Study on Corrosion Fatigue Behavior of 304L Austenite Stainless Steel in 325 °C High-Temperature Water Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
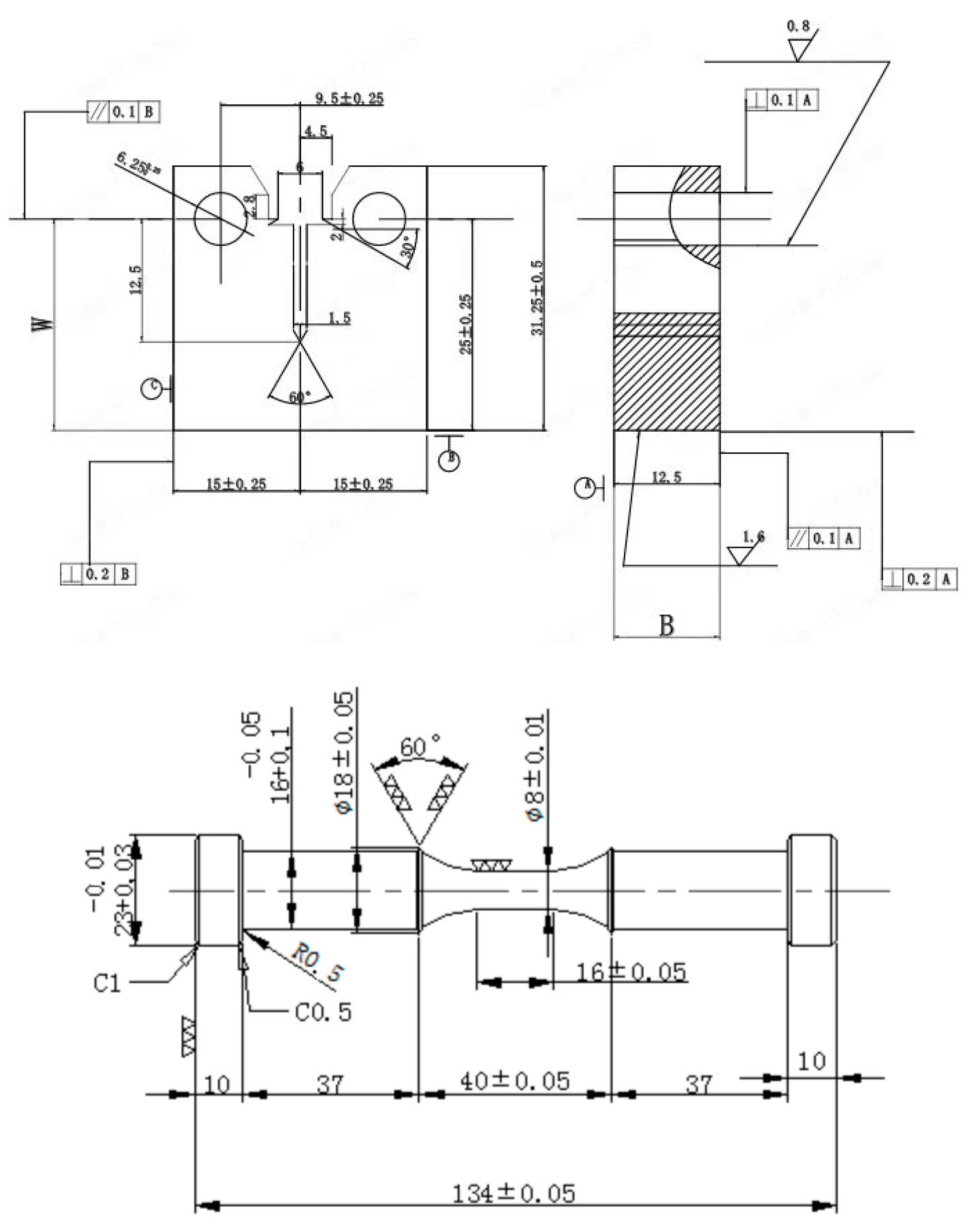
2.2. Specimen Design
2.3. Crack Growth Rate Test
2.4. TEM and EDS Test
2.5. EBSD Observation
3. Results and Discussion
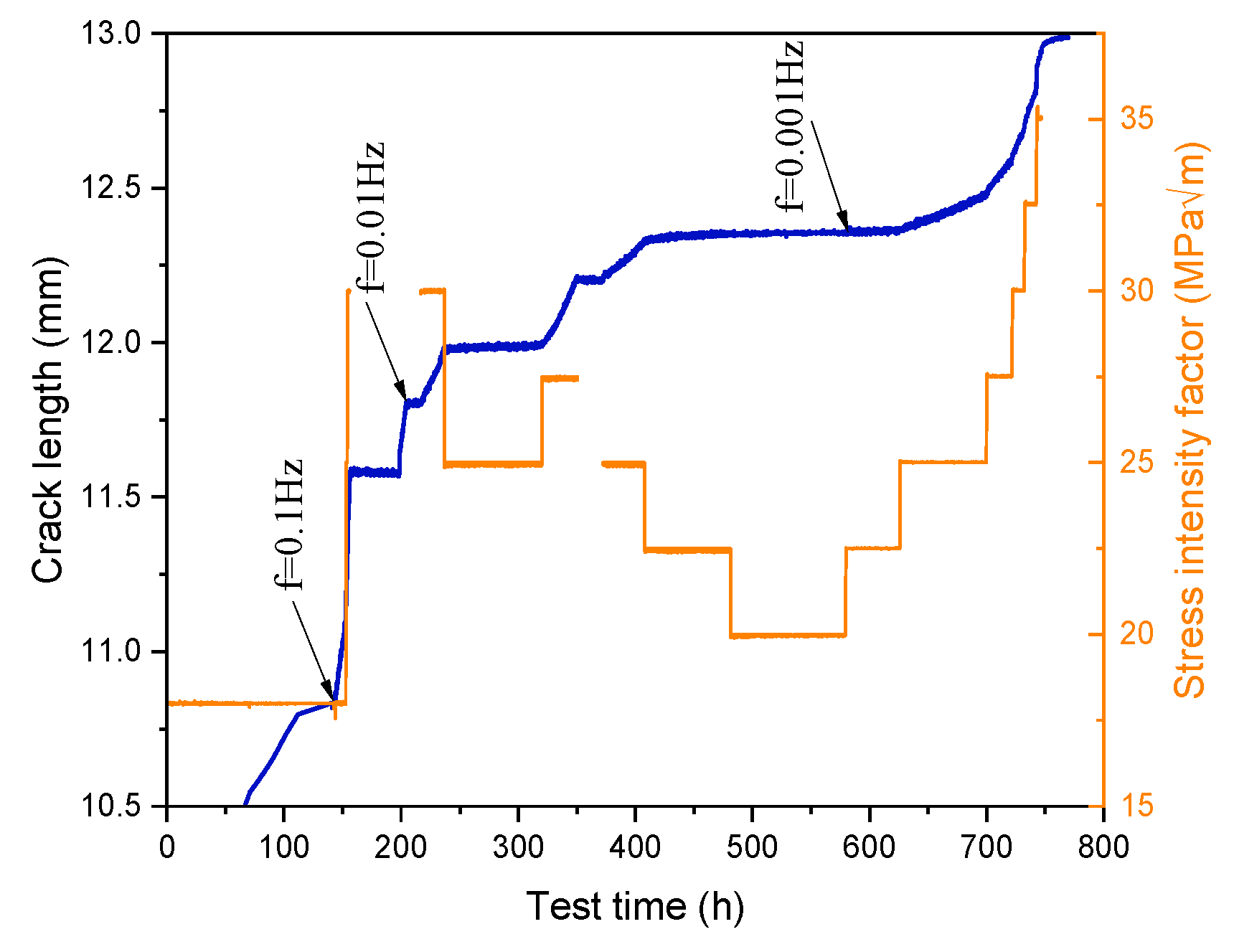
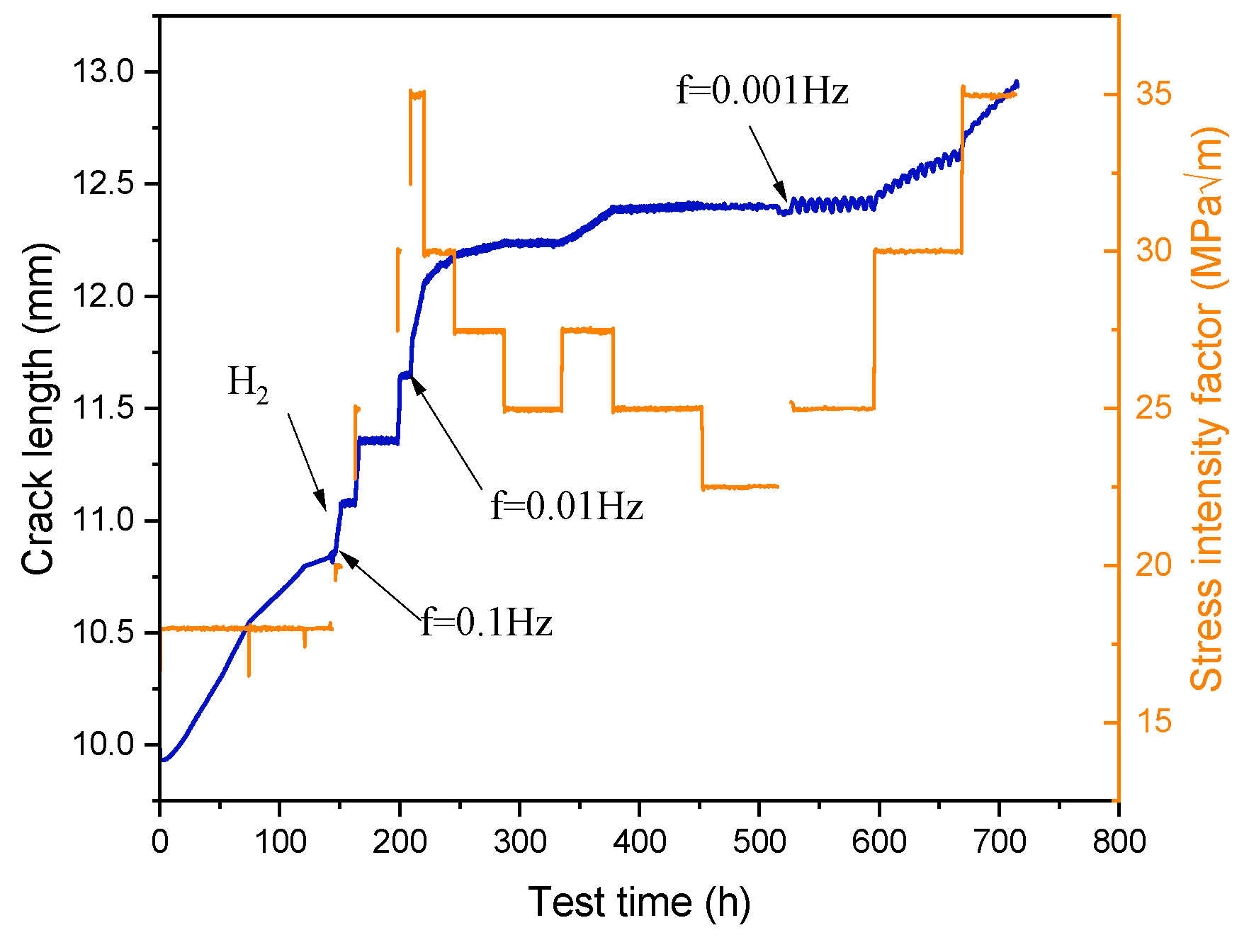
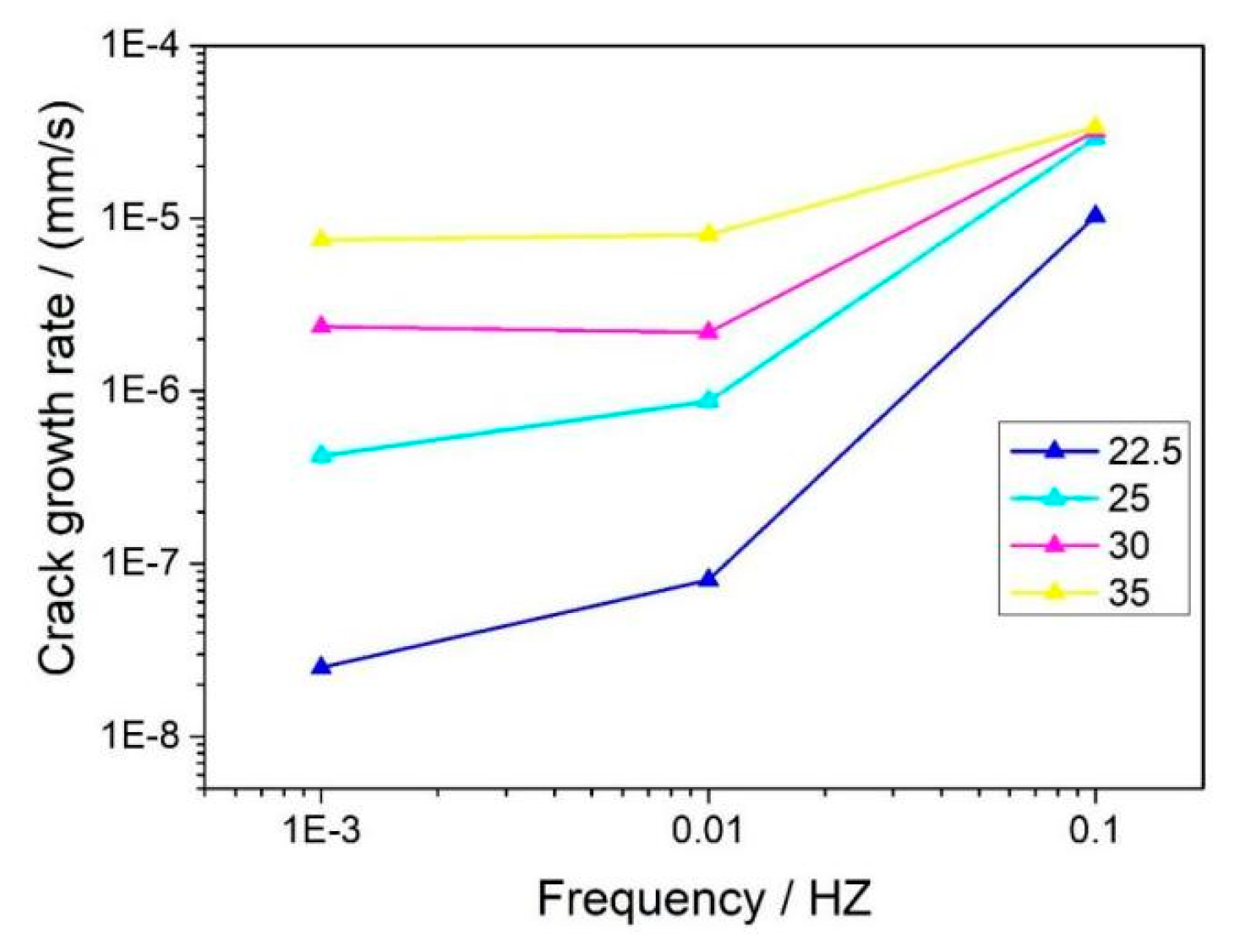
3.1. Crack Growth Rate
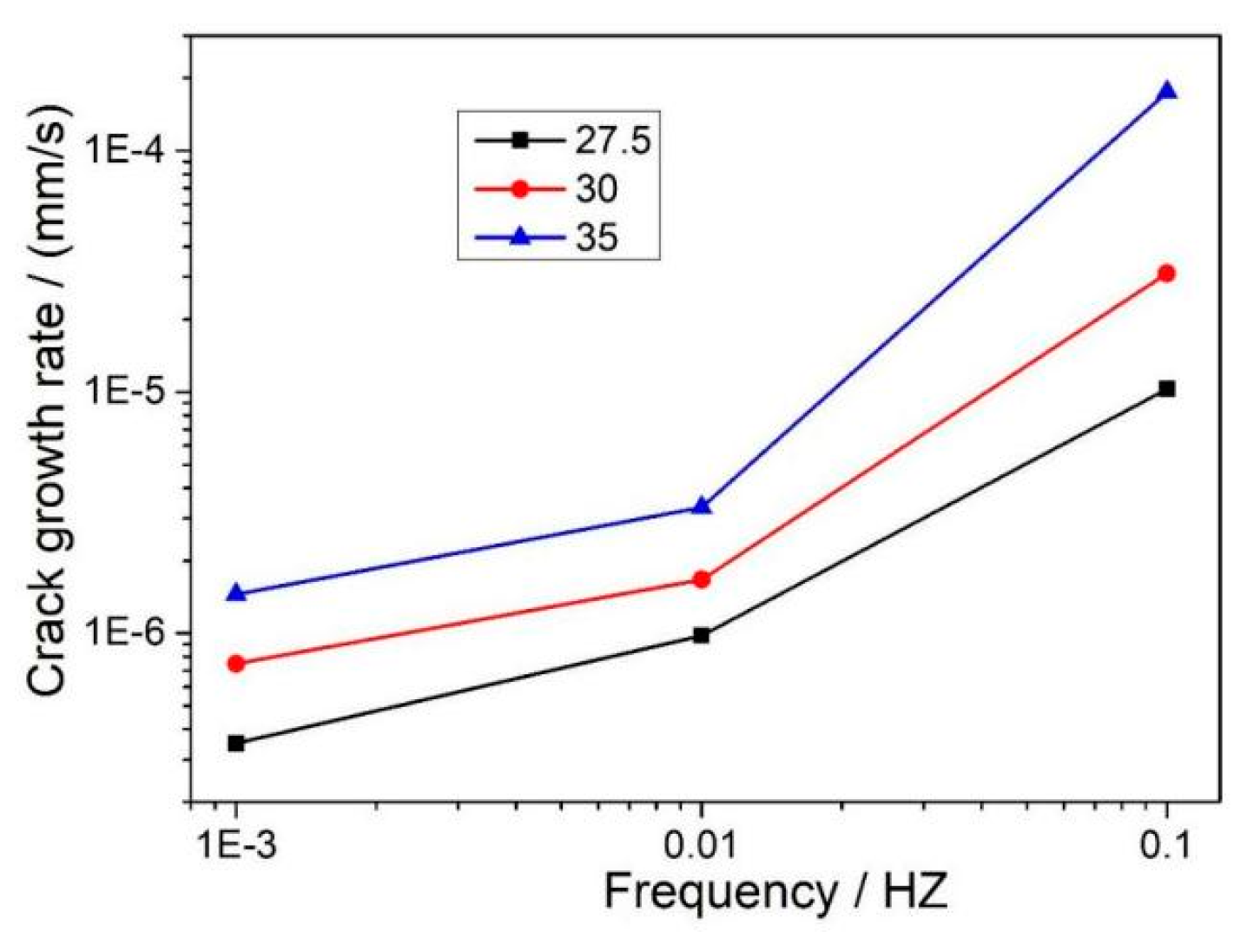
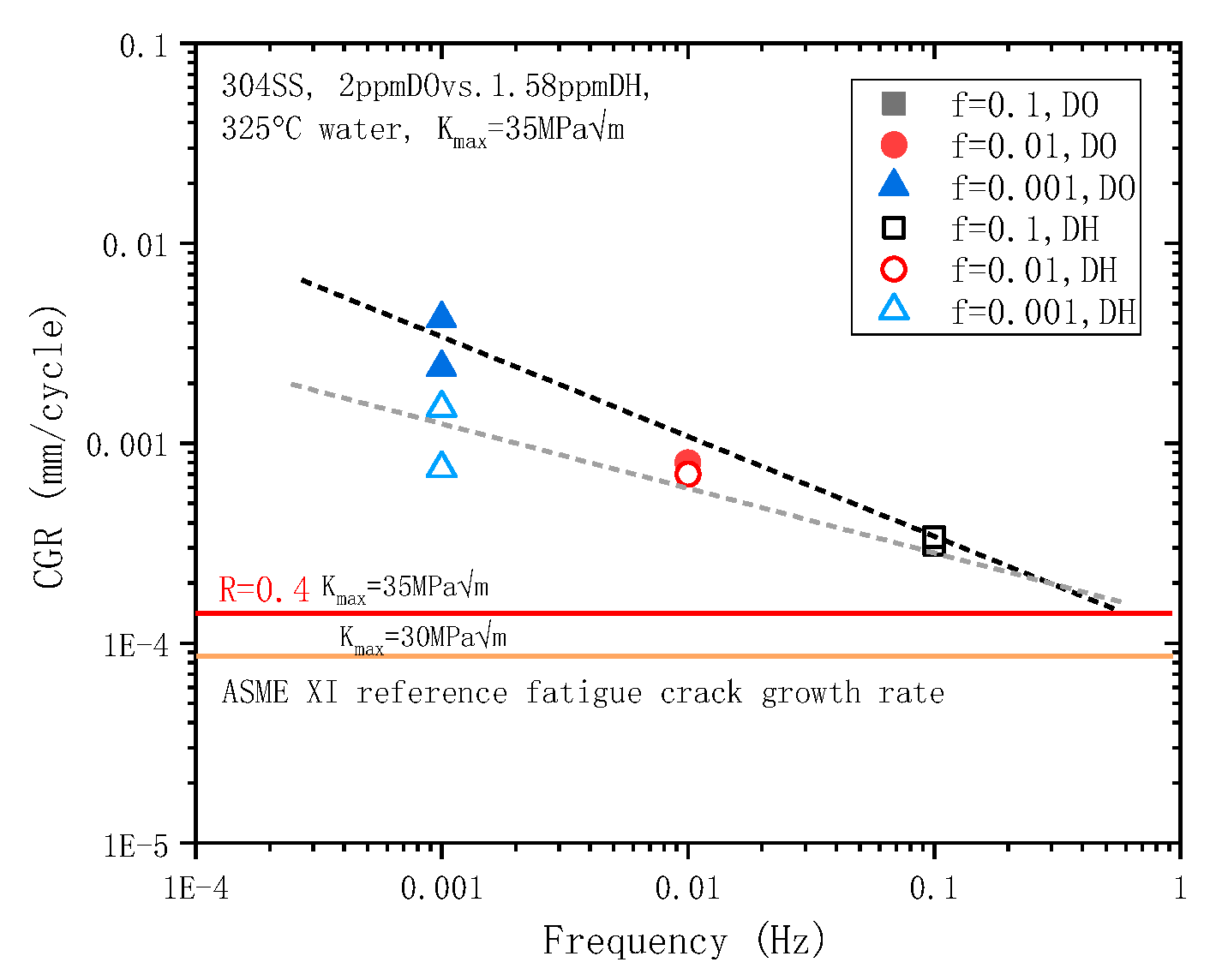
3.1.1. Effect of K and f on CGR
3.1.2. Analysis of CGR Equation
3.2. Effect of Fatigue Loading on Crack Tip Characteristic
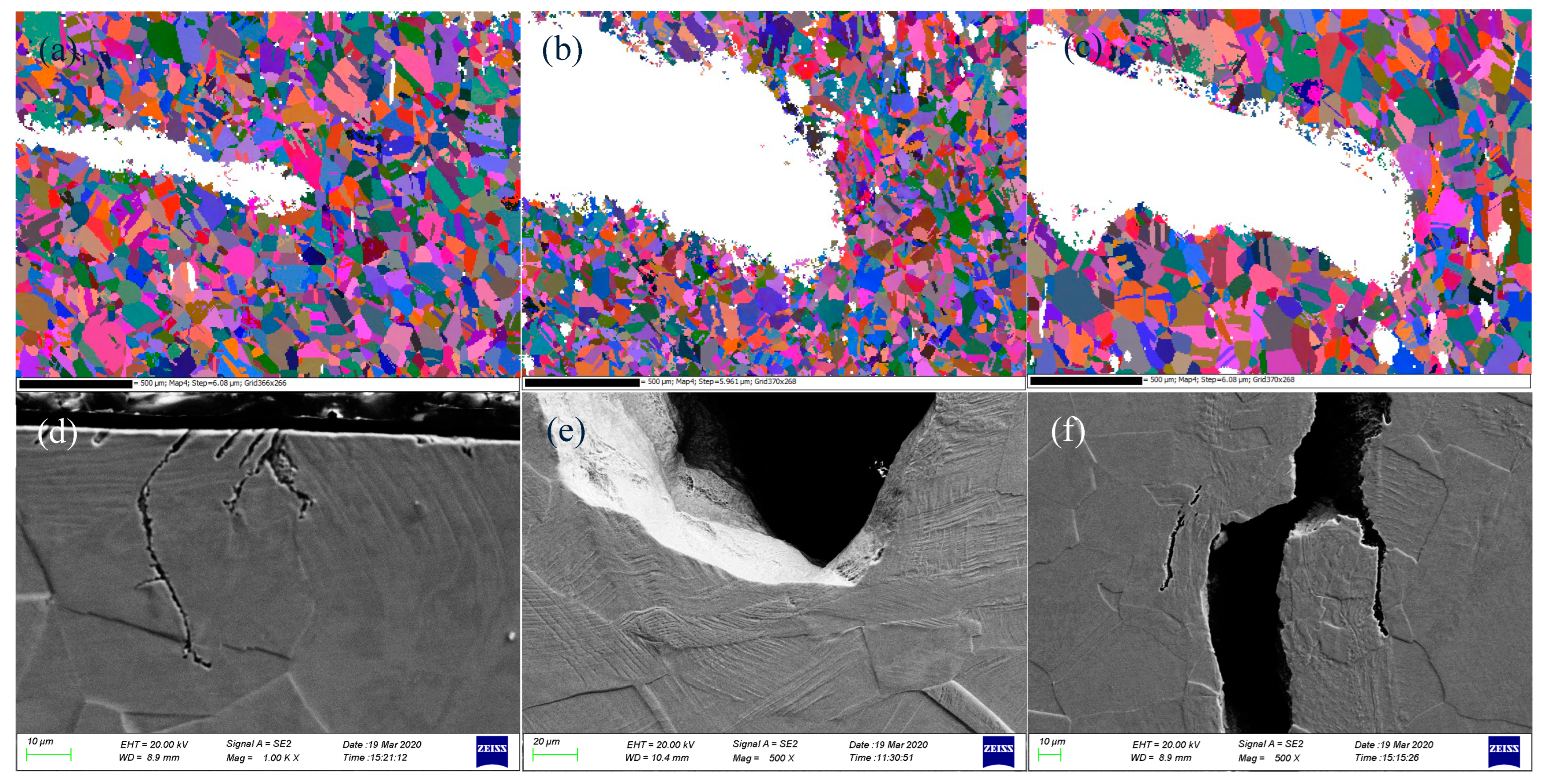
3.2.1. Overall Morphology of the Crack Tip
3.2.2. Fracture Morphology of the Crack Tip
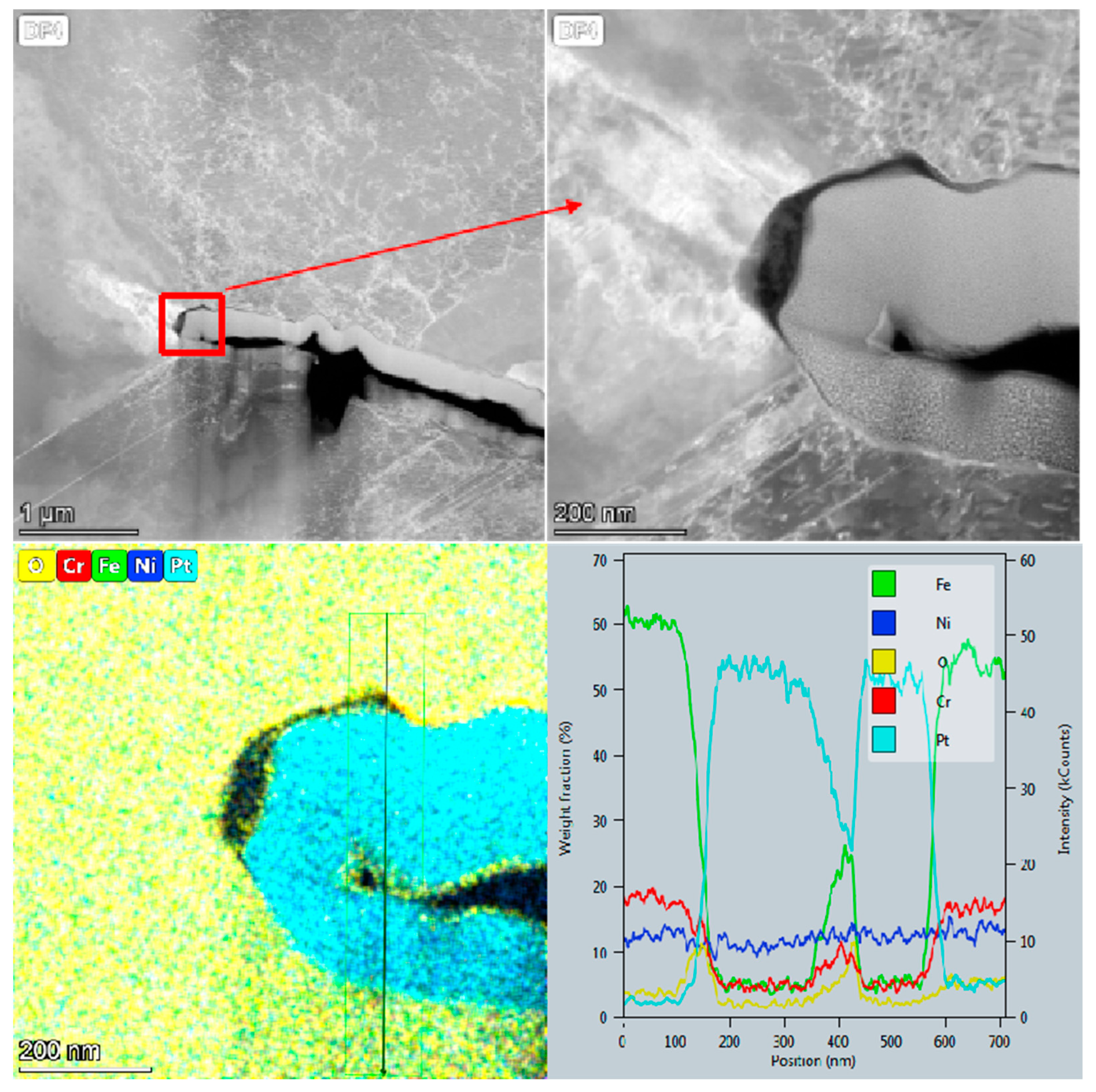
3.2.3. Oxidation Behavior at the Crack Tip
3.3. Mechanism of Crack Growth at Crack Tip
4. Conclusions
- (1)
- In a 325 °C high-temperature water environment, the CGR of 304L SS increased with increasing the stress intensity factor K, stress level, and fatigue frequency. Compared to DH in a high-temperature water environment, DO significantly increases the CGR about an order of magnitude higher.
- (2)
- Under high tensile cyclic stress levels, the crack tip of 304L SS in a high-temperature water environment is sharper, with more secondary cracks on the fracture surface, while the crack tip under low tensile cyclic stress levels is blunter with relatively fewer secondary cracks.
- (3)
