Corrosion Behavior of 30 ppi TAD3D/5A05Al Composite in Neutral Salt Spray Corrosion
Abstract
:1. Introduction
2. Materials and Methods
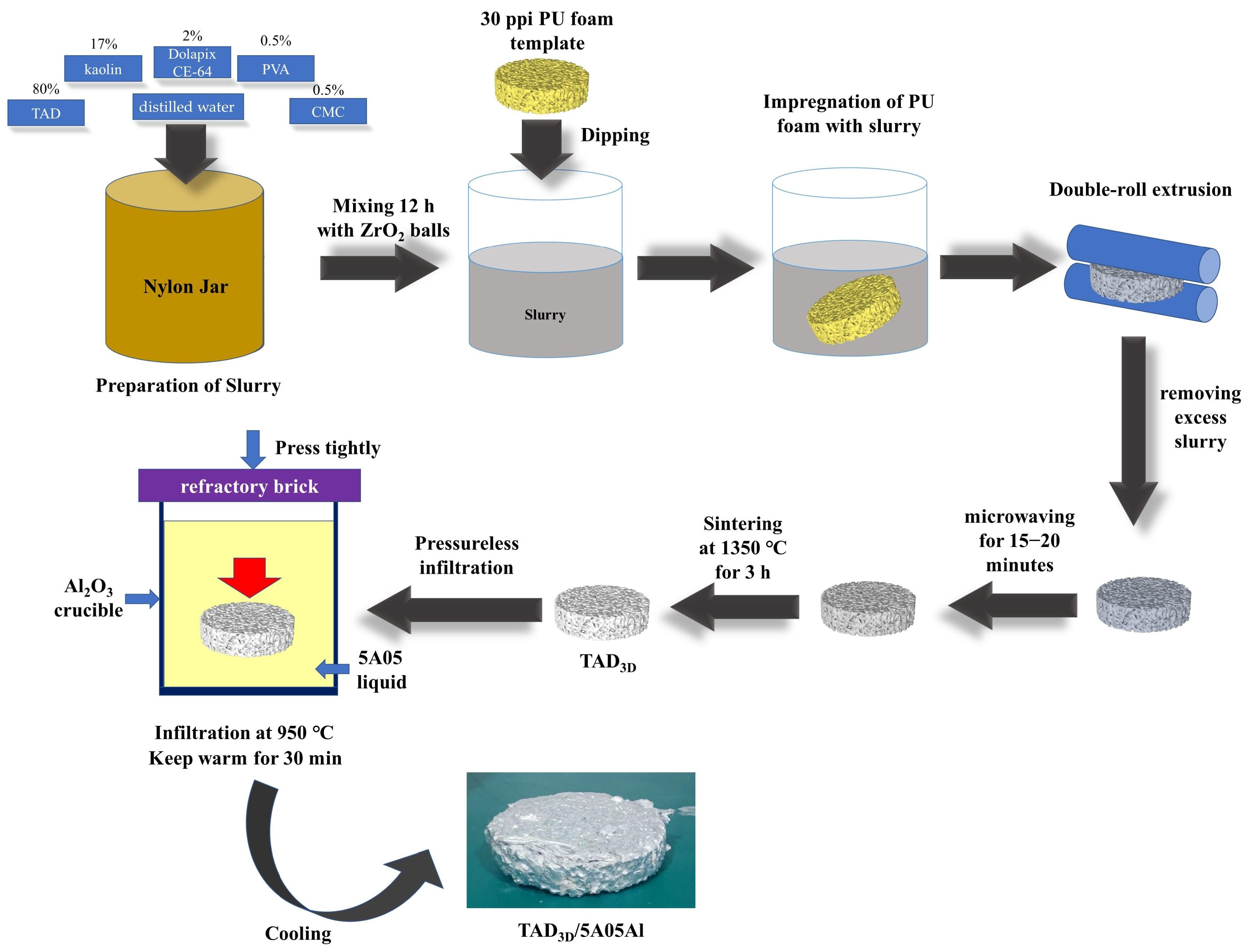
2.1. Preparation of Composite Materials
2.2. Neutral Salt Spray Corrosion
2.3. Surface Analysis
2.4. Electrochemical Measurement
3. Results and Discussion
