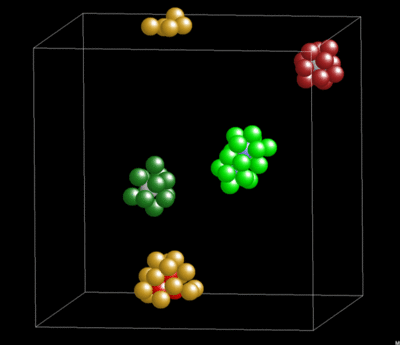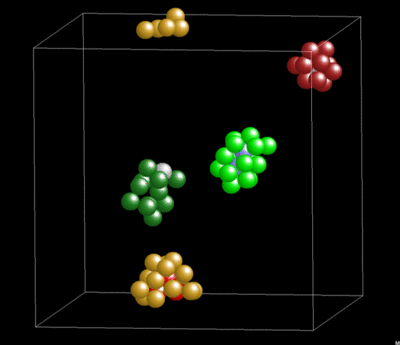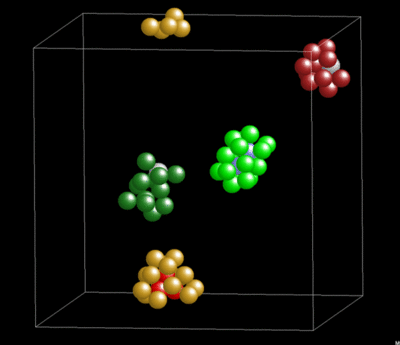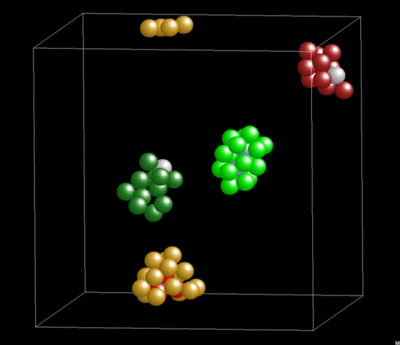
Dynamics and Geometry of Icosahedral Order in Liquid and Glassy Phases of Metallic Glasses
Abstract
:1. Introduction
2. Methods
2.1. Interatomic Potentials
2.2. Simulation Procedure
2.3. Icosahedral Symmetry
3. Results and Discussion
3.1. Icosahedral Order in Zr-Cu System
3.1.1. Icosahedral Medium-range Order in Zr-Cu Metallic Glasses

3.1.2. Geometrical Feature of Icosahedral Network

3.1.3. Icosahedral Order in Supercooled Liquids

3.1.4. Lifetime of Icosahedral Clusters


3.1.5. Lifetime and Cluster Bonding


3.2. Icosahedral Order in Model System
3.2.1. Atomic Size Effect on Glass-forming Ranges of Model Binary System

3.2.2. Geometrical Feature of Icosahedral Network

3.2.3. Atomic Size Ratio Dependence of Icosahedral Order

3.2.4. Cluster Lifetime and Atomic Size difference

3.2.5. Icosahedral Network in Supercooled Liquids

3.2.6. Unit for 5-Fold Symmetry

3.2.7. Geometric Change in Solidification
3.2.8. Geometric Change in Relaxation


3.3. Icosahedral Order and Regge Calculus
3.3.1. Geometric Consideration Based on DRP Model

3.3.2. Regge Calculus


3.3.3. Variety of Tetrahedral Building Blocks: Binary Case


3.3.4. Icosahedral Network and Low Distortion 5-Rings

3.3.5. Geometry Change in Relaxation
3.3.6. Low Distortion 5-Rings and Alloy Compositions of Good Glass-Former


3.3.7. Variety of Tetrahedral Building Blocks: Ternary Case

- (1)
- Multicomponent alloy systems consisting of more than three components,
- (2)
- Significantly different atomic size ratios among the main constituent elements,
- (3)
- Large negative heats of mixing among their elements.
3.3.8. Relation between Icosahedral Clusters and Other Types of Clusters