- A dual-layer oxide film consisting of an outer iron-rich layer and an inner chromium-rich layer is formed in the crack growth path under high tensile cyclic stress levels. However, only a small amount of oxide is observed on the crack surface due to the dissolution of water over a long period of time under lower tensile cyclic stress.
- (4)
- The fatigue crack growth behavior of 304L SS in a high-temperature water environment depends on the interaction between oxidation at the PSB and the base metal.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| stainless steel | SS |
| electron back scatter diffraction | EBSD |
| transmission electron microscope | TEM |
| crack growth rate | CGR |
| dissolved hydrogen | DH |
| dissolved oxygen | DO |
| pressurized water reactor | PWR |
| stress corrosion cracking | SCC |
| Compact type | CT |
| Direct current potential drop | DCPD |
| Scanning electron microscopy | SEM |
| focused ion beam | FIB |
| energy dispersive spectrometer | EDS |
| persistent slip bands | PSBs |
| The American Society of Mechanical Engineers | ASME |
| frequency | f |
References
- Xiao, J.; Yu, Q.Z.; Ting, X.; Hao, W.; Yong, C.; Shao, Y.Q.; Gong, X. Effect of heat treatment on fatigue crack growth behavior of 316NG austenitic stainless steel in deaerated water at 325 °C. J. Nucl. Mater. 2021, 557, 153298. [Google Scholar] [CrossRef]
- Cui, Y.H.; Zhang, J.L. Research of SCC Prediction Model for 304 Stainless Steel in the High-Temperature Water Environment. Strength Mater. 2021, 53, 573–581. [Google Scholar] [CrossRef]
- Wu, H.C.; Yang, B.; Wang, S.L.; Zhang, M.X.; Shi, Y.Z.; Chen, Y.F.; Sun, Y.H. Effect of thermal aging on corrosion fatigue of Z3CN20.09M duplex stainless steel in high temperature water. Mater. Sci. Eng. A 2016, 655, 183–192. [Google Scholar] [CrossRef]
