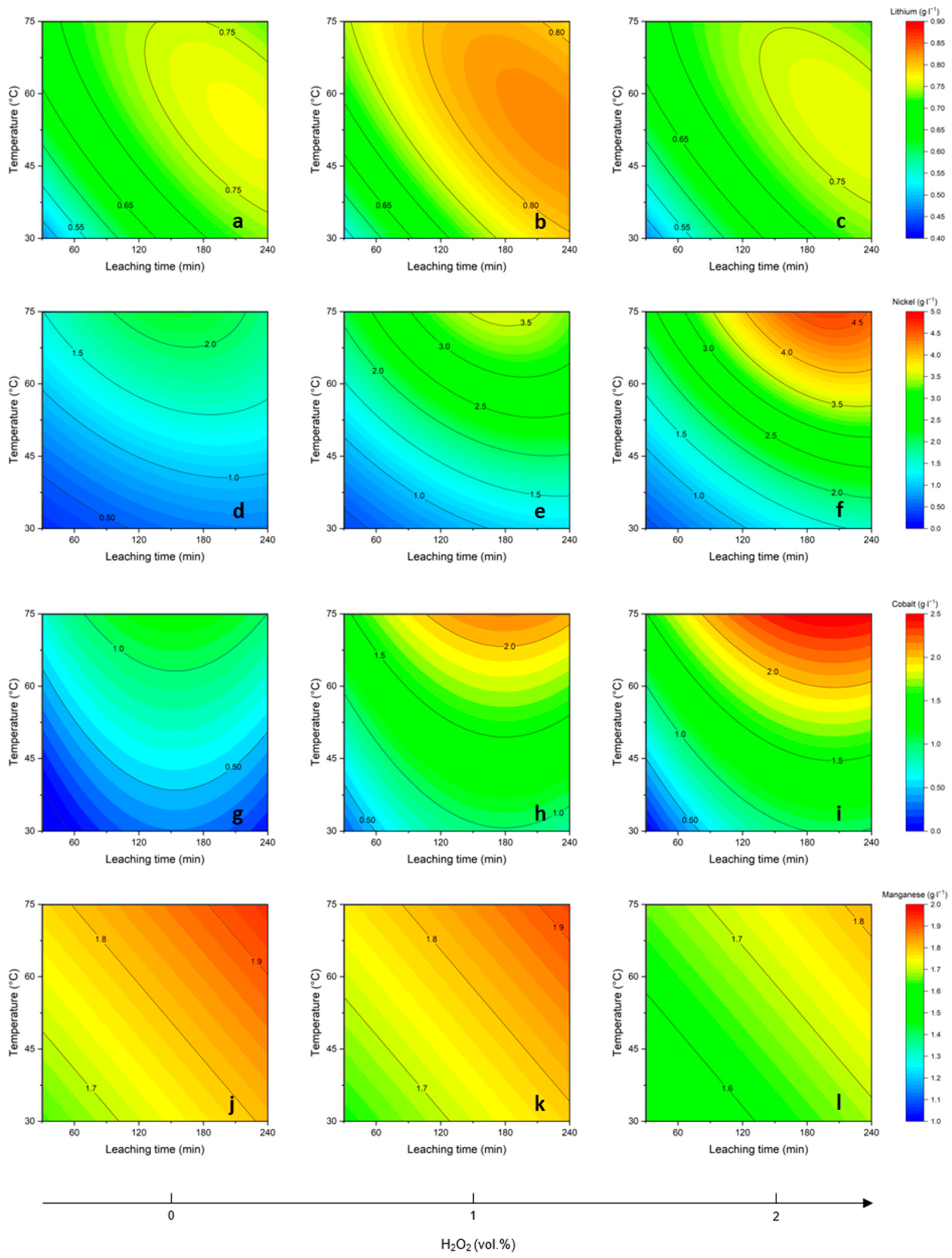
3.2. Parameter Study on the Leaching Behavior of Selected Elements
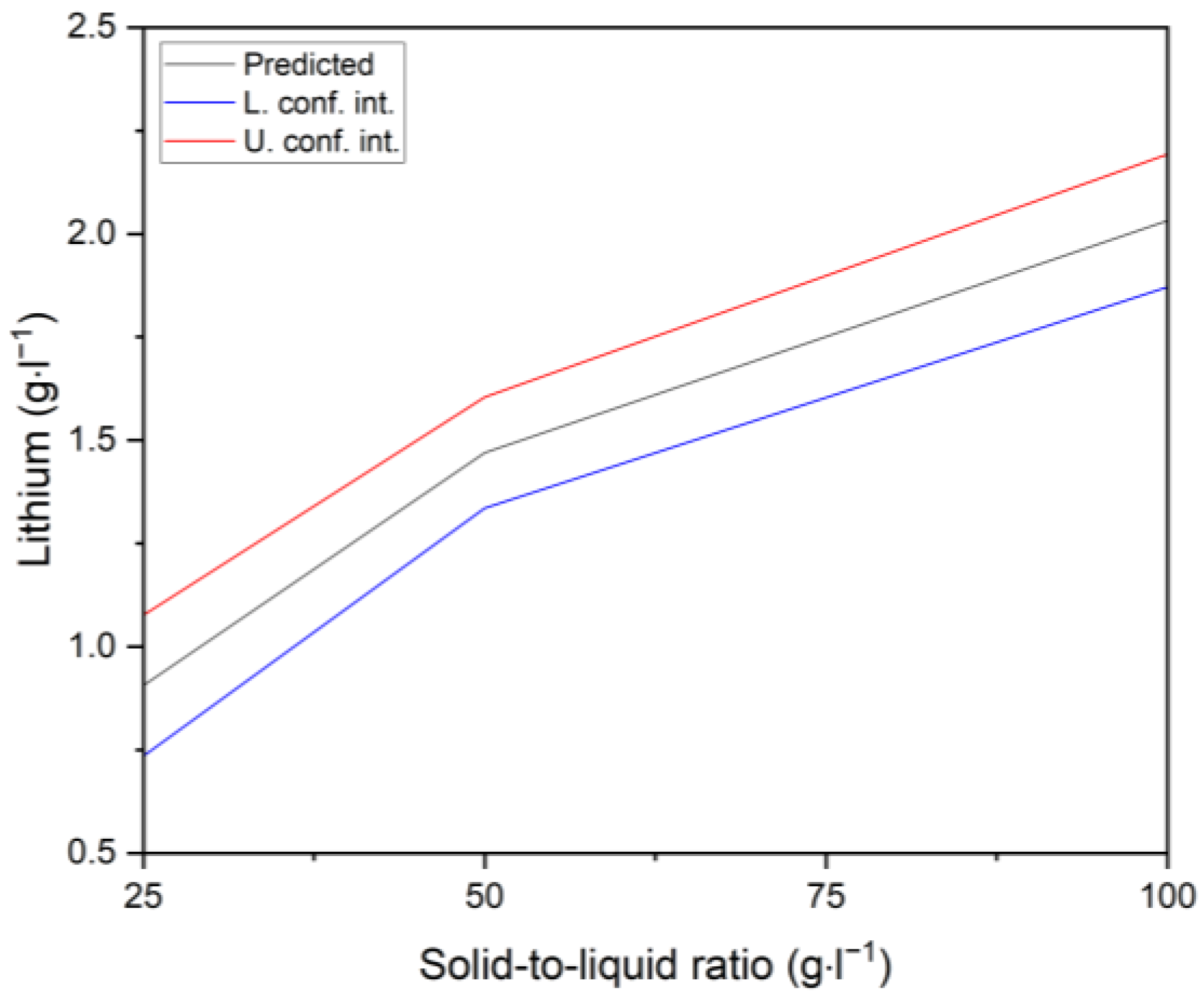
3.2.1. Lithium
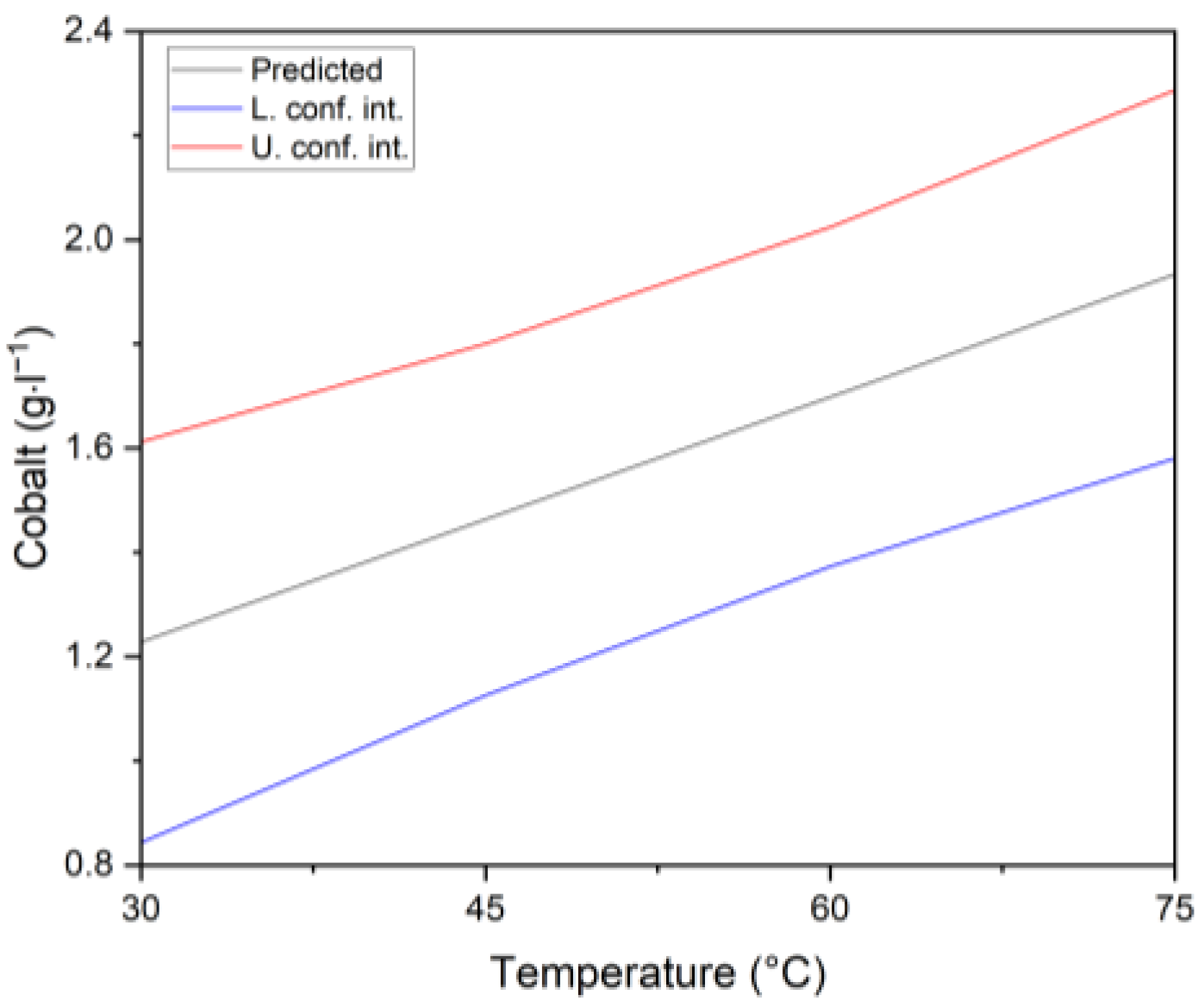
3.2.2. Cobalt
3.2.3. Nickel
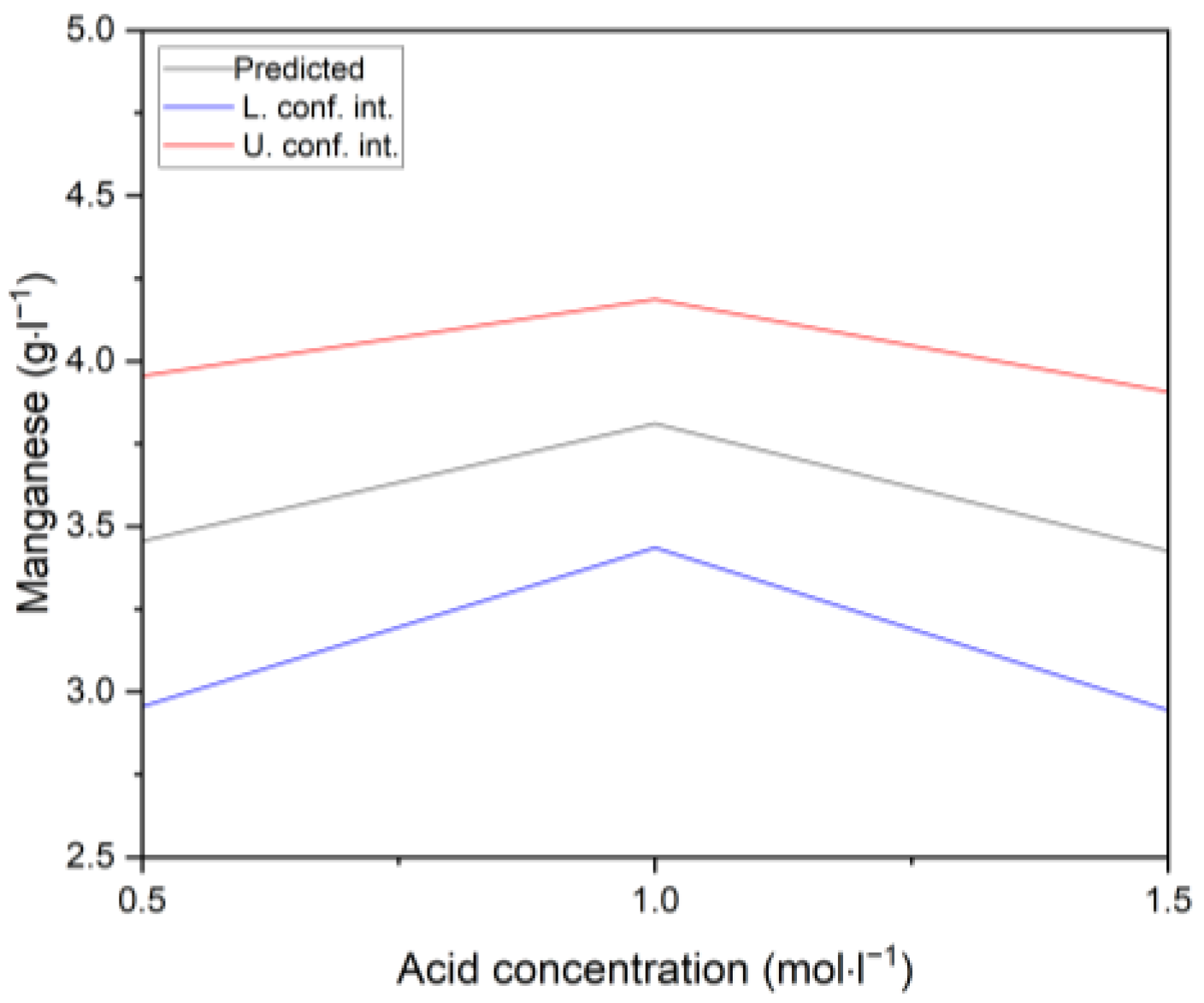
3.2.4. Manganese
3.2.5. Accompanying Elements
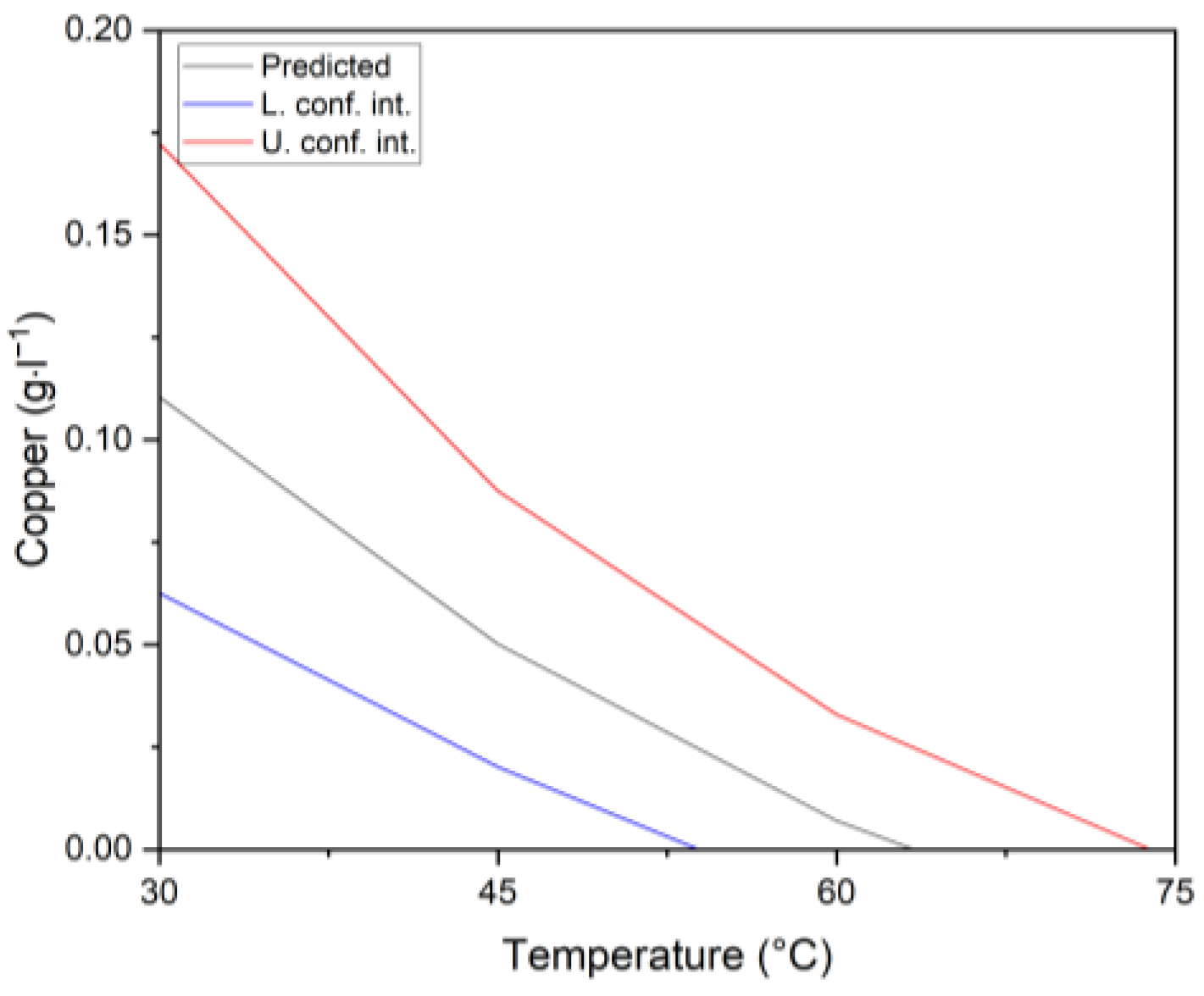
- Copper
- Iron
- Aluminum
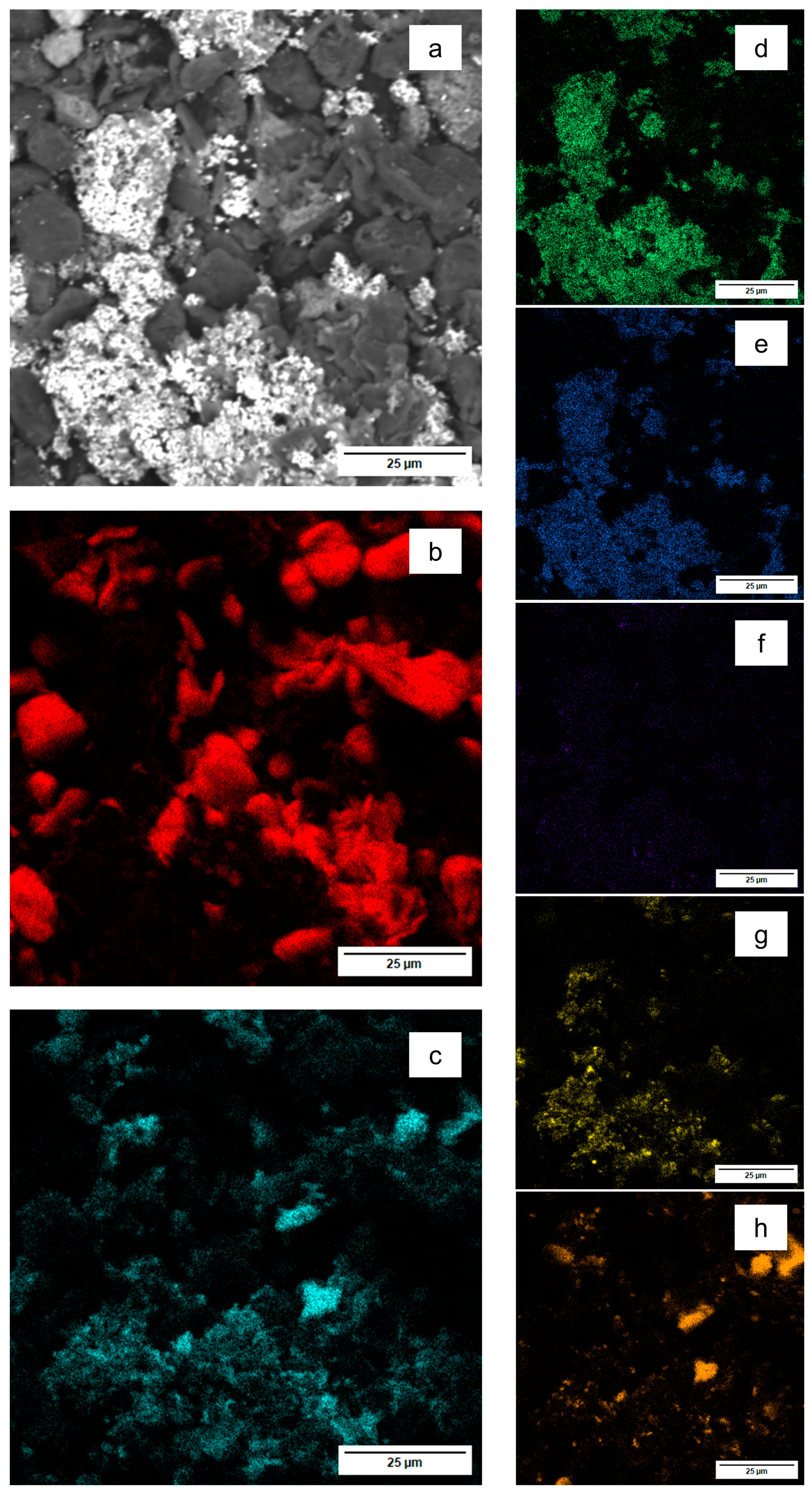
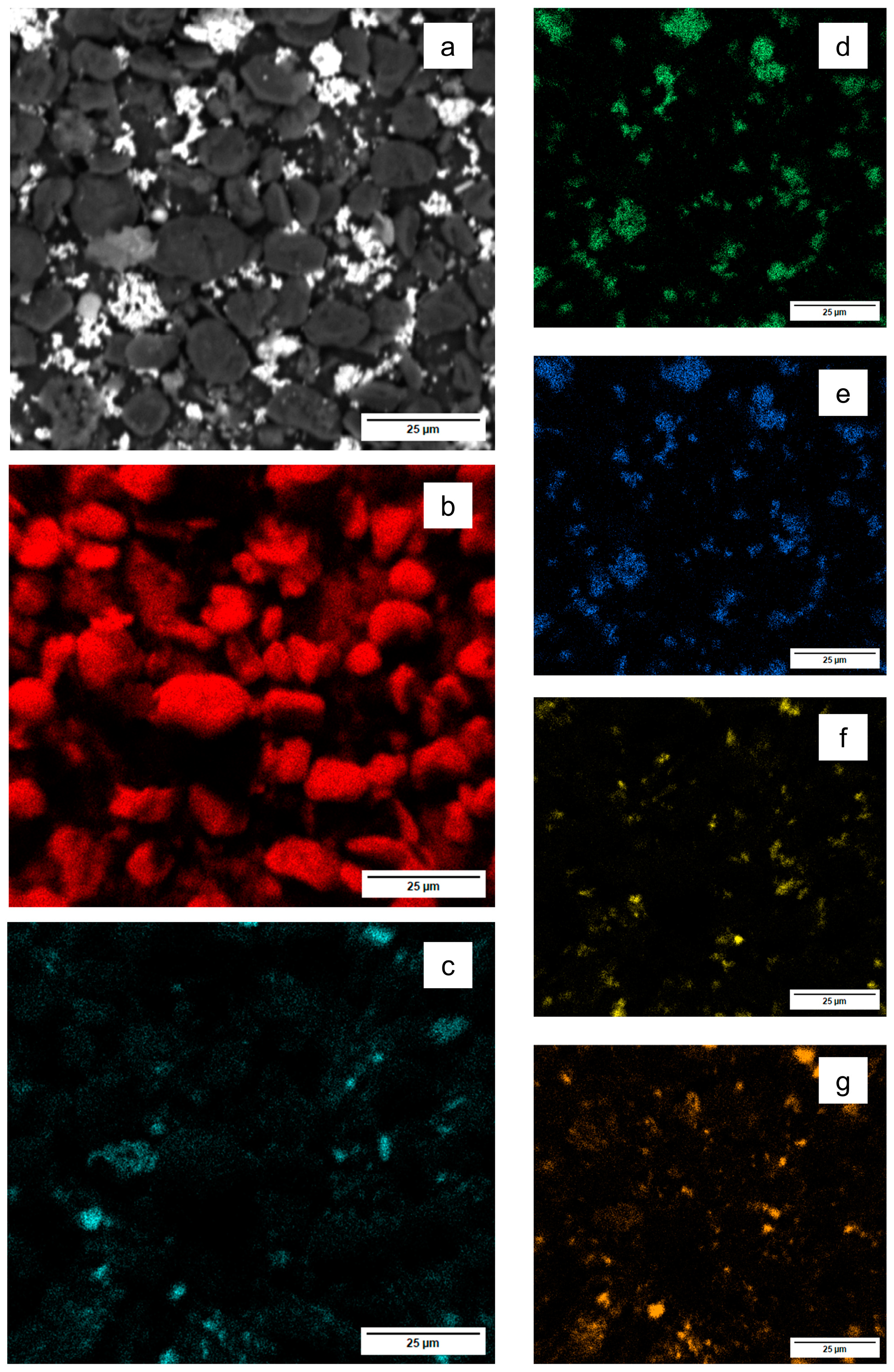
3.3. Filter Residue Analysis
3.3.1. Filter Residue from N3
3.3.2. Filter Residue from N13
3.3.3. N21
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meshram, P.; Mishra, A.; Sahu, R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids—A review. Chemosphere 2020, 242, 125291. [Google Scholar] [CrossRef]