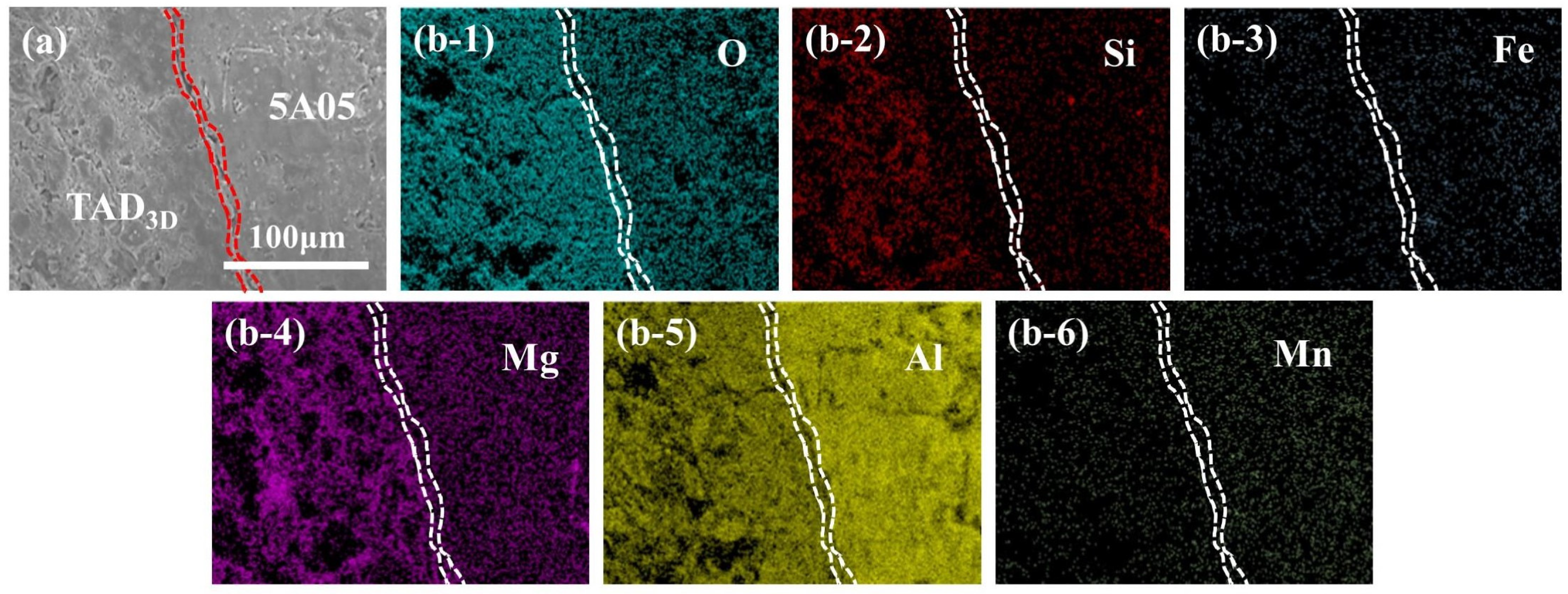
3.1. Microstructure of TAD3D/5A05Al
3.2. Microstructure of 5A05Al Matrix in TAD3D/5A05Al after Removal of NSS Corrosion Products
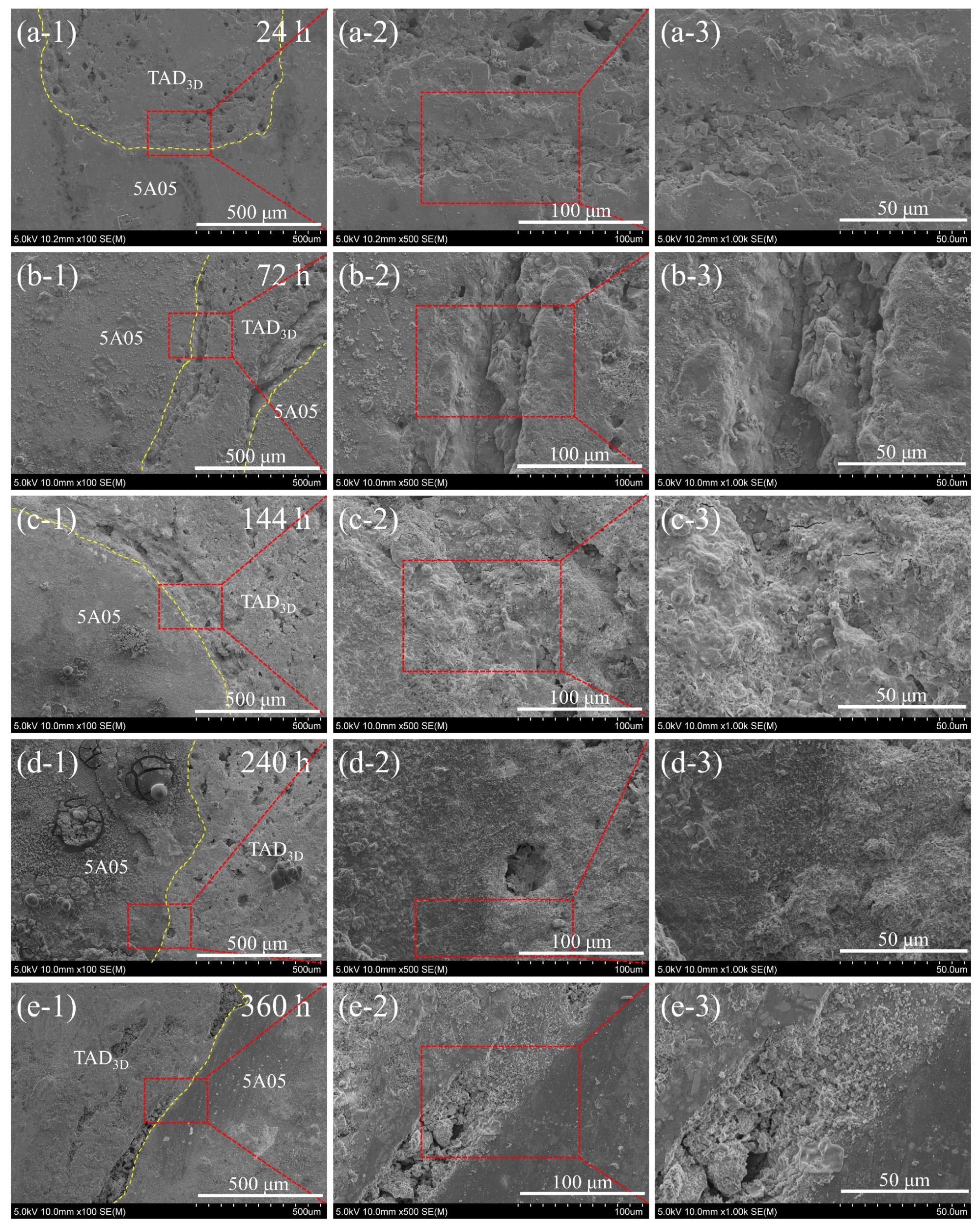
3.3. Microstructure of the Interface in TAD3D/5A05Al with NSS Corrosion Products
3.4. Microstructure of the Interface in TAD3D/5A05Al after Removal of NSS Corrosion Products
