CO–H2 Gas-Based Reduction Behavior of Cr-Rich Electroplating Sludge Mixed with Iron Ore Powder
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
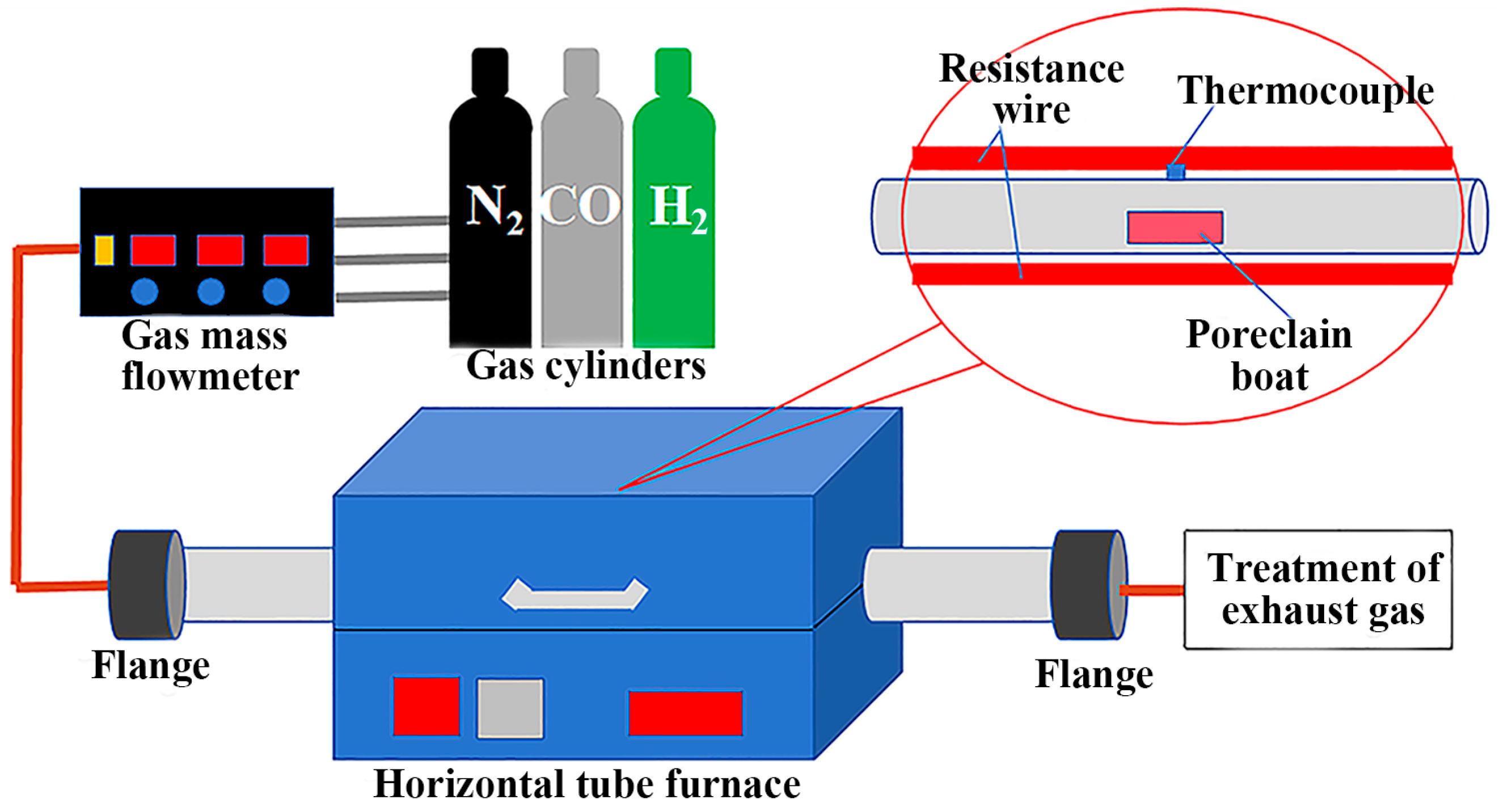
2.2. Experimental Procedure
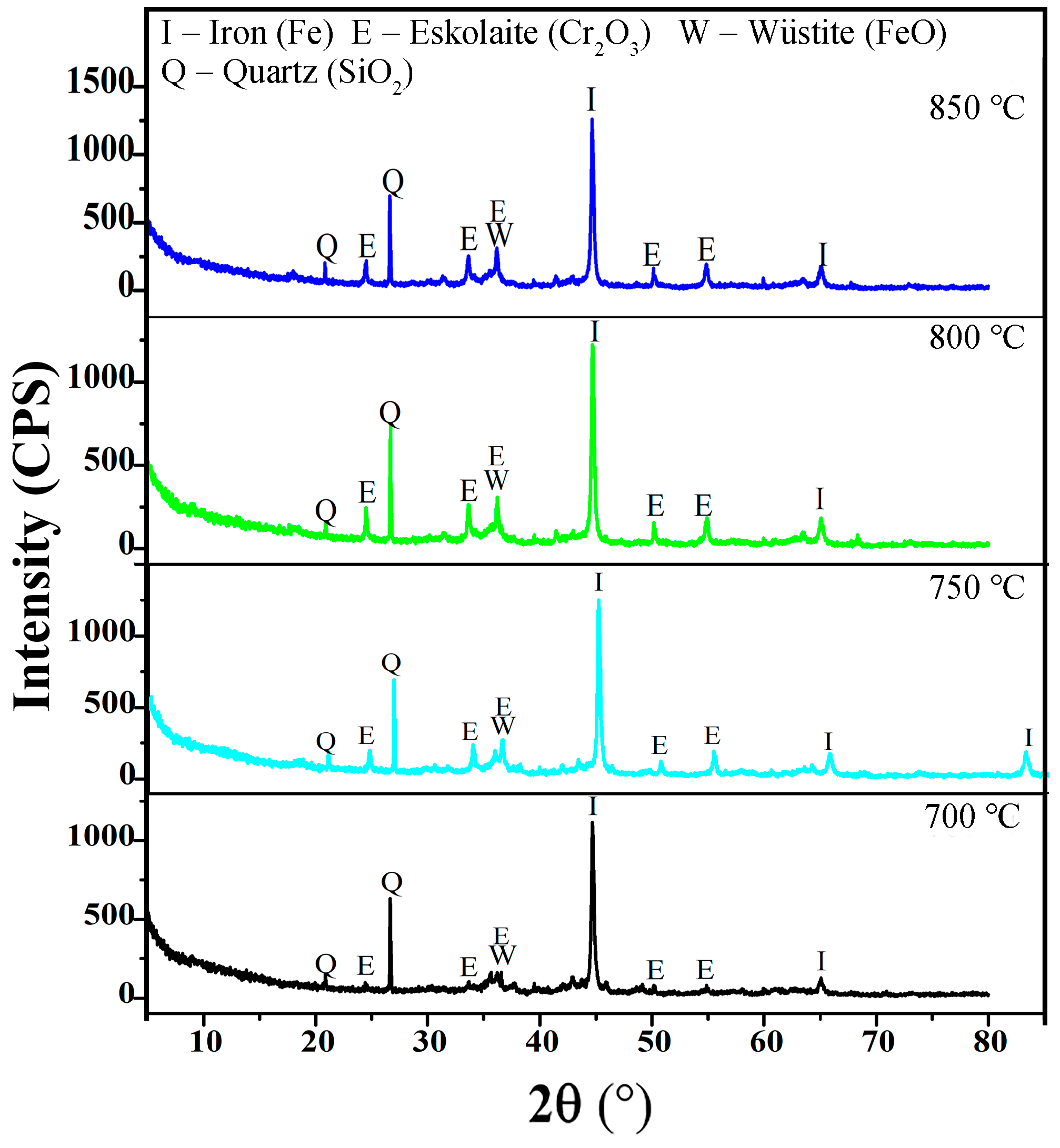
2.3. Characterizations
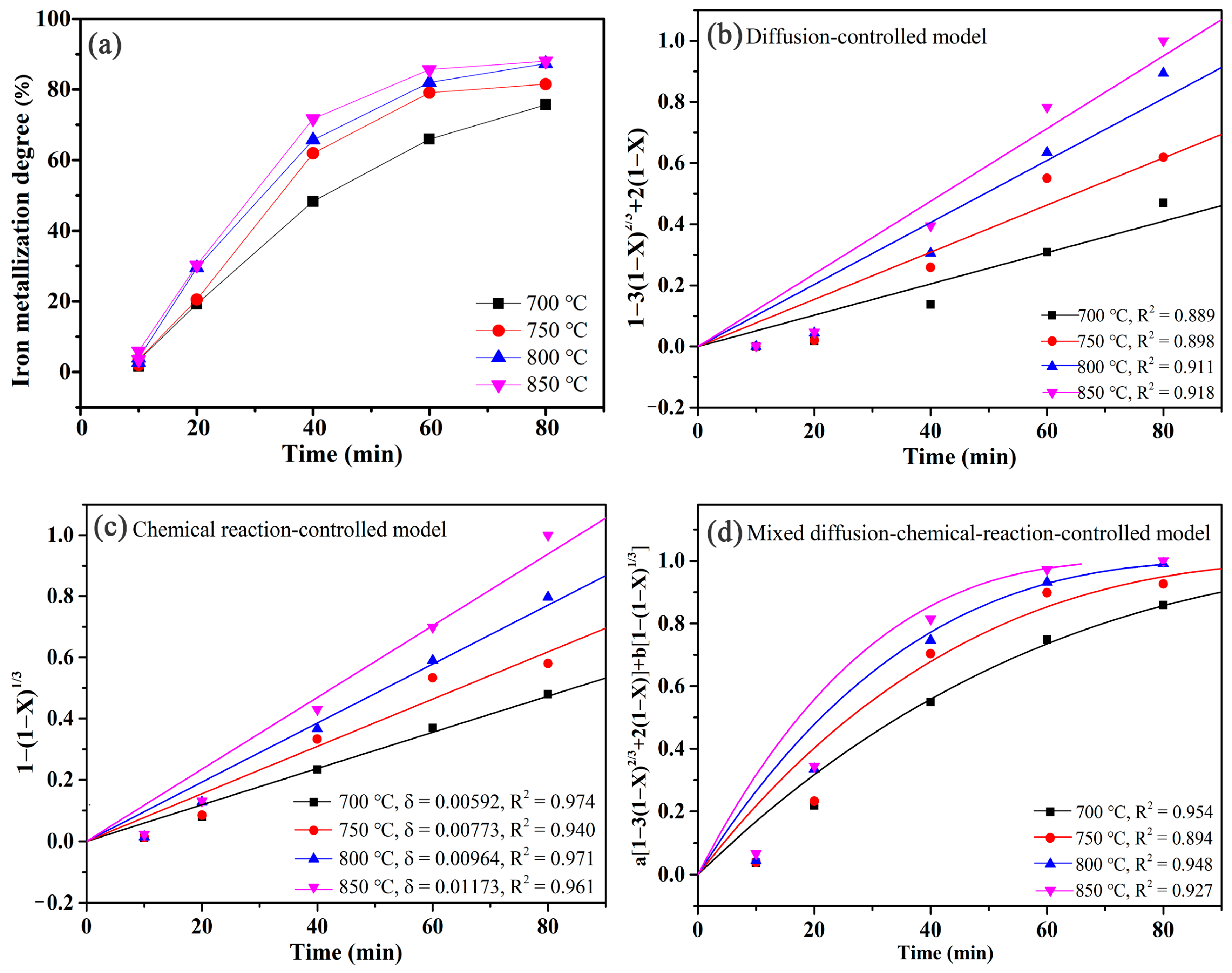
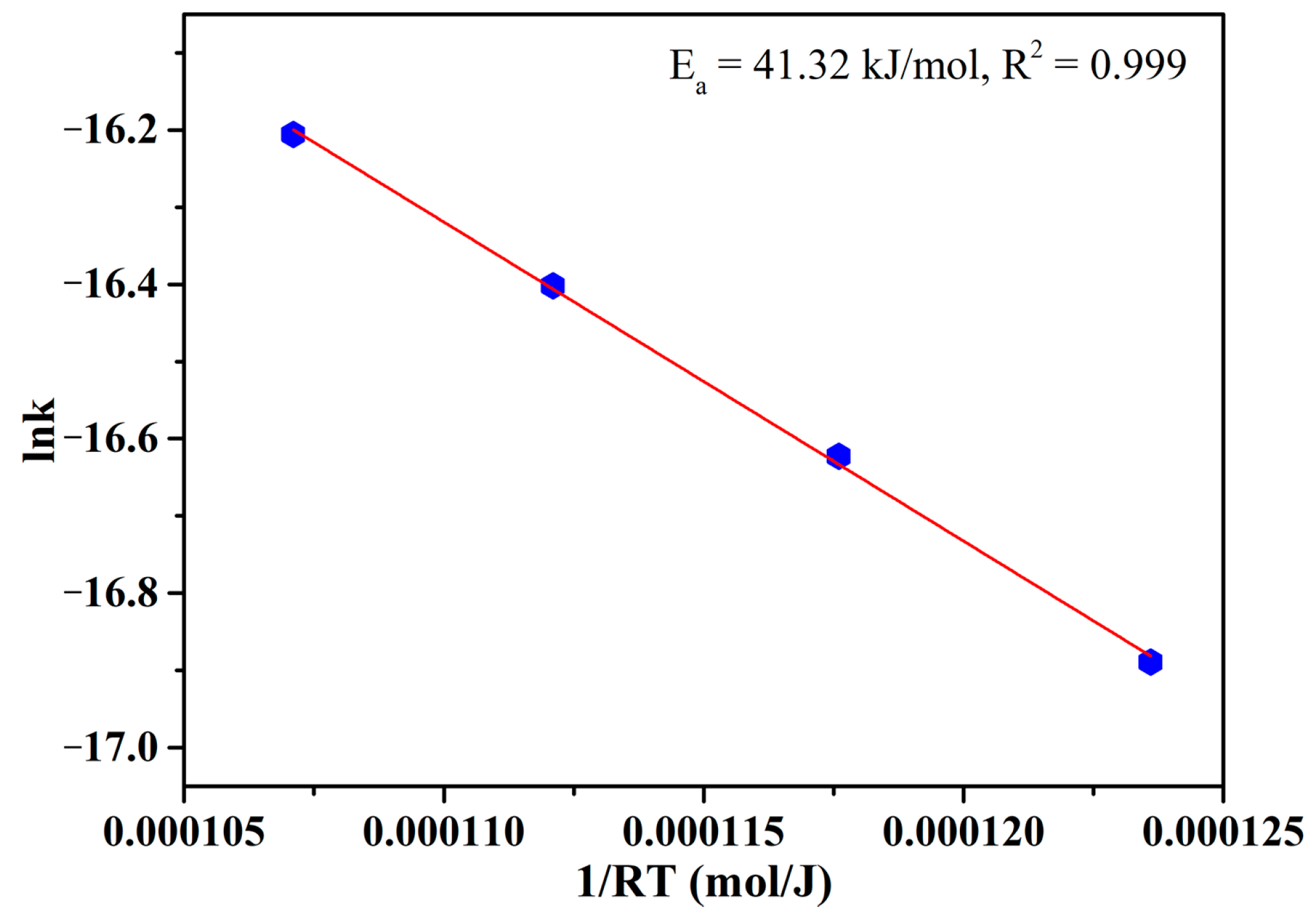
2.4. Kinetic Calculations
3. Results and Discussion
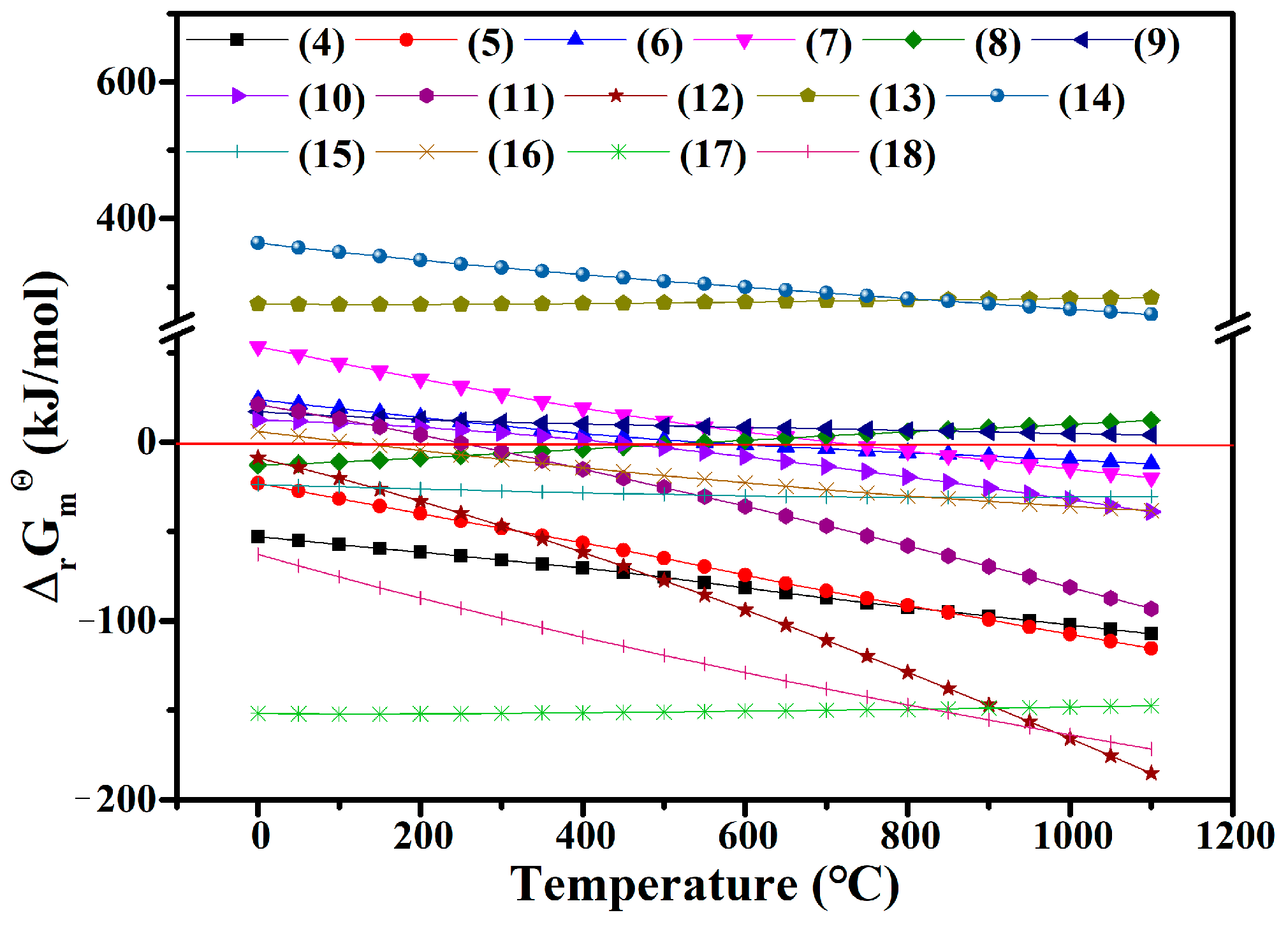
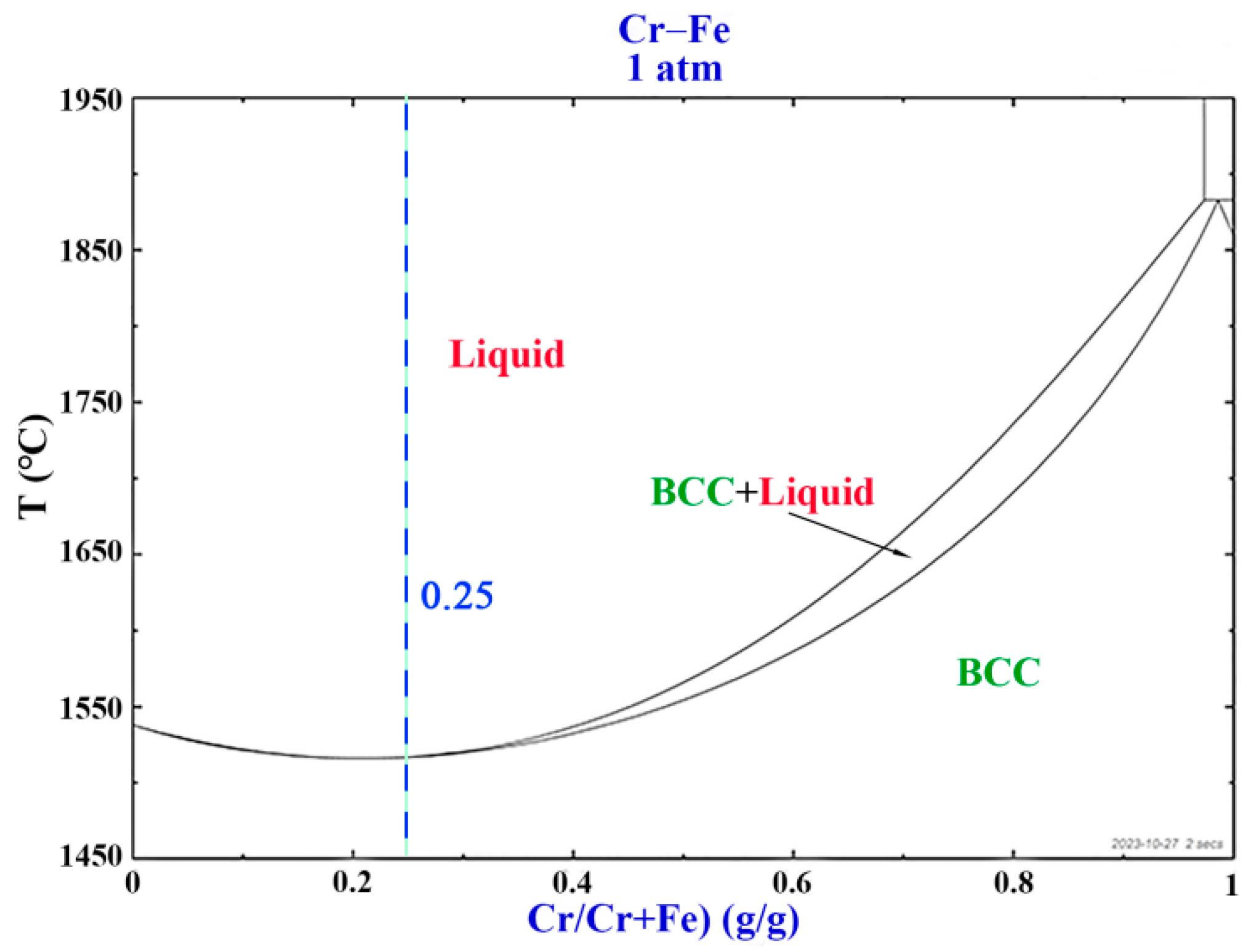
3.1. Thermodynamic Calculations
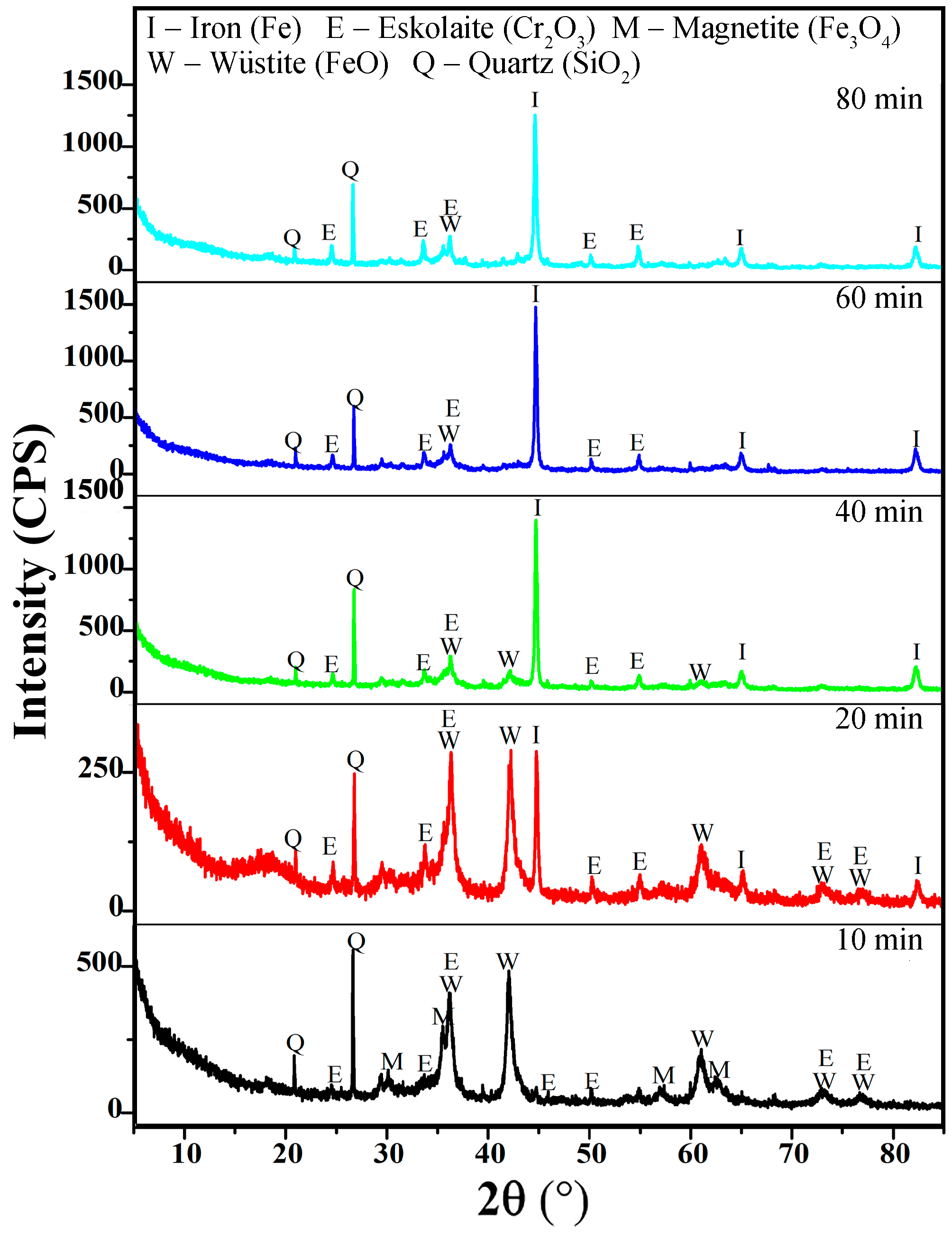
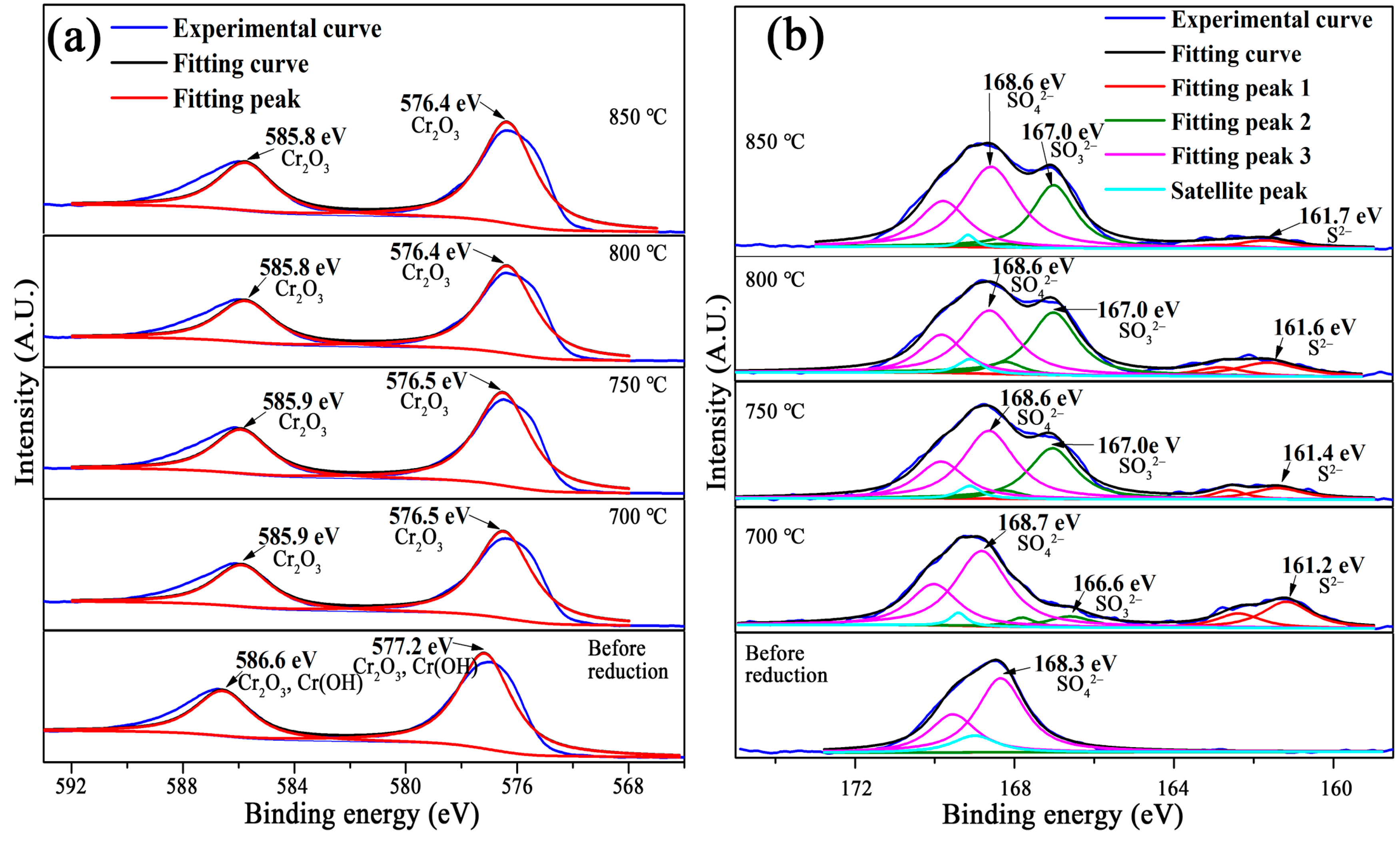
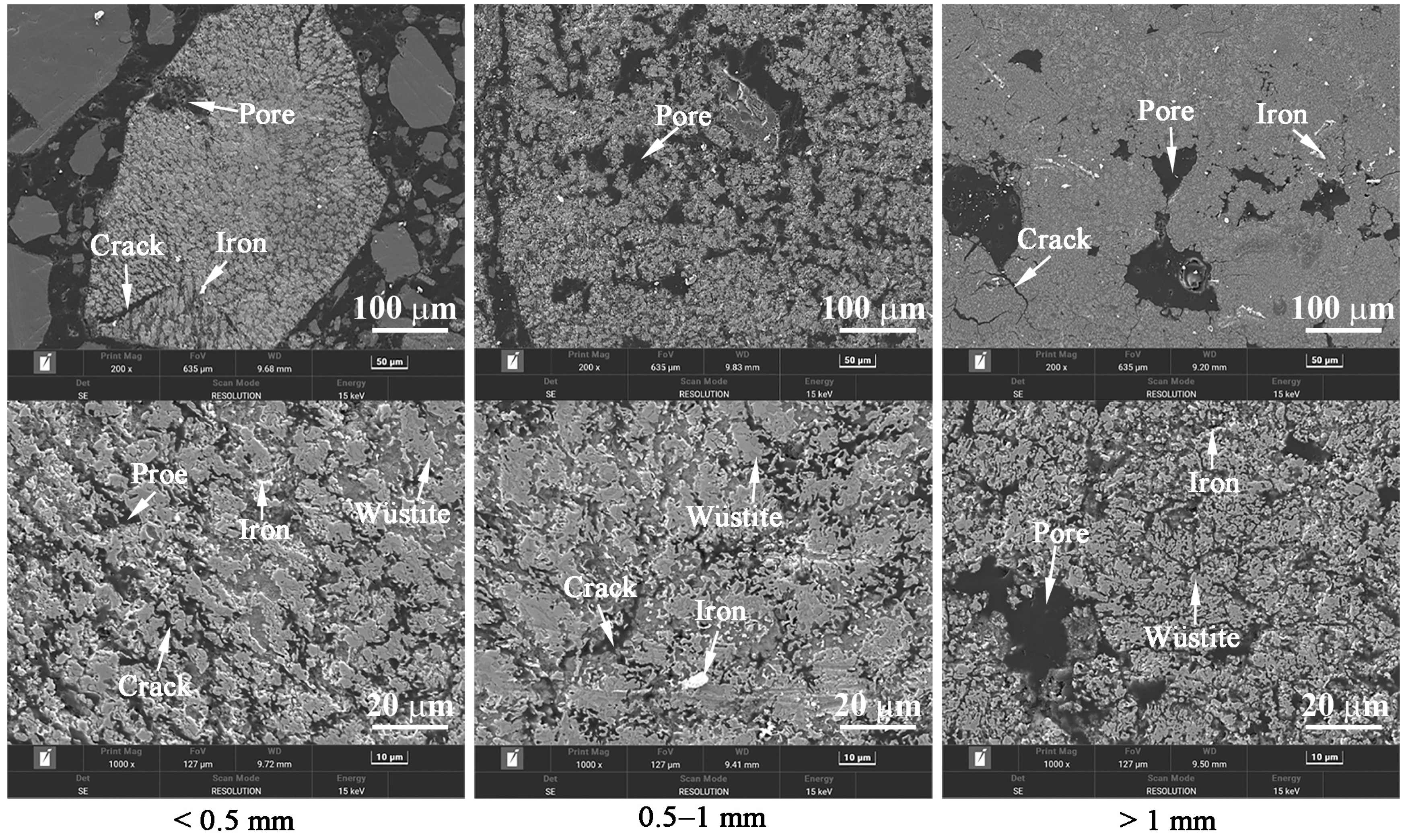
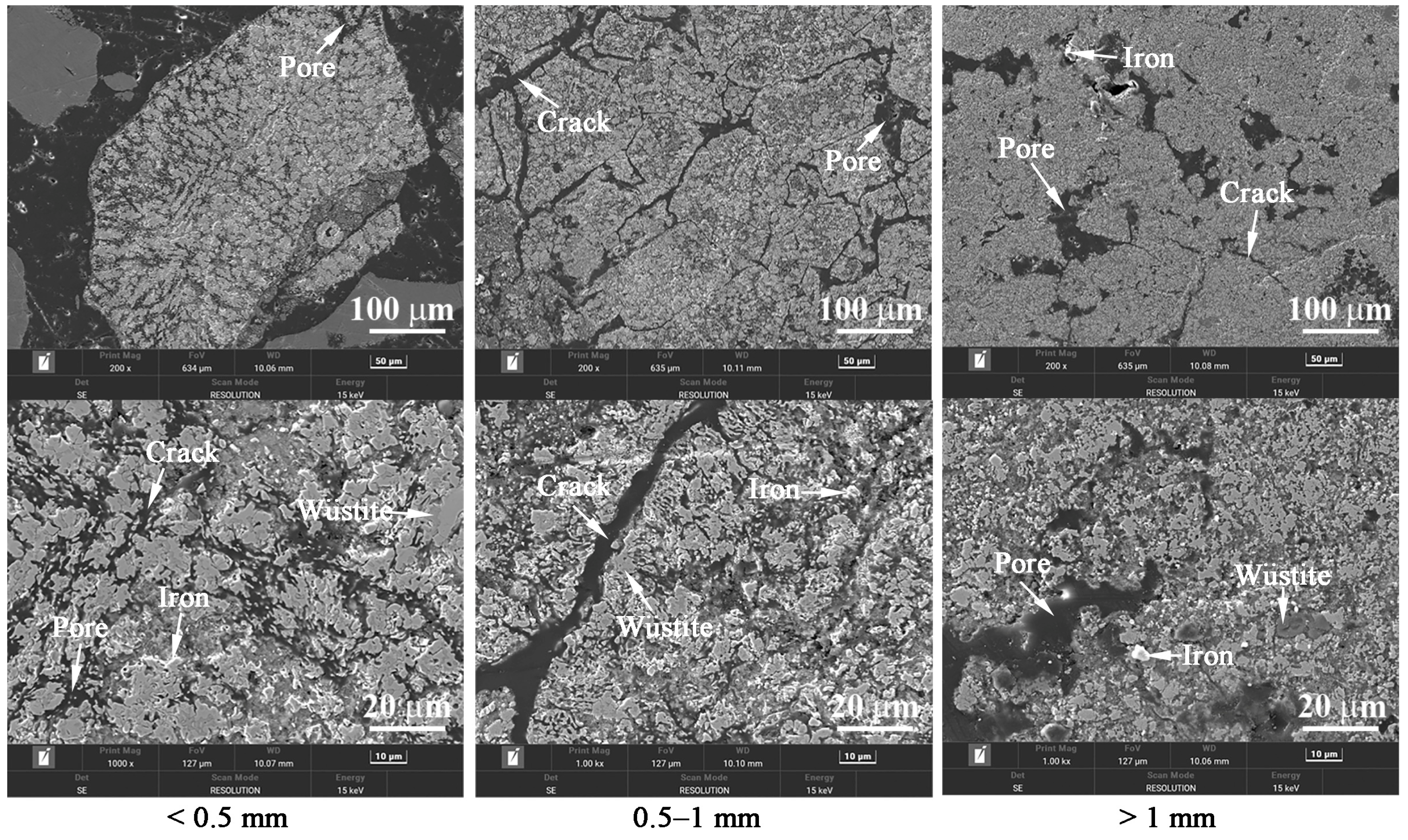
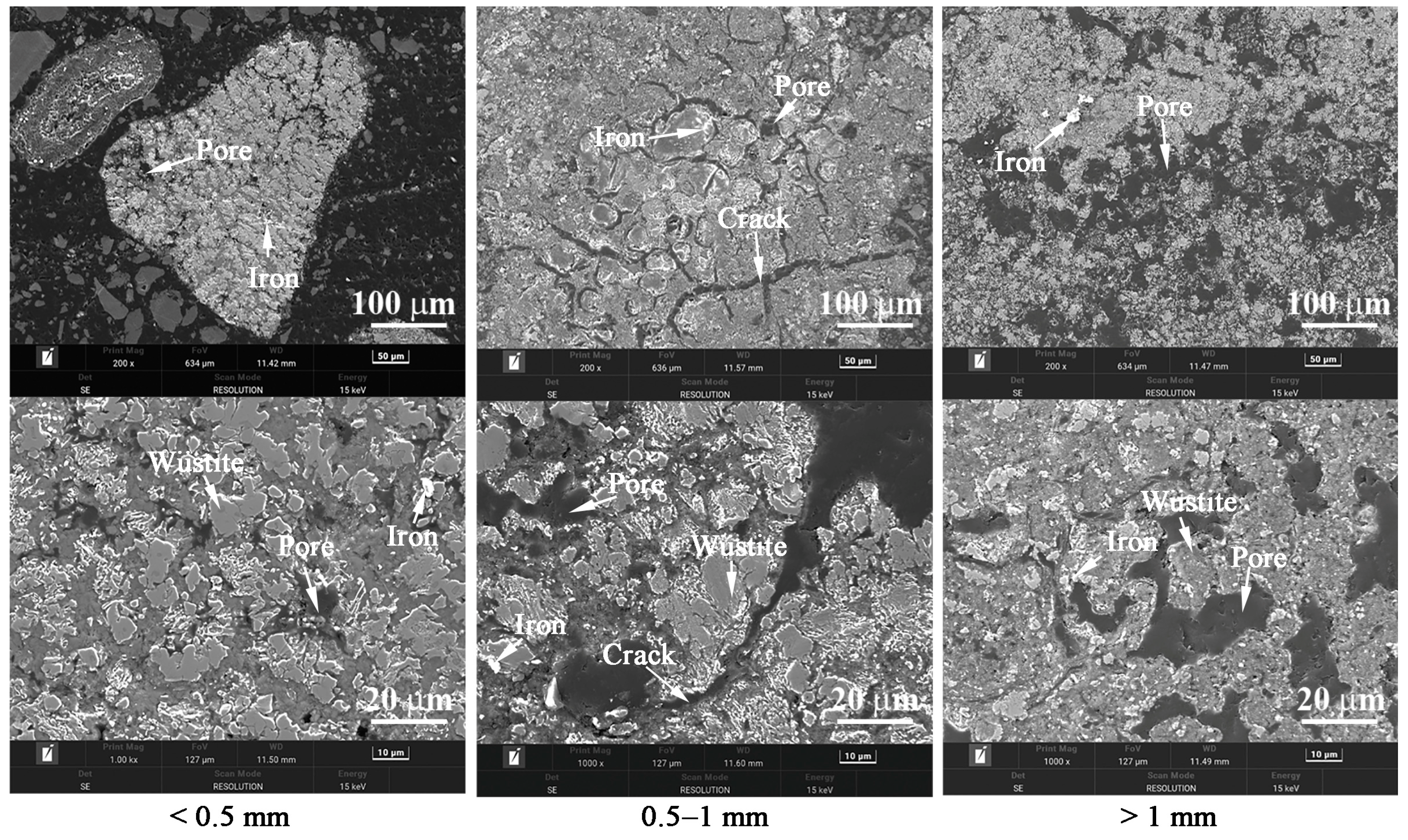
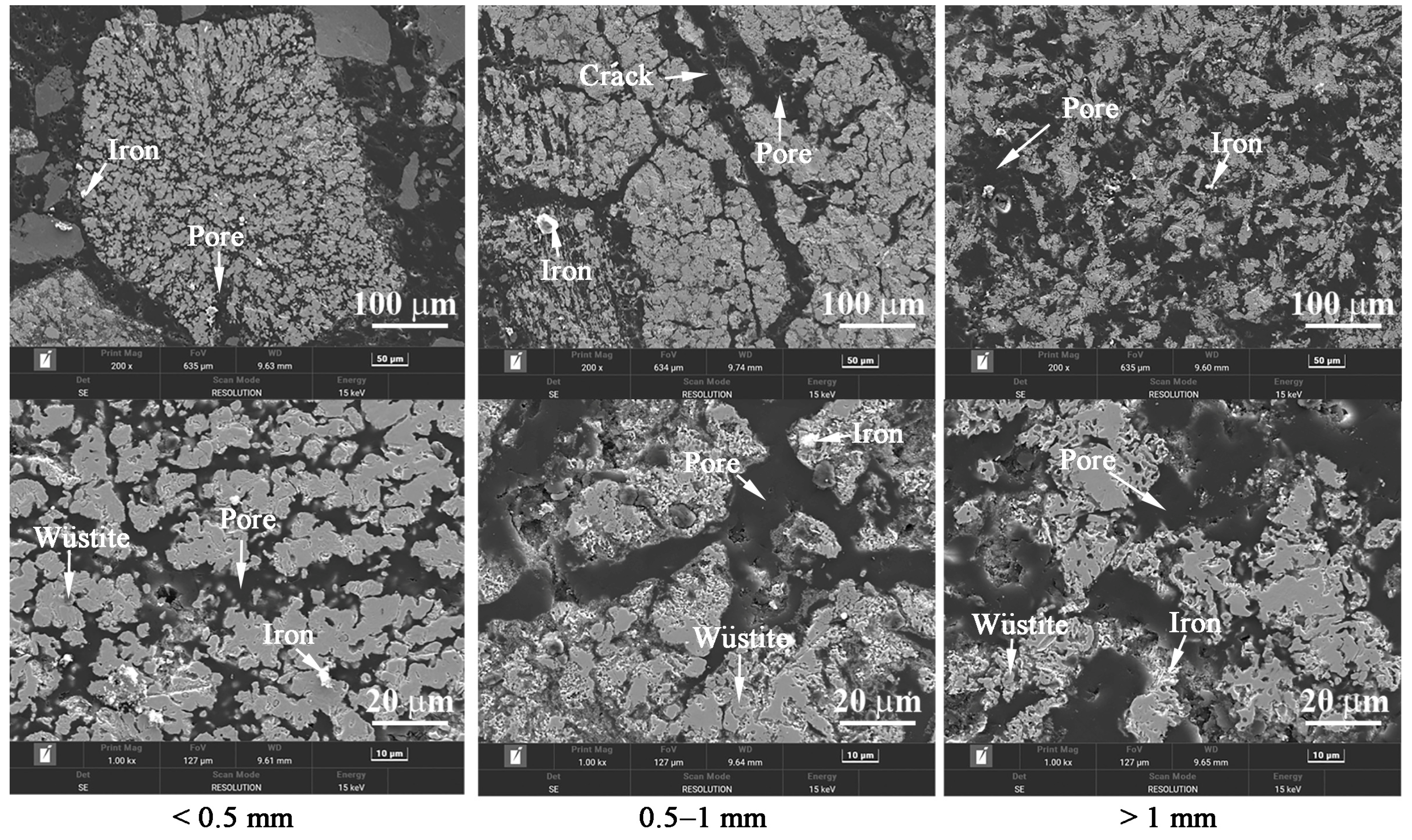
3.2. Effects of Temperature and Time on the Gas-Based Reduction Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yue, Y.; Zhang, J.; Sun, F.; Wu, S.; Pan, Y.; Zhou, J.; Qian, G. Heavy metal leaching and distribution in glass products from the co-melting treatment of electroplating sludge and MSWI fly ash. J. Environ. Manag. 2019, 232, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Zhang, Z. Approaches for electroplating sludge treatment and disposal technology: Reduction, pretreatment and reuse. J. Environ. Manag. 