- Seifert, H.P.; Ritter, S.; Leber, H.J. Corrosion fatigue crack growth behaviour of austenitic stainless steels under light water reactor conditions. Corros. Sci. 2012, 55, 61–75. [Google Scholar] [CrossRef]
- Chiang, M.F.; Young, M.C.; Huang, J.Y. Effects of hydrogen water chemistry on corrosion fatigue behavior of cold-worked 304L stainless steel in simulated BWR coolant environments. J. Nucl. Mater. 2011, 411, 83–89. [Google Scholar] [CrossRef]
- Jinlong, L.; Hongyun, L.; Tongxiang, L. Influence of pre-deformation and oxidation in high temperature water on corrosion resistance of type 304 stainless steel. J. Nucl. Mater. 2015, 466, 154–161. [Google Scholar] [CrossRef]
- Mukahiwaa, K.; Sceninia, F.; Burkea, M.G.; Plattsb, N.; Ticeb, D.R.; Stairmandb, J.W. Corrosion fatigue and microstructural characterisation of Type 316 austenitic stainless steels tested in PWR primary water. Corros. Sci. 2018, 131, 57–70. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, S.; Wu, H.; Yang, C.; Hu, Y.; Fang, K.; Jiang, H. Combined effects of machining-induced residual stress and external load on SCC initiation and early propagation of 316 stainless steel in high temperature high pressure water. Corros. Sci. 2021, 190, 109644. [Google Scholar] [CrossRef]
- Gao, J.; Ziyu, Z.; Jibo, T.; Xinqiang, W.; Xiang, W.; En-Hou, H.; Ke, W. Differences of corrosion fatigue behaviors among 316LN base metal, 316LN heat-affected zone and 308L weld metal in a safe-end weld joint in borated and lithiated high-temperature water. Int. J. Fatigue 2021, 148, 106223. [Google Scholar] [CrossRef]
- Zhang, M.; Chenxin, Z.; Huanchun, W.; Yang, B. Effects of Grain Boundary Engineering on the Microstructure and Corrosion Fatigue Properties of 316L Austenitic Stainless Steel. Front. Mater. 2022, 9, 931848. [Google Scholar] [CrossRef]