3.5. PDP of TAD3D/5A05Al with NSS Corrosion Products
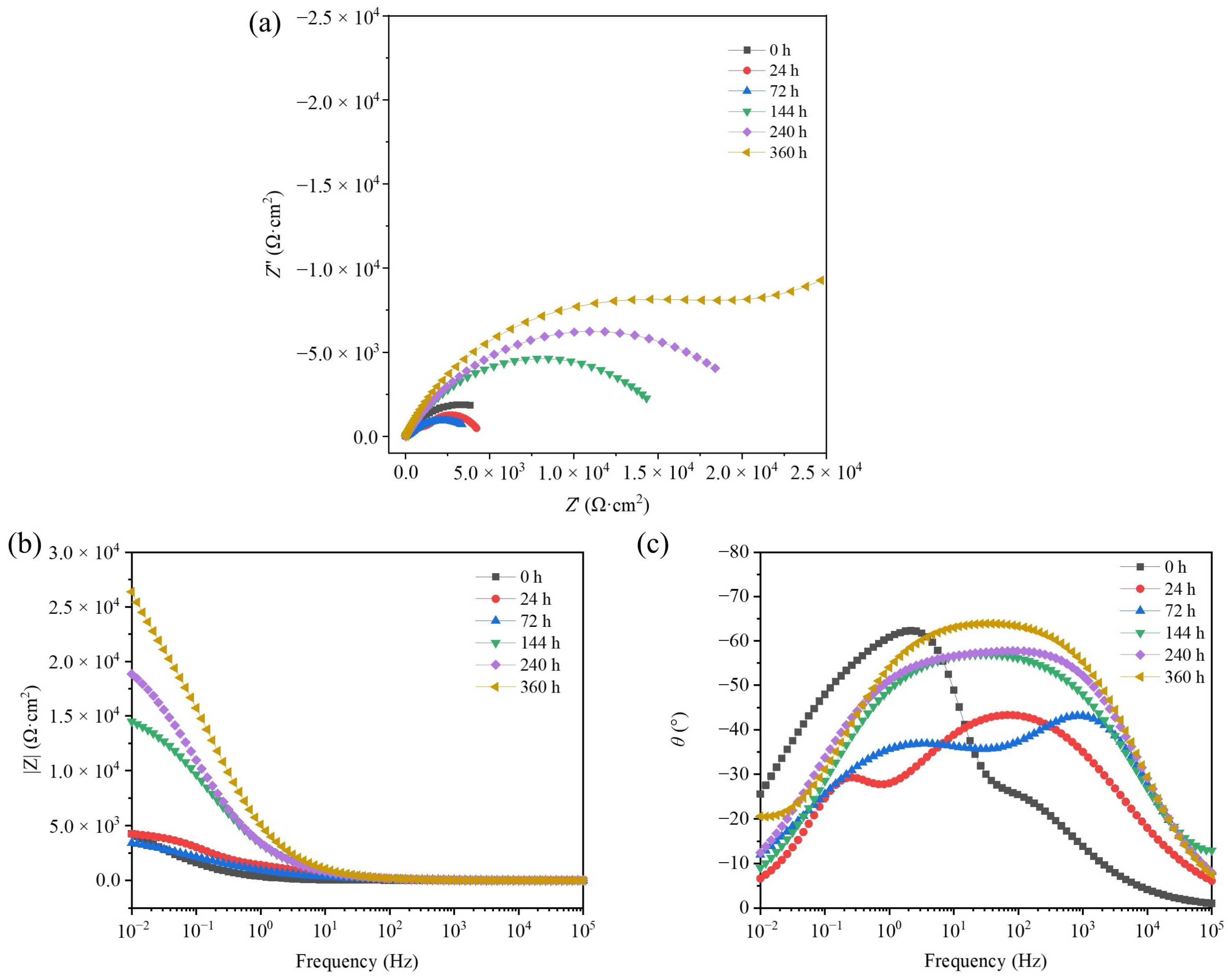
3.6. EIS of TAD3D/5A05Al with NSS Corrosion Products
4. Conclusions
- A TAD3D skeleton with a compressive strength of about 2~5 MPa and pores of about 30 ppi was obtained via the sacrificial template method with TAD and kaolin as raw materials. After pressureless infiltration, TAD3D can be well bonded with the 5A05Al interface to form TAD3D/5A05 composites.
- After 24 to 360 h NSS corrosion, the corrosion of the 5A05 matrix was mainly pitting corrosion. The pits expanded and deepened with the increase in corrosion time, and there was a trend of mutual connection.