- Kresse, C.; Bastian, D.; Bookhagen, B. Lithium-Ionen-Batterierecycling in Deutschland und Europa; Bundesanstalt für Geowissenschaften und Rohstoffe: Hannover, Germany, 2022. [Google Scholar]
- Zechmeister, A.; Anderl, M.; Bartel, A.; Frei, E.; Gugele, B.; Gössl, M.; Mayer, S.; Heinfellner, H.; Heller, C.; Heuberet, A.; et al. Klimaschutzbericht 2022; Report; Umweltbundesamt: Wien, Austria, 2022. [Google Scholar]
- Statista: Annual Greenhouse Gas Emissions in the European Union (EU-27) from 1990 to 2021, by Sector. Available online: https://www.statista.com/statistics/1171183/ghg-emissions-sector-european-union-eu/ (accessed on 6 June 2023).
- IEA. Global Electric Vehicle Outlook 2022; IEA: Paris, France, 2022. [Google Scholar]
- Presse—Und Informationsamt der Bundesregierung: EU-Umweltrat: Nur Noch CO2-Frei Fahren. Available online: https://www.bundesregierung.de/breg-de/schwerpunkte/europa/verbrennermotoren-2058450 (accessed on 29 March 2022).
- Vertretung in Deutschland: EU-Staaten Stimmen—Endgültig—Für Emissionsfreie Autos ab 2035. Available online: https://germany.representation.ec.europa.eu/news/eu-staaten-stimmen-endgultig-fur-emissionsfreie-autos-ab-2035-2023-03-28_de (accessed on 3 April 2023).
- Europäische Kommission. Der Europäische Gründe Deal—Mitteilung der Komission an das Europäische Parlament, den Europäischen Rat, den Rat, den Europäischen Wirtschafts- und Sozialausschuss und den Ausschuss der Regionen—Der Eruopäische Grüne Deal; Europäische Kommission: Brussels, Belgium, 2019. [Google Scholar]
- Europäische Kommission. Die Europäische Batterie-Allianz Macht Fortschritte: Gründung einer Europäischen Batterieakademie zur Verbesserung der Fähigkeiten fpr ein Rasch Wachsendes Batterieökosystem in Europa; Europäische Kommission: Brussels, Belgium, 2022. [Google Scholar]