2023, 349, 119535. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Wang, J.; Zhang, C.; Chen, R. Stabilization of heavy metal-contaminated soils by biochar: Challenges and recommendations. Sci. Total Environ. 2020, 729, 139060. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, L.; Yan, B.; Wu, J.; Kong, D.; Romanovski, V.; Ivanets, A.; Li, H.; Chu, S.; Su, X. Removal of chromium from electroplating sludge by roasting-acid leaching and catalytic degradation of antibiotics by its residue. J. Environ. Chem. Eng. 2023, 12, 111754. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, L.; Peng, J.; Ge, Y.; Tian, Z.; Sun, J.; Cheng, H.; Zhou, H. Cleaner utilization of electroplating sludge by bioleaching with a moderately thermophilic consortium: A pilot study. Chemosphere 2019, 232, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Xu, S.; Ding, S.; Lin, W.; Yu, Q.; Zhang, J.; Qian, G. Electroplating sludge-derived metal and sulfur co-doping catalyst and its application in methanol production by CO2 catalytic hydrogenation. Sci Total Environ. 2022, 838, 156032. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ke, Y.; Tang, J.; Lei, X.; Deng, H.; Lin, Z. Upcycling of electroplating sludge into Fe3C-decorated N,P dual-doped porous carbon via microalgae as efficient sulfur host for lithium-sulfur batteries. Surf. Interfaces 2022, 30, 101869. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, T.; Chen, H.; Zhang, Y.; Geng, Z.; Zhu, S.; Xie, X.; Zhang, H.; Gao, Y.; Huo, Y. Stepwise recycling of Fe, Cu, Zn and Ni from real electroplating sludge via coupled acidic leaching and hydrothermal and extraction routes. Environ. Res. 2023, 216, 114462. [Google Scholar]
- Qu, Z.; Su, T.; Zhou, S.; Chen, Y.; Yu, Y.; Xie, X.; Yang, J.; Huo, M.; Bian, D. Stepwise extraction of Fe, Al, Ca, and Zn: A green route to recycle raw electroplating sludge. J. Environ. Manag. 2021, 300, 113700. [Google Scholar] [CrossRef]
- Tian, L.; Chen, L.; Gong, A.; Wu, X.; Cao, C.; Liu, M.; Chen, Z.; Xu, Z.; Liu, Y. Separation and extraction of valuable metals from electroplating sludge by carbothermal reduction and low-carbon reduction refining. JOM 2020, 72, 4149. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, H.; Hu, J. Co-treatment of electroplating sludge, copper slag, and spent cathode carbon for recovering and solidifying heavy metals. J. Hazard. Mater. 2021, 417, 126020. [Google Scholar]