- The main corrosion products were MgAl2O4, Al(OH)3, and Al2O3. With the increase in corrosion time, the corrosion products increased and filled the cracks, pitting pits, and grooves of the composite material surface. During the corrosion process, the corrosion products transferred to the grooves of the composite interface and grew on the ceramic surface. The corrosion products on the ceramic skeleton formed a continuous passivation film with the corrosion products generated on the Al matrix, covering the surface of the composite material. However, the passivation film did not stop the corrosion from continuing. The grooves of the composite interface gradually deepened with the increase in corrosion time, and the corrosion products in the grooves were loose, which may have led to serious local corrosion.
- PDP and EIS analyses showed that for TAD3D/5A05, Ecorr initially decreased then increased and Icorr first rose and then fell. The high-frequency impedance arc radius and the impedance value both decreased before increasing, while the low-frequency phase angle grew, indicating an initial drop then a rise in corrosion resistance. With the progress of corrosion, the corrosion products on the surface of the composite material continued to increase. After 240 h, there were enough corrosion products to form a continuous passivation film. The passivation film can weaken the corrosion effect and increase the corrosion resistance of the composite material, but its corrosion resistance was still weaker than that of the uncorroded sample.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kota, N.; Charan, M.S.; Laha, T.; Roy, S. Review on development of metal/ceramic interpenetrating phase composites and critical analysis of their properties. Ceram. Int. 2022, 48, 1451–1483. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, Y.-L.; Yu, L.; Yang, H.-L.; Li, Z.-S.; Ding, Y.-D.; Fu, G.-F. Fabrication, microstructure, friction and wear properties of SiC3D/Al brake disc-graphite/SiC pad tribo-couple for high-speed train. Trans. Nonferrous Met. Soc. China 2019, 29, 1889–1902. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Z.; Lv, J.; Xu, G.; Zhou, S.; Tang, W.; Wu, Y. Enhanced thermal conductive 3D-SiC/Al-Si-Mg interpenetrating composites fabricated by pressureless infiltration. Ceram. Int. 2017, 43, 1755–1761. [Google Scholar] [CrossRef]
- Bandil, K.; Vashisth, H.; Kumar, S.; Verma, L.; Jamwal, A.; Kumar, D.; Singh, N.; Sadasivuni, K.K.; Gupta, P. Microstructural, mechanical and corrosion behaviour of Al–Si alloy reinforced with SiC metal matrix composite. J. Compos. Mater. 2019, 53, 4215–4223. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Cao, X.; Yan, Y.; Zhang, C.; Jiang, Y. Research of Microstructure, Phase, and Mechanical Properties of Aluminum-Dross-Based Porous Ceramics. J. Renew. Mater. 2023, 11, 3057–3072. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Zhao, C.; Xing, Y.; Lang, F.; Hou, X. Experimental Investigation of Crack Propagation and Strain Fields Evolution around a Crack Tip in 5A05 Aluminum Alloy. Metals 2018, 8, 685. [Google Scholar] [CrossRef]
- Yu, L.; Hao, S.; Nong, X.; Cao, X.; Zhang, C.; Liu, Y.; Yan, Y.; Jiang, Y. Comparative Study on the Corrosion Resistance of 6061Al and SiC3D/6061Al Composite in a Chloride Environment. Materials 2021, 14, 7730. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, L.; Etienne, A.; Li, Z.; Chandrashekar, G.T.; Gonapati, S.R.; Awe, S.A.; Jarfors, A.E.W. The effect of Ni and Zr additions on hardness, elastic modulus and wear performance of Al-SiCp composite. Tribol. Int. 2022, 169, 107478. [Google Scholar] [CrossRef]