4. Conclusions
Author Contributions
Conflicts of Interest
References
- Bernal, J.D. A Geometrical Approach to the Structure of Liquids. Nature 1959, 183, 141–147. [Google Scholar]
- Cargill, G.S., III. Dense random packing of hard spheres as a structural model for noncrystalline metallic solids. J. Appl. Phys. 1970, 41, 2248–2250. [Google Scholar] [CrossRef]
- Finney, J.L. Modelling the structures of amorphous metals and alloys. Nature 1977, 266, 309–314. [Google Scholar] [CrossRef]
- Yamamoto, R.; Doyama, M. The polyhedron and cavity analyses of a structural model of amorphous iron. J. Phys. F Metal Phys. 1979, 9, 617–627. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T.; Masumoto, T. Zr-Al-Ni amorphous alloys with high glass transition temperature and significant supercooled liquid region. Mater. Trans. JIM 1990, 31, 177–183. [Google Scholar] [CrossRef]
- Parker, A.; Johnson, W.L. A highly processable metallic glass: Zr41.2Ti13.8Cu12.5Ni10Be22.5. Appl. Phys. Lett. 1993, 63, 2342–2344. [Google Scholar]
- Saida, J.; Sanada, T.; Sato, S.; Imafuku, M.; Matsubara, E.; Inoue, A. Local structure study in Zr-based metallic glasses. Mater. Trans. 2007, 48, 1703–1707. [Google Scholar] [CrossRef]
- Hui, X.; Gao, R.; Chen, G.L.; Shang, S.L.; Wang, Y.; Liu, Z.K. Short-to-medium-range order in Mg65Cu25Y10 metallic glass. Phys. Lett. A 2008, 372, 3078–3084. [Google Scholar] [CrossRef]
- Shen, Y.T.; Kim, T.H.; Gangopadhy, A.K.; Kelton, K.F. Icosahedral order, frustration, and the glass transition: Evidence from time-dependent nucleation and supercooled liquid structure studies. Phys. Rev. Lett. 2009, 102, 057801. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Guan, P.-F.; Fujita, T.; Hirotsu, Y.; Inoue, A.; Yavari, A.R.; Sakurai, T.; Chen, M.-W. Direct observation of local atomic order in a metallic glass. Nat. Mater. 2011, 10, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Melgarejo, Z.H.; Kalay, Y.E.; Kalay, I.; Kramer, M.J.; Stone, D.S.; Voyles, P.M. Nanoscale Structure and Structural Relaxation in Zr50Cu45Al5 Bulk Metallic Glass. Phys. Rev. Lett. 2012, 108, 195505. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.C.Y.; Neish, M.J.; Stokol, G.; Buckley, G.A.; Smillie, L.A.; de Jonge, M.D.; Ott, R.T.; Kramer, M.J.; Bourgeois, L.J. Systematic Mapping of Icosahedral Short-Range Order in a Melt-Spun Zr36Cu64 Metallic Glass. Phys. Rev. Lett. 2013, 110, 205505. [Google Scholar] [CrossRef] [PubMed]
- Miracle, D.B. A structural model for metallic glasses. Nat. Mater. 2004, 3, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.W.; Luo, W.K.; Alamgir, F.M.; Bai, J.M.; Ma, E. Atomic packing and short-to-medium-range order in metallic glasses. Nature 2006, 439, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Shimono, M.; Onodera, H. Icosahedral order in supercooled liquids and glassy alloys. Mat. Sci. Forum 2007, 539–543, 2031–2035. [Google Scholar] [CrossRef]
- Wakeda, M.; Shibutani, Y. Icosahedral clustering with medium-range order and local elastic properties of amorphous metals. Acta Mater. 2010, 58, 3963–3969. [Google Scholar] [CrossRef]
- Xie, Z.-C.; Gao, T.-H.; Guo, X.-T.; Qin, X.-M.; Xie, Q. Growth of icosahedral mediumrange order in liquid TiAl alloy during rapid solidification. J. Non-Cryst. Solids 2014, 394, 16–21. [Google Scholar] [CrossRef]
- Li, M.Z.; Wang, C.Z.; Hao, S.G.; Kramer, M.J.; Ho, K.M. Structural heterogeneity and medium-range order in ZrxCu100−x metallic glasses. Phys. Rev. B 2009, 80, 184201. [Google Scholar] [CrossRef]
- Lee, M.; Kim, H.-K.; Lee, J.-C. Icosahedral medium-range orders and backbone formation in an amorphous alloy. Met. Mater. Int. 2010, 16, 877–881. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ma, E.; Sheng, H.W. Atomic level structure in multicomponent bulk metallic glass. Phys. Rev. Lett. 2009, 102, 245501. [Google Scholar] [CrossRef] [PubMed]
- Lekka, Ch.E.; Evangelakis, G.A. Bonding characteristics and strengthening of CuZr fundamental clusters upon small Al additions from density functional theory calculations. Scr. Mater. 2009, 61, 974–977. [Google Scholar] [CrossRef]