- Wang, J.; Tianyu, Z.; Yaolei, H.; Jinna, M.B.; Fei, X.; Kai, C.; Du, D.; Andresen, P.L.; Zhang, L.; Zhang, M. Environment assisted cracking of 308L weld metal in high temperature water. J. Nucl. Mater. 2021, 557, 153275. [Google Scholar] [CrossRef]
- Lu, Z.; Shoji, T.; Meng, F.; Qiu, Y.; Dan, T.; Xue, H. Effects of water chemistry and loading conditions on stress corrosion cracking of cold-rolled 316NG stainless steel in high temperature water. Corros. Sci. 2011, 53, 247–262. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Zhang, T.; Wang, Y. Study on the mechanism of high-cycle corrosion fatigue crack initiation in X80 steel. Corros. Sci. 2012, 57, 99–103. [Google Scholar] [CrossRef]
- Srinivasan, V.S.; Sandhya, R.; Valsan, M.; Bhanu Sankara Rao, K.; Mannan, S.L. Comparative evaluation of strain controlled low cycle fatigue behaviour of solution annealed and prior cold worked 316L(N) stainless steel. Int. J. Fatigue 2004, 26, 1295–1302. [Google Scholar] [CrossRef]
- Meng, F.; Lu, Z.; Shoji, T.; Wang, J.; Han, E.; Ke, W. Stress corrosion cracking of uni-directionally cold worked 316NG stainless steel in simulated PWR primary water with various dissolved hydrogen concentrations. Corros. Sci. 2011, 53, 2558–2565. [Google Scholar] [CrossRef]
- Du, D.; Wang, J.; Chen, K.; Zhang, L.; Andresen, P.L. Environmentally assisted cracking of forged 316LN stainless steel and its weld in high temperature water. Corros. Sci. 2019, 147, 69–80. [Google Scholar] [CrossRef]