- Europäische Kommission. Verordnung des Europäischen Parlaments über Batterien und Altbatterien, zur Aufhebung der Richtlinie 2006/66/EG und zur Änderung der Verordnung (EU) 2019/1020; Europäische Kommission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. Critical Raw Materials: Ensuring Secure and Sustainable Supply Chains for EU´s Green and Digital Future; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- 2023/0081 (COD); Verordnung des Europäischen Parlaments und des Rates zur Schaffung eines Rahmens für Maßnahmen zur Stärkung des Europäischen Ökosystems der Fertigung von Netto-Null-Technologieprodukten (Netto-Null-Industrie-Verordnung). European Commission: Brussels, Belgium, 2023.
- Blengini, G.A.; Latunussa, C.E.L.; Eynard, U.; de Matos, C.T.; Wittmer, D.; Georgitzikis, K.; Pavel, C.; Carrara, S.; Mancini, L.; Unguru, M.; et al. Study on the EU’s List of Critical Raw Materials; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling End-of-Life Electric Vehicle Lithium-Ion Batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Bernhart, W. Recycling von Lithium-Ionen-Batterien im Kontext von Technologie- und Preisentwicklungen. ATZelektronik 2019, 14, 38–43. [Google Scholar] [CrossRef]
- Statista. Lithiumcarbonat: Preisentwicklung in den Jahren 2012 bis 2022. Available online: https://de.statista.com/statistik/daten/studie/1323981/umfrage/preisentwicklung-fuer-lithiumcarbonat/?locale=de (accessed on 3 April 2023).
- Statista. Entwicklung des Aluminiumpreises Weltweit in den Jahren 1960 bis 2022. Available online: https://de.statista.com/statistik/daten/studie/432518/umfrage/aluminiumpreis-weltweit/?locale=de (accessed on 3 April 2023).