- Wang, H.; Jiao, S.; Zhang, G. Preparation of ferrosilicochromium by silicothermic reduction of Cr-bearing electroplating sludge. J. Sustain. Metall. 2023, 9, 303–313. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Liu, Y.; Zhu, S.; Liang, D.; Sun, T.; Xie, X.; Wang, X. Resource utilization of hazardous Cr/Fe-rich sludge: Synthesis of erdite flocculant to treat real electroplating wastewater. J. Environ. Health Sci. Eng. 2022, 20, 509–519. [Google Scholar]
- Tran, T.H.; Tran, Q.M.; Le, T.V.; Pham, T.T.; Le, V.T.; Nguyen, M.K. Removal of Cu (II) by calcinated electroplating sludge. Heliyon 2022, 7, e07092. [Google Scholar] [CrossRef]
- Matović, L.; Vujasin, R.; Kumrić, K.; Krstić, S.; Wu, Y.; Kabtamu, D.M.; Devečerski, A. Designing of technological scheme for conversion of Cr-rich electroplating sludge into the black ceramic pigments of consistent composition, following the principles of circular economy. J. Environ. Chem. Eng. 2021, 9, 105038. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, R.; Yu, J.; Wang, H.; Guo, Q.; Liu, K.; Chen, H.; Chi, R. Sequential recovery of Cu(II), Cr(III), and Zn(II) from electroplating sludge leaching solution by an on-line biosorption method with dosage controlling. J. Clean. Prod. 2022, 337, 130427. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, Y.; Zheng, J.; Zhou, J.; Wu, Z.; Deng, H.; Wu, X.; Lin, Z. Understanding and controlling the key phase transformation for selective extracting Ni and Cu from Cr-containing electroplating sludge. Surf. Interfaces 2021, 24, 101090. [Google Scholar] [CrossRef]
- Tian, B.; Cui, Y.; Qin, Z.; Wen, L.; Li, Z.; Chu, H.; Xin, B. Indirect bioleaching recovery of valuable metals from electroplating sludge and optimization of various parameters using response surface methodology (RSM). J. Environ. Manag. 2022, 312, 114927. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, L.; Lin, C.; Xie, X.; Yong, X.; Wu, X.; Zhou, J.; Jia, H.; Wei, P. Extracting heavy metals from electroplating sludge by acid and bioelectrical leaching using Acidithiobacillus ferrooxidans. Hydrometallurgy 2022, 191, 105225. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Jiao, S.; Zhang, G. Recovery of Cu-Fe-S matte from electroplating sludge via the sulfurization-smelting method. J. Environ. Chem. Eng. 2022, 10, 108266. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, P.; Chen, L.; Zhang, D.; Liu, W.; Yang, T. Recovery of Cu and Ni in electroplating sludge by a low-temperature alkaline smelting technique. J. Sustain. Metall. 2022, 8, 1026–1040. [Google Scholar] [CrossRef]