- Wang, Y.; Normand, B.; Mary, N.; Yu, M.; Liao, H. Effects of ceramic particle size on microstructure and the corrosion behavior of cold sprayed SiCp/Al 5056 composite coatings. Surf. Coat. Technol. 2017, 315, 314–325. [Google Scholar] [CrossRef]
- Lee, T.; Chae, H.; Shin, S.; Cho, S.; Lee, S.-K.; Lee, S.Y.; An, K.; Ryu, H.J. Overcoming brittleness of high volume fraction Al/SiCp composites by controlling interface characteristics. Mater. Des. 2022, 222, 111038. [Google Scholar] [CrossRef]
- Ramadan, S.; Taha, M.A.; El-Meligy, W.M.; Saudi, H.A.; Zawrah, M.F. Influence of Graphene Content on Sinterability and Physico-Mechanical Characteristics of Al/Graphene Composites Prepared via Powder Metallurgy. Biointerface Res. Appl. Chem. 2022, 13, 192. [Google Scholar] [CrossRef]
- Ayodele, O.O.; Babalola, B.J.; Olubambi, P.A. Characterization, nanomechanical, and wear attributes of sintered Al–TiB2 composites. J. Mater. Res. Technol. 2023, 24, 4153–4167. [Google Scholar] [CrossRef]
- Ji, Y.-Y.; Xu, Y.-Z.; Zhang, B.-B.; Behnamian, Y.; Xia, D.-H.; Hu, W.-B. Review of micro-scale and atomic-scale corrosion mechanisms of second phases in aluminum alloys. Trans. Nonferrous Met. Soc. China 2021, 31, 3205–3227. [Google Scholar] [CrossRef]
- Senthilkumar, J.; Balasubramanian, M.; Balasubramanian, V. Studies on effect of powder metallurgy on the corrosion behaviour of Al-Al2O3p composites. Adv. Mater. Process. Technol. 2023, 9, 859–874. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, P.; Zhang, C.; Jin, F.; Li, Y.; Cao, X.; Yu, L. Simulation and Experimental of Infiltration and Solidification Process for Al(2)O(3(3D))/5083Al Interpenetrating Phase Composite for High Speed Train Prepared by Low-Pressure Infiltration. Materials 2023, 16, 6634. [Google Scholar] [CrossRef]
- Bhuyan, P.; Kota, N.; Bairagi, D.; Sahasrabudhe, S.; Roy, S.; Mandal, S. Unveiling the corrosion perspective of Al-Si alloy/SiC foam interpenetrating phase composite. Mater. Today Commun. 2023, 35, 105495. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Z.; Lv, J.; Xu, G.; Zhou, S.; Tang, W.; Wu, Y. Multimodal particle distribution in 3D-SiC/Al-Si-Mg interpenetrating composite fabricated by pressureless infiltration. Ceram. Int. 2018, 44, 19851–19858. [Google Scholar] [CrossRef]
- Vijayan, K.; Ramalingam, S.; Raeez Akthar Sadik, M.; Prasanth, A.S.; Nampoothiri, J.; Pablo Escobedo-Diaz, J.; Shankar, K. Fabrication of Co-Continuous ceramic composite (C4) through gas pressure infiltration technique. Mater. Today Proc. 2021, 46, 1013–1016. [Google Scholar] [CrossRef]
- Asar, A.; Zaki, W. A comprehensive review of the mechanisms and structure of interpenetrating phase composites with emphasis on metal-metal and polymer-metal variants. Compos. Part B Eng. 2024, 275, 111314. [Google Scholar] [CrossRef]
- Roy, S.; Albrecht, P.; Weidenmann, K.A. Influence of Ceramic Freeze-Casting Temperature on the Anisotropic Thermal Expansion Behavior of Corresponding Interpenetrating Metal/Ceramic Composites. J. Mater. Eng. Perform. 2023, 32, 8795–8806. [Google Scholar] [CrossRef]
- Sousa, E.; Silveira, C.B.; Fey, T.; Greil, P.; Hotza, D.; de Oliveira, A.P.N. LZSA glass ceramic foams prepared by replication process. Adv. Appl. Ceram. 2013, 104, 22–29. [Google Scholar] [CrossRef]
- Liu, J.; Binner, J.; Higginson, R. Dry sliding wear behaviour of co-continuous ceramic foam/aluminium alloy interpenetrating composites produced by pressureless infiltration. Wear 2012, 276–277, 94–104. [Google Scholar] [CrossRef]
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2019.