- Statista. Durchschnittlicher Preis für Mangan Weltweit in den Jahren 2018 bis 2022. Available online: https://de.statista.com/statistik/daten/studie/1361220/umfrage/durchschnittlicher-preis-fuer-graphit-weltweit/?locale=de (accessed on 3 April 2023).
- Statista. Durchschnittlicher Preis für Kupfer Weltweit in den Jahren 1960 bis 2022. Available online: https://de.statista.com/statistik/daten/studie/432911/umfrage/durchschnittlicher-preis-fuer-kupfer-weltweit/?locale=de (accessed on 3 April 2023).
- Statista. Durchschnittlicher Preis für Kobalt Weltweit in den Jahren 2018 bis 2022. Available online: https://de.statista.com/statistik/daten/studie/1366505/umfrage/durchschnittlicher-preis-fuer-indium-weltweit/?locale=de (accessed on 3 April 2023).
- Statista. Durchschnittlicher Nickelpreis Weltweit in den Jahren 1960 bis 2022. Available online: https://de.statista.com/statistik/daten/studie/260594/umfrage/durchschnittlicher-nickelpreis/?locale=de (accessed on 3 April 2023).
- Windisch-Kern, S.; Gerold, E.; Nigl, T.; Jandric, A.; Altendorfer, M.; Rutrecht, B.; Scherhaufer, S.; Raupenstrauch, H.; Pomberger, R.; Antrekowitsch, H.; et al. Recycling chains for lithium-ion batteries: A critical examination of current challenges, opportunities and process dependencies. Waste Manag. 2022, 138, 125–139. [Google Scholar] [CrossRef]
- Jin, S.; Mu, D.; Lu, Z.; Li, R.; Liu, Z.; Wang, Y.; Tian, S.; Dai, C. A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances. J. Clean. Prod. 2022, 340, 130535. [Google Scholar] [CrossRef]
- Li, J.; Lai, Y.; Zhu, X.; Liao, Q.; Xia, A.; Huang, Y.; Zhu, X. Pyrolysis kinetics and reaction mechanism of the electrode materials during the spent LiCoO2 batteries recovery process. J. Hazard. Mater. 2020, 398, 122955. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Sharma, P.; Pandey, A.; Jang, M.; Jeon, B.-H.; Kim, S.H. Recycling of cathode material from spent lithium-ion batteries: Challenges and future perspectives. J. Hazard. Mater. 2022, 429, 128312. [Google Scholar] [CrossRef] [PubMed]
- Shaw-Stewart, J.; Alvarez-Reguera, A.; Greszta, A.; Marco, J.; Masood, M.; Sommerville, R.; Kendrick, E. Aqueous solution discharge of cylindrical lithium-ion cells. Sustain. Mater. Technol. 2019, 22, e00110. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xu, S.; He, Y.; Liu, X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy 2016, 165, 390–396. [Google Scholar] [CrossRef]
- Lombardo, G.; Ebin, B.; Steenari, B.M.; Alemrajabi, M.; Karlsson, I.; Petranikova, M. Comparison of the effects of incineration, vacuum pyrolysis and dynamic pyrolysis on the composition of NMC-lithium battery cathode-material production scraps and separation of the current collector. Resour. Conserv. Recycl. 2021, 164, 105142. [Google Scholar] [CrossRef]
- Nigl, T.; Nigl, T.; Rutrecht, B.; Altendorfer, M.; Scherhaufer, S.; Meyer, I.; Sommer, M.; Beigl, P. Lithium-Ionen-Batterien—Kreislaufwirtschaftliche Herausforderungen am Ende des Lebenszyklus und im Recycling. BHM Berg-Hüttenmännische Mon. 2021, 166, 144–149. [Google Scholar] [CrossRef]
- Doose, S.; Mayer, J.K.; Michalowski, P.; Kwade, A. Challenges in Ecofriendly Battery Recycling and Closed Material Cycles: A Perspective on Future Lithium Battery Generations. Metals 2021, 11, 291. [Google Scholar] [CrossRef]
- Gerold, E.; Schinnerl, C.; Antrekowitsch, H. Critical Evaluation of the Potential of Organic Acids for the Environmentally Friendly Recycling of Spent Lithium-Ion Batteries. Recycling 2022, 7, 4. [Google Scholar] [CrossRef]
- Gerold, E.; Luidold, S.; Antrekowitsch, H. Separation and Efficient Recovery of Lithium from Spent Lithium-Ion Batteries. Metals 2021, 11, 1091. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Mohanty, A.; Devi, N. A Review on Green Method of Extraction and Recovery of Energy Critical Element Cobalt from Spent Lithium-Ion Batteries (LIBs). Miner. Process. Extr. Metall. Rev. 2023, 44, 52–63. [Google Scholar] [CrossRef]
- Punt, T.; Akdogan, G.; Bradshaw, S.; van Wyk, P. Development of a novel solvent extraction process using citric acid for lithium-ion battery recycling. Miner. Eng. 2021, 173, 107204. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Zhang, Y.; Dong, P.; Wang, D.; Pai, K.V.; Manjanna, J.; Santhosh, G.; Duan, J.; Zhou, Z.; Xiao, J. Effective and environmentally friendly recycling process designed for LiCoO2 cathode powders of spent Li-ion batteries using mixture of mild organic acids. Waste Manag. 2018, 78, 51–57. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Xue, Q.; Fan, E.; Wu, F.; Chen, R. Economical recycling process for spent lithium-ion batteries and macro- and micro-scale mechanistic study. J. Power Sources 2018, 377, 70–79. [Google Scholar] [CrossRef]
- Zheng, Q.; Watanabe, M.; Iwatate, Y.; Alzuma, D.; Shibazaki, K.; Hiraga, Y.; Kishita, A.; Nakayasu, Y. Hydrothermal leaching of ternary and binary lithium-ion battery cathode materials with citric acid and the kinetic study. J. Supercrit. Fluids 2020, 165, 104990. [Google Scholar] [CrossRef]
- Nurqomariah, A.; Fajaryanto, R. Leaching and kinetics process of cobalt from used lithium ion batteries with organic citric acid. E3S Web Conf. 2018, 67, 3036. [Google Scholar]