- Ding, J.; Cheng, Y.-Q.; Ma, E. Full icosahedra dominate local order in Cu64Zr34 metallic glass and supercooled liquid. Acta Mater. 2014, 69, 343–354. [Google Scholar] [CrossRef]
- Frank, F.C.; Kasper, J.S. Complex alloy structures regarded as sphere packings. I. Definitions and basic principles. Acta Cryst. 1958, 11, 184–190. [Google Scholar] [CrossRef]
- Leocmach, M.; Tanaka, H. Roles of icosahedral and crystal-like order in the hard spheres glass transition. Nat. Commun. 2012, 3, 974. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, U.R.; Schrøder, T.B.; Dyre, J.C.; Harrowell, P. Geometry of Slow Structural Fluctuations in a Supercooled Binary Alloy. Phys. Rev. Lett. 2010, 104, 105701. [Google Scholar] [CrossRef] [PubMed]
- Malins, A.; Eggers, J.; Royall, C.P.; Williams, S.R.; Tanaka, H. Identification of long-lived clusters and their link to slow dynamics in a model glass former. J. Chem. Phys. 2013, 138, 12A535. [Google Scholar] [CrossRef] [PubMed]
- Finnis, M.W.; Sinclair, J.E. A Simple Empirical N-Body Potential for Transition Metals. Pilos. Mag. A 1984, 50, 45–55. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Barefoot, J.R.; Jarrett, R.N.; Tien, J.K. Modeling of γ/γ′ phase equilibrium in the Nickel-Aluminum system. Acta Metall. 1984, 32, 1519–1525. [Google Scholar] [CrossRef]
- Rosato, V.; Guillope, M.; Legrand, B. Thermodynamical and structural properties of f.c.c. transition metals using a simple tight-binding model. Philos. Mag. A 1989, 59, 321–336. [Google Scholar] [CrossRef]
- Shimono, M.; Onodera, H. Molecular dynamics study on structural relaxation of metallic glasses. Mat. Sci. Forum 2010, 638–642, 1648–1652. [Google Scholar] [CrossRef]
- Inoue, A. High strength bulk amorphous alloys with low critical cooling rates. Mater. Trans. JIM 1995, 36, 866–875. [Google Scholar] [CrossRef]
- Andersen, H.C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef]
- Shimono, M.; Onodera, H. Geometrical and chemical factors in the glass-forming ability. Scripta Mater. 2001, 44, 1595–1598. [Google Scholar] [CrossRef]
- Shimono, M.; Onodera, H. Structural Relaxation in Supercooled Liquids. Mater. Trans. 2005, 46, 2830–2837. [Google Scholar] [CrossRef]
- Shimono, M.; Onodera, H. Icosahedral symmetry, fragility and stability of supercooled liquid state of metallic glasses. Rev. Métallurgie 2012, 109, 41–46. [Google Scholar] [CrossRef]
- Ohkubo, T.; Kai, H.; Hirotsu, Y. Structural modeling of Pd–Si and Fe–Zr–B amorphous alloys based on the microphase separation model. Mater. Sci. Eng. A 2001, 304–306, 300–304. [Google Scholar] [CrossRef]
- Park, J.; Shibutani, Y. Weighted Voronoi tessellation technique for internal structure of metallic glasses. Intermetallics 2007, 15, 187–192. [Google Scholar] [CrossRef]
- Hansen, J.P.; McDonald, I.R. Theory of Simple Liquids, 3rd ed.; Academic Press: New York, NY, USA, 2006. [Google Scholar]
- Miracle, D.B. The efficient cluster packing model—An atomic structural model for metallic glasses. Acta Mater. 2006, 54, 4317–4336. [Google Scholar] [CrossRef]
- Hopkins, A.B.; Stillinger, F.H.; Torquato, S. Densest local sphere-packing diversity. II. Application to three dimensions. Phys. Rev. E 2011, 83, 011304. [Google Scholar] [CrossRef]
- Regge, T. General relativity without coordinates. Nuovo Cimento 1961, 19, 558–571. [Google Scholar] [CrossRef]
- Nelson, D.R. Order, frustration, and defects in liquids and glasses. Phys. Rev. B 1983, 28, 5515–5535. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimono, M.; Onodera, H. Dynamics and Geometry of Icosahedral Order in Liquid and Glassy Phases of Metallic Glasses. Metals 2015, 5, 1163-1187. https://doi.org/10.3390/met5031163
Shimono M, Onodera H. Dynamics and Geometry of Icosahedral Order in Liquid and Glassy Phases of Metallic Glasses. Metals. 2015; 5(3):1163-1187. https://doi.org/10.3390/met5031163
Chicago/Turabian StyleShimono, Masato, and Hidehiro Onodera. 2015. "Dynamics and Geometry of Icosahedral Order in Liquid and Glassy Phases of Metallic Glasses" Metals 5, no. 3: 1163-1187. https://doi.org/10.3390/met5031163