- Yu, Y.; Ge, J.; Wu, Z.; Lin, J.; Zhu, Z.; Yang, Q.; Liu, X. One-step extraction of CuCl2 from Cu-Ni mixed electroplating sludge by chlorination-mineralization surface-interface phase change modulation. Surf. Interfaces 2023, 37, 102535. [Google Scholar] [CrossRef]
- Gong, J.; Tan, R.; Wang, B.; Wang, Z.; Gong, B.; Mi, X.; Deng, D.; Liu, X.; Liu, C.; Deng, C.; et al. Process and mechanism of strengthening chlorination cascade recovery of valuable metals from electroplating sludge. J. Clean. Prod. 2022, 376, 134330. [Google Scholar] [CrossRef]
- Shibata, E.; Egawa, S.; Nakamura, T. Reduction behavior of chromium oxide in molten slag using aluminum, ferrosilicon and graphite. ISIJ Int. 2002, 42, 609–613. [Google Scholar] [CrossRef]
- Guo, B.; Tan, Y.; Wang, L.; Chen, L.; Wu, Z.; Sasaki, K.; Mechtcherine, V.; Tsang, D.C.W. High-efficiency and low-carbon remediation of zinc contaminated sludge by magnesium oxysulfate cement. J. Hazard. Mater. 2021, 408, 124486. [Google Scholar] [CrossRef]