- Wang, J.; Tianyu, Z.; Kai, C.; Yichen, B.; Wujiang, M.; Miaomiao, Z.; Guo, X.; Andresen, P.L.; Zhang, L. Investigations on the SCC initiation behavior of cold worked 316 L in high temperature oxygenated water at constant loads. Corros. Sci. 2022, 203, 110336. [Google Scholar] [CrossRef]
- Cui, T.; Haiying, D.; Xinhe, X.; Jiarong, M.; Zhanpeng, L.; Tang, Y.; Pan, D.; Lozano-Perez, S.; Shoji, T. Hydrogen-enhanced oxidation of ferrite phase in stainless steel cladding and the contribution to stress corrosion cracking in deaerated high temperature water. J. Nucl. Mater. 2021, 557, 153209. [Google Scholar] [CrossRef]
- Cui, T.; Xinhe, X.; Deng, P.; Junjie, C.; Zhanpeng, L.; Yaping, Z.; Yang, S.; Shoji, T. Determining SCC resistance of stainless steel claddings in high- temperature water by constant load crack growth tests and slow strain rate tests. J. Nucl. Mater. 2024, 588, 154796. [Google Scholar] [CrossRef]
- Xiong, Y.; Watanabe, Y.; Shibayama, Y.; Zhong, X.; Mary, N. Low-cycle fatigue behaviors of 316L austenitic stainless steels with different surface finishing in simulated pressurized water reactor primary water and an oxygenated, borated, lithiated high temperature water. Corros. Sci. 2021, 190, 109709. [Google Scholar] [CrossRef]
- Xu, S.; Wu, X.Q.; Han, E.H.; Ke, W.; Katada, Y. Crack initiation mechanisms for low cycle fatigue of type 316Ti stainless steel in high temperature water. Mater. Sci. Eng. A 2008, 490, 16–25. [Google Scholar] [CrossRef]
- Chopra, O.K.; Shack, W.J. Low-cycle fatigue of piping and pressure vessel steels in LWR environments. Nucl. Eng. Des. 1998, 184, 49–76. [Google Scholar] [CrossRef]
- Huang, J.Y.; Young, M.C.; Jeng, S.L.; Yeh, J.J.; Huang, J.S.; Kuo, R.C. Corrosion fatigue behavior of low alloy steels under simulated BWR coolant conditions. J. Nucl. Mater. 2010, 405, 17–27. [Google Scholar] [CrossRef]