- Li, X.; Xia, W.; Yan, H.; Chen, J.; Li, X. Improving strength and corrosion resistance of high Mg alloyed Al–Mg–Mn alloys through Ce addition. Corros. Eng. Sci. Technol. 2020, 55, 381–391. [Google Scholar] [CrossRef]
- Goswami, R.; Spanos, G.; Pao, P.S.; Holtz, R.L. Microstructural Evolution and Stress Corrosion Cracking Behavior of Al-5083. Metall. Mater. Trans. A 2011, 42, 348–355. [Google Scholar] [CrossRef]
- Engler, O.; Kuhnke, K.; Krupp, H.J.; Hentschel, T. Characterization of Intergranular Corrosion in AA 5xxx Al-Mg Alloys. Pract. Metallogr. 2020, 57, 545–568. [Google Scholar] [CrossRef]
- Grasserbauer, J.; Weißensteiner, I.; Falkinger, G.; Kremmer, T.M.; Uggowitzer, P.J.; Pogatscher, S. Influence of Fe and Mn on the Microstructure Formation in 5xxx Alloys—Part I: Evolution of Primary and Secondary Phases. Materials 2021, 14, 3204. [Google Scholar] [CrossRef]
- Kamunur, K.; Bekmurat, N.K.; Milikhat, B.; Abdulkarimova, R.G. Microstructure and thermal properties of an Al–Mg alloy solidified at high temperature in the argon atmosphere. Combust. Plasma Chem. 2019, 19, 17–23. [Google Scholar] [CrossRef]
- Seth, P.P.; Parkash, O.; Kumar, D. Structure and mechanical behavior of in situ developed Mg(2)Si phase in magnesium and aluminum alloys—A review. RSC Adv 2020, 10, 37327–37345. [Google Scholar] [CrossRef]
- Islam, M.M.; Sawahata, J.; Akimoto, K.; Sakurai, T. Formation of silicon layer through aluminothermic reduction of quartz substrates. Front. Mater. 2022, 9, 977869. [Google Scholar] [CrossRef]






| Composition | Al2O3 | SiO2 | MgO | CaO | TiO2 |
|---|---|---|---|---|---|
| TAD | 87.54 | 3.06 | 5.15 | 1.08 | 0.15 |
| kaolin | 42.23 | 56.52 | 0.55 | 0.49 | 0.21 |
| Elements | Mg | Si | Zn | Mn | Cu | Fe | Al |
|---|---|---|---|---|---|---|---|
| wt.% | 5.02 | 0.10 | 0.04 | 0.43 | 0.05 | 0.21 | Balance |
| NSS Corrosion Durations | Ecorr (V) | Icorr (µA·cm−2) |
|---|---|---|
| 0 h | −0.710 | 0.396 |
| 24 h | −0.849 | 0.525 |
| 72 h | −0.918 | 0.647 |
| 144 h | −0.932 | 0.682 |
| 240 h | −0.759 | 0.153 |
| 360 h | −0.712 | 0.139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yang, H.; Chen, Y.; Fu, G.; Jiang, L. Corrosion Behavior of 30 ppi TAD3D/5A05Al Composite in Neutral Salt Spray Corrosion. Metals 2024, 14, 488. https://doi.org/10.3390/met14050488
Li Z, Yang H, Chen Y, Fu G, Jiang L. Corrosion Behavior of 30 ppi TAD3D/5A05Al Composite in Neutral Salt Spray Corrosion. Metals. 2024; 14(5):488. https://doi.org/10.3390/met14050488
Chicago/Turabian StyleLi, Zishen, Hongliang Yang, Yuxin Chen, Gaofeng Fu, and Lan Jiang. 2024. "Corrosion Behavior of 30 ppi TAD3D/5A05Al Composite in Neutral Salt Spray Corrosion" Metals 14, no. 5: 488. https://doi.org/10.3390/met14050488





