Nano-ZnO Particles’ Effect in Improving the Mechanical Response of Mg-3Al-0.4Ce Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Primary Processing
2.3. Secondary Processing
2.3.1. Pre-Extrusion
2.3.2. Extrusion
2.3.3. Post-Extrusion
2.4. Characterisation
2.4.1. Density and Porosity
2.4.2. Microstructure
2.4.3. X-ray Diffraction
2.4.4. Coefficient of Thermal Expansion
2.4.5. Microhardness
2.4.6. Tensile Test
3. Results and Discussion
3.1. Density and Porosity
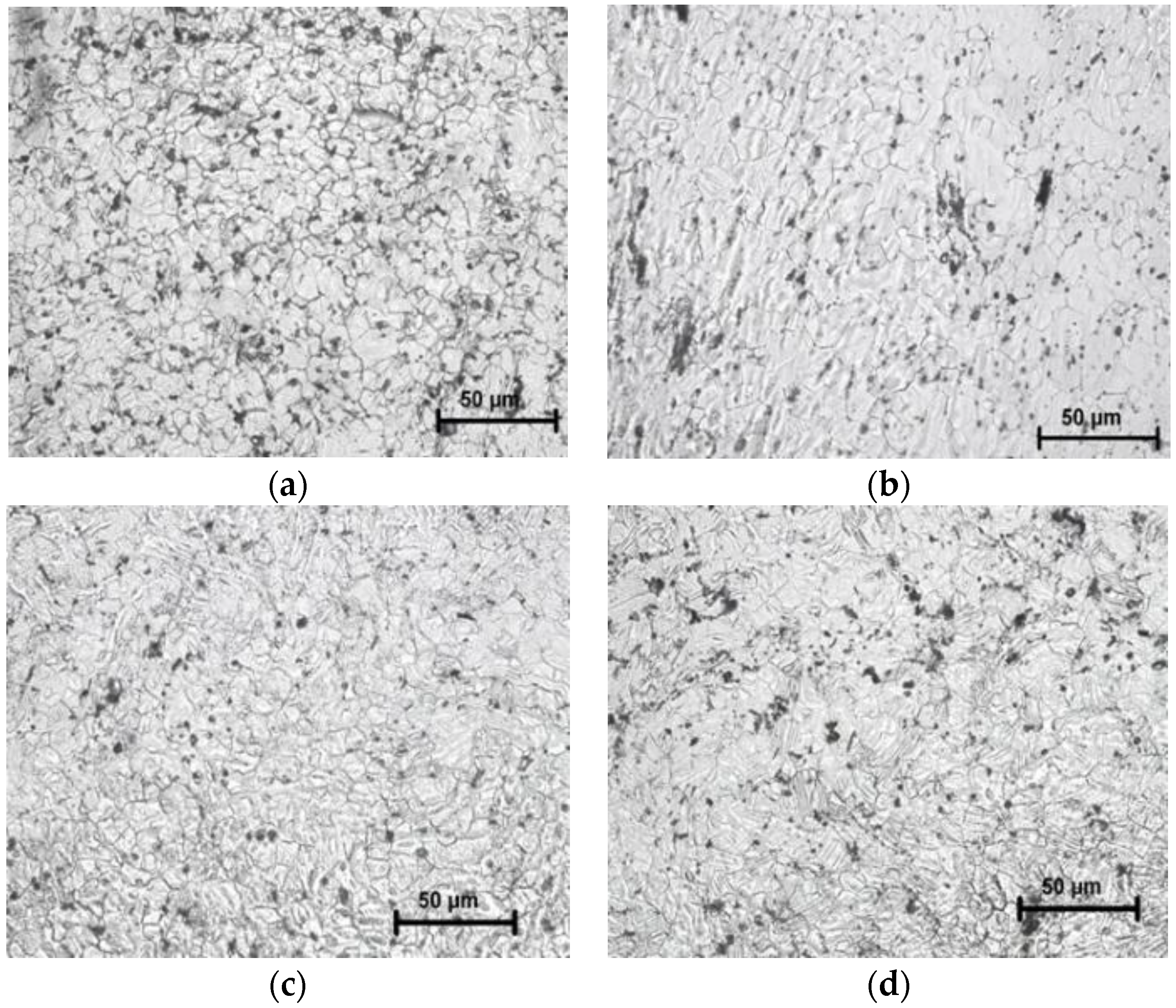
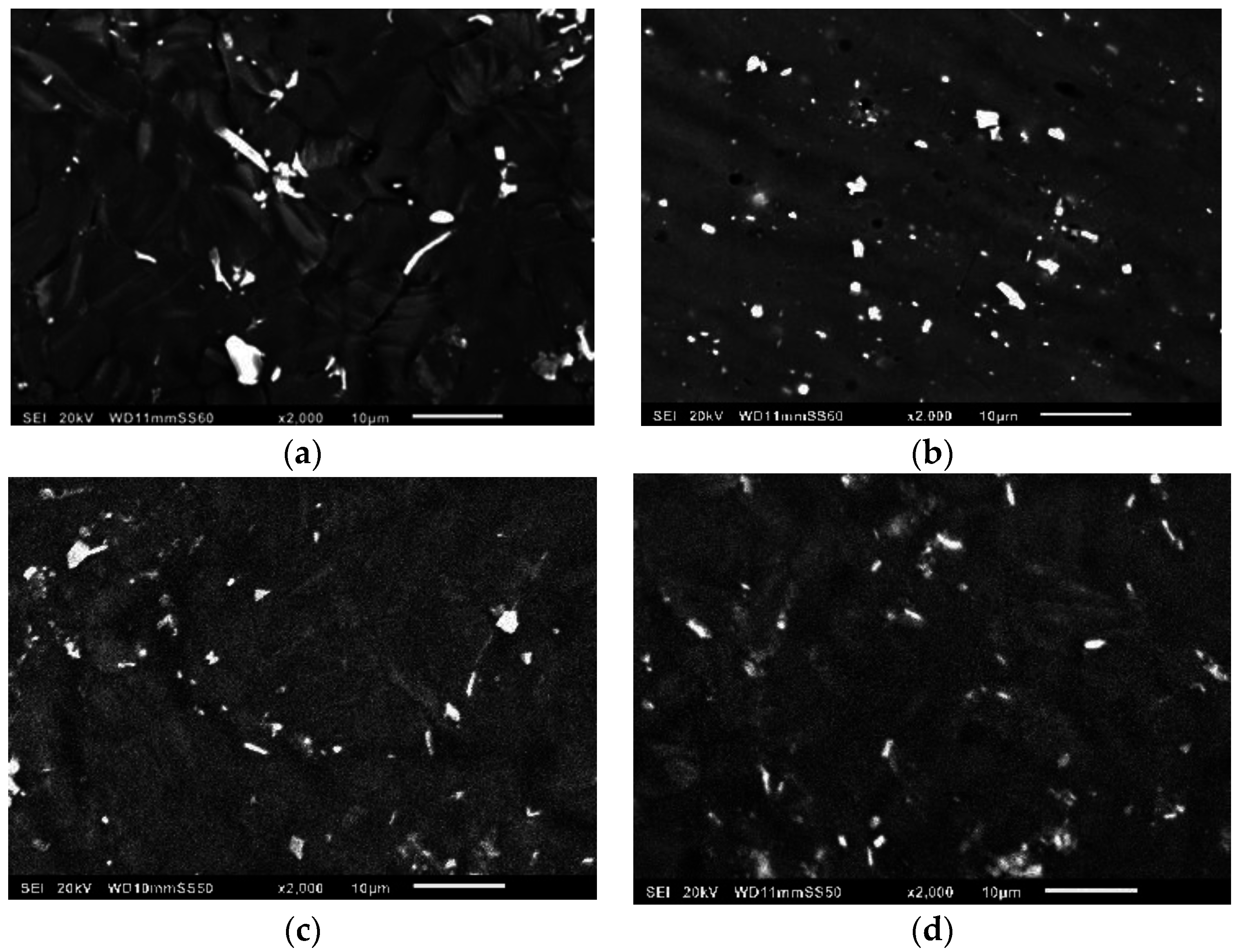
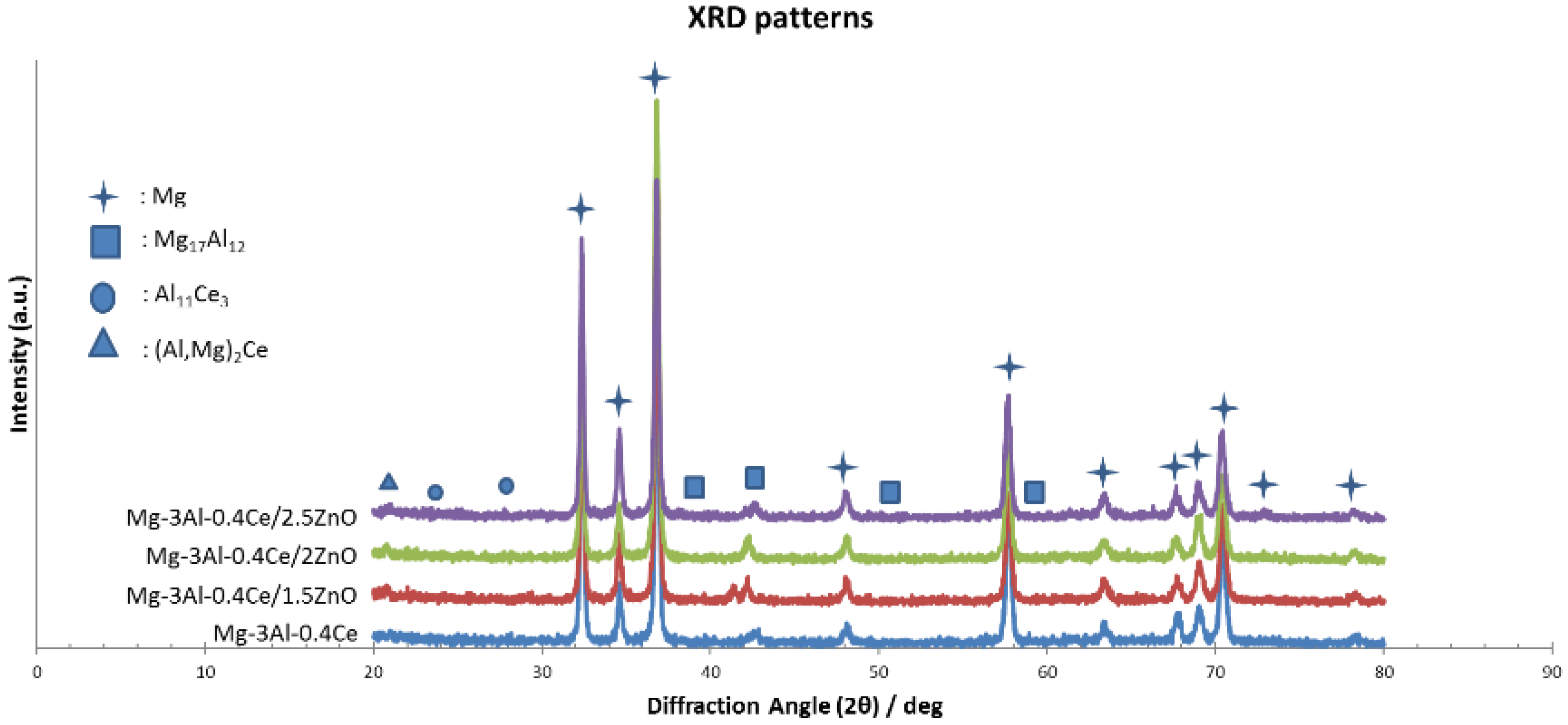
3.2. Microstructure/Phase Analysis
3.2.1. Grain Size Analysis
3.2.2. Microstructure Analysis
3.2.3. Phase Analysis
3.3. Coefficient of Thermal Expansion (CTE)
3.4. Mechanical Properties
3.4.1. Microhardness
3.4.2. Tensile Properties
3.4.3. Fractography
4. Conclusions
- The processing methodology used in this study is capable of synthesizing the Mg-3Al-0.4Ce alloy and its nanocomposites with porosities restricted to ~1%.