- Gao, J.; Tan, J.; Wu, X.; Xia, S. Effect of grain boundary engineering on corrosion fatigue behavior of 316LN stainless steel in borated and lithiated high-temperature water. Corros. Sci. 2019, 152, 190–201. [Google Scholar] [CrossRef]
- Li, Y.F.; Xiao, J.; Chen, Y.; Zhou, J.; Qiu, S.Y.; Xu, Q. Fatigue crack growth of 316NG austenitic stainless steel welds at 325 °C. J. Nucl. Mater. 2018, 499, 353–360. [Google Scholar] [CrossRef]
- Lukáš, T.; Ivo, K.; Veronika, M.; Tomáš, V.; Jaroslav, P.; Hutař, P.; Šmíd, M. Advantageous Description of Short Fatigue Crack Growth Rates in Austenitic Stainless Steels with Distinct Properties. Metals 2019, 475, 1–20. [Google Scholar]
- Su, T.; Huang, Y.; Xuan, F. Stress corrosion cracking growth rate prediction model for nuclear power turbine rotor steel in a simulated environment. J. Mater. Res. Technol. 2023, 23, 830–844. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, J.; Wu, X.; Han, E.; Ke, W. Corrosion fatigue crack growth behavior of 316LN stainless steel in high-temperature pressurized water. Nucl. Eng. Technol. 2021, 53, 2977–2981. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, J.; Wu, X.; Han, E.; Ke, W.; Rao, J. Corrosion fatigue behavior and crack-tip characteristic of 316LN stainless steel in high-temperature pressurized water. J. Nucl. Mater. 2019, 518, 21–29. [Google Scholar] [CrossRef]
- Al-Rubaie, K.S.; Godefroid, L.B.; Lopes, J.A.M. Statistical modeling of fatigue crack growth rate in Inconel alloy 600. Int. J. Fatigue 2007, 29, 931–940. [Google Scholar] [CrossRef]