- Musariri, B.; Akdogan, G.; Dorfling, C.; Bradshaw, S. Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Miner. Eng. 2019, 137, 108–117. [Google Scholar] [CrossRef]
- Choi, J.-W.; Cho, C.-W.; Yun, Y.-S. Organic acid-based linear free energy relationship models for green leaching of strategic metals from spent lithium-ion batteries and improvement of leaching performance. J. Hazard. Mater. 2022, 423, 127214. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Guan, Y.; Fan, E.; Wu, F.; Chen, R. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manag. 2018, 71, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Liu, Q.; Kim, R.; Wang, J.; Liu, G.; Ghahreman, A. Selective recovery of valuable metals from industrial waste lithium-ion batteries using citric acid under reductive conditions: Leaching optimization and kinetic analysis. Hydrometallurgy 2020, 191, 105160. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Zhai, L.; Zhang, X.; Curtiss, L.; Jin, Y.; Wu, F.; Chen, R.; Amine, K. A facile recovery process for cathodes from spent lithium iron phosphate batteries by using oxalic acid. CSEE J. Power Energy Syst. 2018, 4, 219–225. [Google Scholar] [CrossRef]
- Verma, A.; Johnson, G.H.; Corbin, D.R.; Shiflett, M.B. Separation of Lithium and Cobalt from LiCoO2: A Unique Critical Metals Recovery Process Utilizing Oxalate Chemistry. ACS Sustain. Chem. Eng. 2020, 8, 6100–6108. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.; Santhosh, G.; Manjanna, J. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co. Hydrometallurgy 2016, 161, 54–57. [Google Scholar] [CrossRef]
- He, L.-P.; Sun, S.Y.; Mu, Y.Y.; Song, X.F.; Yu, J.G. Recovery of Lithium, Nickel, Cobalt, and Manganese from Spent Lithium-Ion Batteries Using l -Tartaric Acid as a Leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Setiawan, H.; Petrus, H.T.B.M.; Perdana, I. Reaction kinetics modeling for lithium and cobalt recovery from spent lithium-ion batteries using acetic acid. Int. J. Miner. Metall. Mater. 2019, 26, 98–107. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Rashchi, F.; Vahidi, E. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: Process optimization and kinetic aspects. Waste Manag. 2017, 64, 244–254. [Google Scholar] [CrossRef]
- Ning, P.; Meng, Q.; Dong, P.; Duan, J.; Xu, M.; Lin, Y.; Zhang, Y. Recycling of cathode material from spent lithium ion batteries using an ultrasound-assisted DL-malic acid leaching system. Waste Manag. 2020, 103, 52–60. [Google Scholar] [CrossRef]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable Recovery of Cathode Materials from Spent Lithium-Ion Batteries Using Lactic Acid Leaching System. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Gerold, E.; Luidold, S.; Antrekowitsch, H. Decomposition of hydrogen peroxide in selected organic acids. In Proceedings of the European Metallurgical Conference, Salzburg, Austria, 30 June–27 July 2021; pp. 1–13. [Google Scholar]
- Gerold, E.; Lerchbammer, R.; Antrekowitsch, H. Parameter Study on the Recycling of LFP Cathode Material Using Hydrometallurgical Methods. Metals 2022, 12, 1706. [Google Scholar] [CrossRef]
- Roy, J.J.; Cao, B.; Madhavi, S. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef]
- Sethurajan, M.; Gaydardzhiev, S. Bioprocessing of spent lithium ion batteries for critical metals recovery—A review. Resour. Conserv. Recycl. 2021, 165, 105225. [Google Scholar] [CrossRef]
- Do, M.P.; Jegan Roy, J.; Cao, B.; Srinivasan, M. Green Closed-Loop Cathode Regeneration from Spent NMC-Based Lithium-Ion Batteries through Bioleaching. ACS Sustain. Chem. Eng. 2022, 10, 2634–2644. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger. Waste Manag. 2017, 60, 666–679. [Google Scholar] [CrossRef] [Green Version]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, Z.; Zhang, X.; Yang, E.; Tu, Y. A green process to recover valuable metals from the spent ternary lithium-ion batteries. Sep. Purif. Technol. 2022, 299, 121782. [Google Scholar] [CrossRef]
- Sidiq, A.L.; Floweri, O.; Karunawan, J.; Abdillah, O.B.; Santosa, S.P.; Iskandar, F. NCM cathode active materials reproduced from end-of-life Li-ion batteries using a simple and green hydrometallurgical recycling process. Mater. Res. Bull. 2022, 153, 111901. [Google Scholar] [CrossRef]
- Liang, Z.; Ding, X.; Cai, C.; Peng, G.; Hu, J.; Yang, X.; Chen, S.; Liu, L.; Hou, H.; Liang, S.; et al. Acetate acid and glucose assisted subcritical reaction for metal recovery from spent lithium ion batteries. J. Clean. Prod. 2022, 369, 133281. [Google Scholar] [CrossRef]
- Chen, X.; Fan, B.; Xu, L.; Zhou, T.; Kong, J. An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries. J. Clean. Prod. 2016, 112, 3562–3570. [Google Scholar] [CrossRef]
- Yao, L.; Xi, Y.; Han, H.; Li, W.; Wang, C.; Feng, Y. LiMn2O4 prepared from waste lithium ion batteries through sol-gel process. J. Alloys Compd. 2021, 868, 159222. [Google Scholar] [CrossRef]