- Plaul, F.J.; Böhm, C.; Schenk, J.L. Fluidized-bed technology for the production of iron products for steelmaking. J. S. Afr. Inst. Min. Metall. 2009, 109, 121–128. [Google Scholar]
- Schenk, J.L. Recent status of fluidized bed technologies for producing iron input materials for steelmaking. Particuology 2011, 9, 14–23. [Google Scholar] [CrossRef]
- Ma, J.; Li, C.; Hu, L.; Kong, W.; Lu, Q.; Zhang, J. Structural regulation of electroplating sludge by a meta-organic framework synthesis method for an enhanced denitrification activity. J. Mater. Cycles Waste 2021, 23, 614–621. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, Z.; Tian, R.; Tang, H.; Ye, L.; Rao, M.; Li, G. Assessment of thermal stability of chromium-rich electroplating sludge. J. Therm. Anal. Calorim. 2023, 148, 10335–10344. [Google Scholar] [CrossRef]
- GB/T 6730.5-2022; Iron Ores—Determination of Total Iron Content—Titrimetric Methods after Titanium (III) Chloride Reduction. Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- GB/T 38812.2-2020; Direct Reduced Iron—Determination of Metallic Iron Content—The Potassium Dichromate Titrimetric Method after Decomposition of Sample by Ferric Chloride. Standardization Administration of the People’s Republic of China: Beijing, China, 2020.