- Vainionpa, A.; Seppanen, T.; Que, Z. Effects of pressurized water reactor environment and cyclic loading parameters on the low cycle fatigue behavior of 304L stainless steel. Int. J. Fatigue 2024, 182, 108231. [Google Scholar] [CrossRef]
- Han, Y.; Han, E.; Peng, Q.; Ke, W. Effects of electropolishing on corrosion and stress corrosion cracking of Alloy 182 in high temperature water. Corros. Sci. 2017, 121 (Suppl. C), 1–10. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Fang, K.; Zhang, W.; Wang, X. Effect of surface machining on the corrosion behaviour of 316 austenitic stainless steel in simulated PWR water. Corros. Sci. 2017, 126 (Suppl. C), 104–120. [Google Scholar] [CrossRef]
- Cissé, S.; Laffont, L.; Tanguy, B.; Lafont, M.; Andrieu, E. Effect of surface preparation on the corrosion of austenitic stainless steel 304L in high temperature steam and simulated PWR primary water. Corros. Sci. 2012, 56, 209–216. [Google Scholar] [CrossRef]
- Xu, J.; Wu, X.; Han, E. The evolution of electrochemical behaviour and oxide film properties of 304 stainless steel in high temperature aqueous environment. Electrochim. Acta 2012, 71, 219–226. [Google Scholar] [CrossRef]
- Ti, W.; Wu, H.; Li, C.; Zhang, G.; Xue, F.; Wang, X. Effect of Machining on the Oxide Film Formation of Austenite Stainless Steel in 300 °C High Temperature Water. Mater. Trans. 2020, 61, 1122–1129. [Google Scholar] [CrossRef]
- Cheng, X.; Feng, Z.; Li, C.; Dong, C.; Li, X. Investigation of oxide film formation on 316L stainless steel in high-temperature aqueous environments. Electrochim. Acta 2011, 56, 5860–5865. [Google Scholar] [CrossRef]