- Singh, O.V.; Kumar, R. Biotechnological production of gluconic acid: Future implications. Appl. Microbiol. Biotechnol. 2007, 75, 713–722. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Li, C.; Lin, J.; Gao, L.; Lin, H.; Lin, J. Modeling and simulation of enzymatic gluconic acid production using immobilized enzyme and CSTR-PFTR circulation reaction system. Biotechnol. Lett. 2018, 40, 649–657. [Google Scholar] [CrossRef]
- Anastassiadis, S.; Morgunov, I.G. Gluconic acid production. Recent Pat. Biotechnol. 2007, 1, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Antonick, P.J.; Hu, Z.; Fujita, Y.; Reed, D.W.; Das, G.; Wu, L.; Shivaramaiah, R.; Kim, P.; Eslamimanesh, A.; Riman, R.E. Bio- and mineral acid leaching of rare earth elements from synthetic phosphogypsum. J. Chem. Thermodyn. 2019, 132, 491–496. [Google Scholar] [CrossRef]
- Mouna, H.M.; Baral, S.S. A bio-hydrometallurgical approach towards leaching of lanthanum from the spent fluid catalytic cracking catalyst using Aspergillus niger. Hydrometallurgy 2019, 184, 175–182. [Google Scholar]
- Naseri, T.; Mousavi, S.M.; Kuchta, K. Environmentally sustainable and cost-effective recycling of Mn-rich Li-ion cells waste: Effect of carbon sources on the leaching efficiency of metals using fungal metabolites. Waste Manag. 2023, 157, 47–59. [Google Scholar] [CrossRef]
- Liu, Q.; Bai, J.F.; Gu, W.H.; Peng, S.J.; Wang, L.C.; Wang, J.W.; Li, H.X. Leaching of copper from waste printed circuit boards using Phanerochaete chrysosporium fungi. Hydrometallurgy 2020, 196, 105427. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Hajdu-Rahkama, R.; Puhakka, J.A. High-rate and-yield continuous fluidized-bed bioconversion of glucose-to-gluconic acid for enhanced metal leaching. Chem. Eng. J. 2023, 462, 142088. [Google Scholar] [CrossRef]
- Roshanfar, M.; Golmohammadzadeh, R.; Rashchi, F. An environmentally friendly method for recovery of lithium and cobalt from spent lithium-ion batteries using gluconic and lactic acids. J. Environ. Chem. Eng. 2019, 7, 102794. [Google Scholar] [CrossRef]
- Fan, E.; Shi, P.; Zhang, X.; Lin, J.; Wu, F.; Li, L.; Chen, R. Glucose oxidase-based biocatalytic acid-leaching process for recovering valuable metals from spent lithium-ion batteries. Waste Manag. 2020, 114, 166–173. [Google Scholar] [CrossRef]
- Gerold, E.; Antrekowitsch, H. Potenzialabschätzung von Synergieeffekten zur simultanen Rückgewinnung von Wertmetallen aus komplexen, metallhaltigen Reststoffen. Osterr. Wasser-Abfallwirtsch. 2022, 74, 22–31. [Google Scholar] [CrossRef]
- Tao, R.; Xing, P.; Li, H.; Cun, Z.; Wang, C.; Ma, S.; Sun, Z. Kinetics study and recycling strategies in different stages of full-component pyrolysis of spent LiNixCoyMnzO2 lithium-ion batteries. Waste Manag. 2023, 155, 8–18. [Google Scholar] [CrossRef]



| Element | Li | Ni | Co | Mn | Cu | Al | Fe |
|---|---|---|---|---|---|---|---|
| wt % | 3.4 | 22.2 | 6.2 | 7.3 | 5.9 | 4.4 | 0.5 |
| Parameter | Minimum Value | Maximum Value |
|---|---|---|
| C6H10O10 (mol/L) | 0.5 | 1.5 |
| H2O2 (vol %) | 0 | 2 |
| Temperature (°C) | 30 | 75 |
| S/L ratio (g/L) | 25 | 100 |
| Leaching time (min) | 30 | 240 |
| No. | Acid Concentration | Oxidant Concentration | Temperature | Solid/Liquid Ratio | Leaching Time |
|---|---|---|---|---|---|
| - | (mol·L−1) | (vol %) | (°C) | (g·L−1) | (min) |
| N1 | 0.5 | 2 | 30 | 25 | 30 |
| N2 | 0.5 | 0 | 60 | 25 | 30 |
| N3 | 1.5 | 2 | 60 | 25 | 30 |
| N4 | 1 | 1 | 75 | 25 | 30 |
| N5 | 1.5 | 1 | 30 | 50 | 30 |
| N6 | 0.5 | 0 | 30 | 100 | 30 |
| N7 | 1.5 | 2 | 30 | 100 | 30 |
| N8 | 1.5 | 0 | 75 | 100 | 30 |
| N9 | 0.5 | 2 | 75 | 100 | 30 |
| N10 | 1.5 | 0 | 30 | 25 | 60 |
| N11 | 1.5 | 0 | 75 | 25 | 60 |
| N12 | 0.5 | 0 | 75 | 50 | 60 |
| N13 | 1.5 | 2 | 75 | 50 | 60 |
| N14 | 1 | 1 | 45 | 100 | 60 |
| N15 | 1.5 | 0 | 60 | 100 | 60 |
| N16 | 1 | 2 | 30 | 25 | 120 |
| N17 | 1.5 | 2 | 45 | 100 | 120 |
| N18 | 0.5 | 1 | 60 | 100 | 120 |
| N19 | 0.5 | 0 | 30 | 25 | 240 |
| N20 | 1.5 | 2 | 30 | 25 | 240 |
| N21 | 1.5 | 0 | 75 | 25 | 240 |
| N22 | 0.5 | 2 | 75 | 25 | 240 |
| N23 | 0.5 | 2 | 45 | 50 | 240 |
| N24 | 1 | 0 | 60 | 50 | 240 |
| N25 | 1.5 | 0 | 30 | 100 | 240 |
| N26 | 0.5 | 2 | 30 | 100 | 240 |
| N27 | 0.5 | 0 | 75 | 100 | 240 |
| N28 | 1.5 | 1 | 75 | 100 | 240 |
| N29 | 1 | 2 | 75 | 100 | 240 |
| N30 | 1 | 1 | 60 | 50 | 120 |
| N31 | 1 | 1 | 60 | 50 | 120 |
| N32 | 1 | 1 | 60 | 50 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerchbammer, R.; Gerold, E.; Antrekowitsch, H. Gluconic Acid Leaching of Spent Lithium-Ion Batteries as an Environmentally Friendly Approach to Achieve High Leaching Efficiencies in the Recycling of NMC Active Material. Metals 2023, 13, 1330. https://doi.org/10.3390/met13081330
Lerchbammer R, Gerold E, Antrekowitsch H. Gluconic Acid Leaching of Spent Lithium-Ion Batteries as an Environmentally Friendly Approach to Achieve High Leaching Efficiencies in the Recycling of NMC Active Material. Metals. 2023; 13(8):1330. https://doi.org/10.3390/met13081330
Chicago/Turabian StyleLerchbammer, Reinhard, Eva Gerold, and Helmut Antrekowitsch. 2023. "Gluconic Acid Leaching of Spent Lithium-Ion Batteries as an Environmentally Friendly Approach to Achieve High Leaching Efficiencies in the Recycling of NMC Active Material" Metals 13, no. 8: 1330. https://doi.org/10.3390/met13081330