- Yang, J.; Martens, W.N.; Frost, R.L. Transition of chromium oxyhydroxide nanomaterials to chromium oxide: A hot-stage Raman spectroscopic study. J. Raman Spectrosc. 2010, 42, 1142–1146. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, C.; Xie, J.; Wang, Z. The evolution of dehydration and thermal decomposition of nanocrystalline and amorphous chromium hydroxide. J. Anal. Appl. Pyrol. 2016, 118, 225–230. [Google Scholar] [CrossRef]
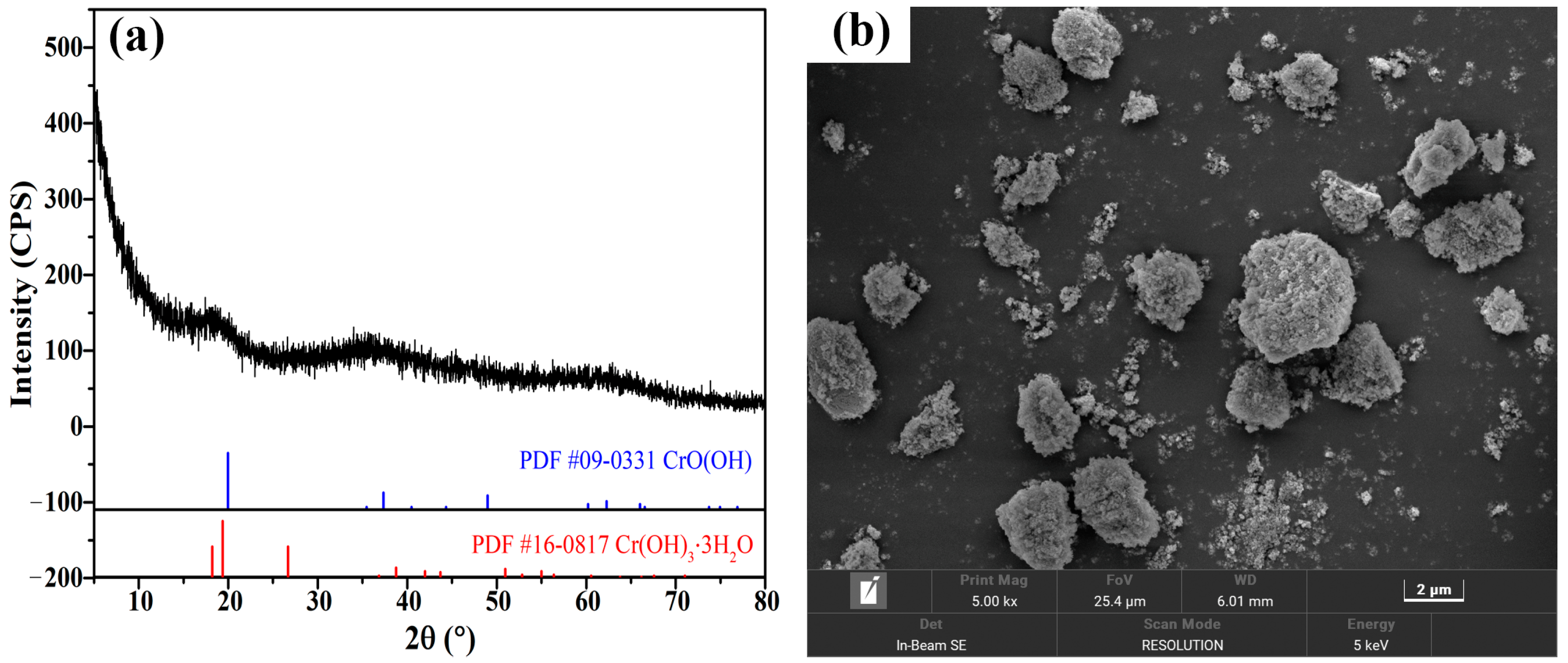
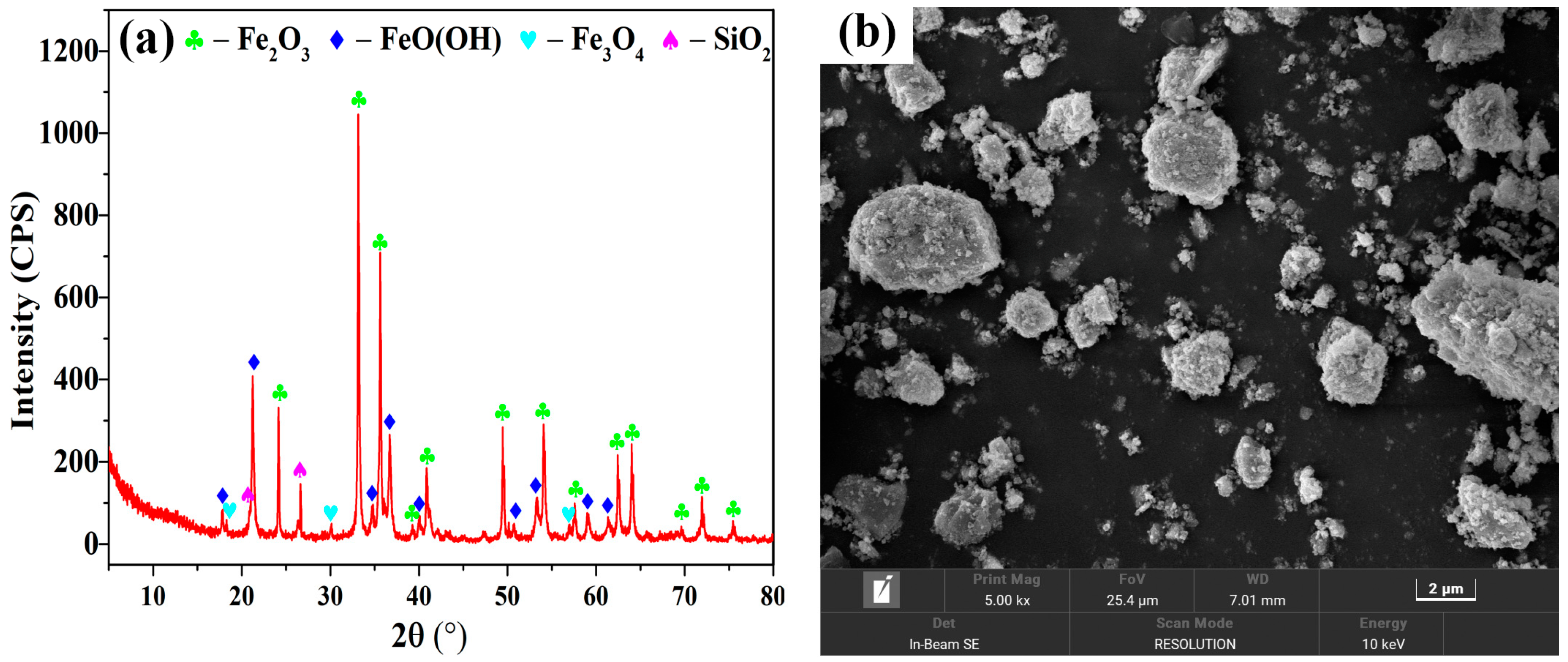

| Component | Cr | Fe | Ca | S | Cu | Zn | Pb | Ni |
| Content | 36.85 | 4.64 | 2.32 | 2.03 | 0.24 | 0.24 | 0.22 | 0.03 |
| Component | Mg | Al | Si | P | Cl | Na | K | LOI * |
| Content | 0.31 | 0.17 | 0.22 | 0.08 | 0.01 | 0.03 | 0.01 | 25.48 |
| Particle Size (mm) | <0.075 | 0.075–0.15 | 0.15–0.5 | 0.5–1 | 1–3 | >3 |
| Proportion | 9.22 | 14.06 | 15.48 | 34.27 | 15.95 | 11.05 |
| Component | TFe | FeO | Ca | Mg | Al | Si | Cr | Cu |
| Content | 59.59 | 2.82 | 0.29 | 0.16 | 0.91 | 1.92 | 0.058 | 0.042 |
| Component | Zn | Pb | K | Na | S | P | LOI | |
| Content | 0.034 | 0.008 | 0.022 | 0.019 | 0.063 | 0.051 | 5.72 |
| Particle Size (mm) | <0.15 | 0.15–0.5 | 0.5–1 | 1–3 | >3 |
| Proportion | 10.52 | 43.20 | 24.27 | 15.89 | 6.12 |
| No. | Reaction Equation | Standard Gibbs Free Energy Change |
|---|---|---|
| (4) | ||
| (5) | ||
| (6) | ||
| (7) | ||
| (8) | ||
| (9) | ||
| (10) | ||
| (11) | ||
| (12) | ||
| (13) | ||
| (14) | ||
| (15) | ||
| (16) | ||
| (17) | ||
| (18) |
| Component | Cr | Fe | Ca | Si | Al | Mg | S | Zn | Pb |
| Content | 15.26 | 45.84 | 5.52 | 2.93 | 1.24 | 0.84 | 0.879 | 0.123 | 0.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Peng, Z.; Yi, L.; Rao, M. CO–H2 Gas-Based Reduction Behavior of Cr-Rich Electroplating Sludge Mixed with Iron Ore Powder. Metals 2024, 14, 325. https://doi.org/10.3390/met14030325
Zhang J, Peng Z, Yi L, Rao M. CO–H2 Gas-Based Reduction Behavior of Cr-Rich Electroplating Sludge Mixed with Iron Ore Powder. Metals. 2024; 14(3):325. https://doi.org/10.3390/met14030325
Chicago/Turabian StyleZhang, Jian, Zhiwei Peng, Lingyun Yi, and Mingjun Rao. 2024. "CO–H2 Gas-Based Reduction Behavior of Cr-Rich Electroplating Sludge Mixed with Iron Ore Powder" Metals 14, no. 3: 325. https://doi.org/10.3390/met14030325