- Xiao, J.; Qiu, S.Y.; Chen, Y.; Lin, Z.X.; Xu, Q.; Xie, H.Y. Effects of dissolved oxygen on corrosion fatigue cracking of Alloy 690(TT) in pressurized water reactor environments. Int. J. Fatigue 2015, 74, 65–70. [Google Scholar] [CrossRef]
- Kamaya, M. Environmental effect on fatigue strength of stainless steel in PWR primary water—Role of crack growth acceleration in fatigue life reduction. Int. J. Fatigue 2013, 55, 102–111. [Google Scholar] [CrossRef]






| Element | C | Si | Mn | P | S | Cr | Ni | Mo | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 304L | 0.014 | 0.41 | 0.72 | 0.016 | 0.0035 | 18.61 | 8.41 | 0.25 | Balance |
| Stress Rate | Frequency | Dissolved Oxygen | Dissolved Hydrogen | pH | Conductivity | Temperature | Pressure |
|---|---|---|---|---|---|---|---|
| R = 0.3 | 0.1, 0.01 | 2 ppm | 1.58 ppm | 6.6 | 0.15 µS/cm | 325 °C | 10.5 MPa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Liu, X.; Xu, C.; Li, Y.; Yin, J.; Jin, X.; Jia, W.; Qian, W.; Wang, P.; Zhang, Y. Study on Corrosion Fatigue Behavior of 304L Austenite Stainless Steel in 325 °C High-Temperature Water Environment. Metals 2024, 14, 489. https://doi.org/10.3390/met14050489
Wu H, Liu X, Xu C, Li Y, Yin J, Jin X, Jia W, Qian W, Wang P, Zhang Y. Study on Corrosion Fatigue Behavior of 304L Austenite Stainless Steel in 325 °C High-Temperature Water Environment. Metals. 2024; 14(5):489. https://doi.org/10.3390/met14050489
Chicago/Turabian StyleWu, Huanchun, Xiangbing Liu, Chaoliang Xu, Yuanfei Li, Jian Yin, Xiao Jin, Wenqing Jia, Wangjie Qian, Peng Wang, and Yanwei Zhang. 2024. "Study on Corrosion Fatigue Behavior of 304L Austenite Stainless Steel in 325 °C High-Temperature Water Environment" Metals 14, no. 5: 489. https://doi.org/10.3390/met14050489