- The addition of ZnO nanoparticles reduced the CTE of the Mg-3Al-0.4Ce alloy, resulting in more dimensionally stable nanocomposites.
- The addition of ZnO nano-particulates corresponded with the increase in the microhardness of the Mg-3Al-0.4Ce alloy. A maximum microhardness was realized in the case of the Mg-3Al-0.4Ce/2.5ZnO nanocomposite, with a value of 161 Hv.
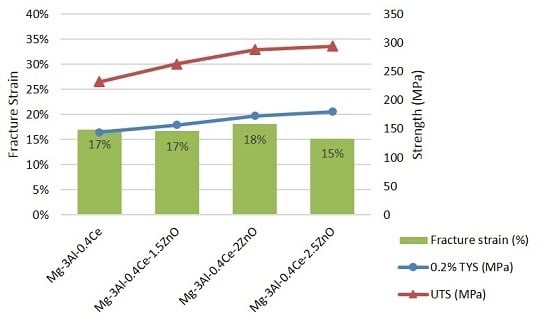
- The addition of an increasing amount of ZnO nanoparticles led to an increase in the 0.2% offset yield strength and ultimate tensile strength, while strain to fracture remained unaffected. A maximum yield strength of 180 MPa and ultimate tensile strength of 294 MPa was achieved for the Mg-3Al-0.4Ce/2.5ZnO nanocomposite.
- These superior mechanical properties, achieved with the addition of the ZnO nanoparticles, are attributed to the uniform distribution of the secondary phases and the presence of ZnO reinforcement.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- King, J.F. Magnesium: Commodity or exotic? Mater. Sci. Technol. 2007, 23, 1–14. [Google Scholar] [CrossRef]
- Alam, M.E.; Han, S.; Nguyen, Q.B.; Hamouda, A.M.S.; Gupta, M. Development of new magnesium based alloys and their nanocomposites. J. Alloy. Compd. 2011, 509, 8522–8529. [Google Scholar] [CrossRef]
- Tekumalla, S.; Seetharaman, S.; Almajid, A.; Gupta, M. Mechanical Properties of Magnesium-Rare Earth Alloy Systems: A Review. Metals 2015, 5, 1. [Google Scholar] [CrossRef]
- Rokhlin, L.L. Structure and properties of alloys of the Mg-REM system. Met. Sci. Heat Treat. 2006, 48, 487–490. [Google Scholar] [CrossRef]
- Tekumalla, S.; Seetharaman, S.; Bau, N.Q.; Wong, W.L.E.; Goh, C.S.; Shabadi, R.; Gupta, M. Influence of Cerium on the Deformation and Corrosion of Magnesium. J. Eng. Mater. Technol. 2016, 138, 031011. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Nayak, U.P.; Sabat, R.; Suwas, S.; Almajid, A.; Gupta, M. Nano-ZnO particle addition to monolithic magnesium for enhanced tensile and compressive response. J. Alloy. Compd. 2014, 615, 211–219. [Google Scholar] [CrossRef]
- Gupta, M.; Wong, W.L.E. Magnesium-based nanocomposites: Lightweight materials of the future. Mater. Charact. 2015, 105, 30–46. [Google Scholar] [CrossRef]
- Luo, A.A.; Wu, W.; Mishra, R.K.; Jin, L.; Sachdev, A.K.; Ding, W. Microstructure and Mechanical Properties of Extruded Magnesium-Aluminum-Cerium Alloy Tubes. Metall. Mater. Trans. A 2010, 41, 2662–2674. [Google Scholar] [CrossRef]
- Song, Y.L.; Liu, Y.H.; Wang, S.H.; Yu, S.R.; Zhu, X.Y. Effect of cerium addition on microstructure and corrosion resistance of die cast AZ91 magnesium alloy. Mater. Corros. 2007, 58, 189–192. [Google Scholar] [CrossRef]
- Zhang, J.; Leng, Z.; Zhang, M.; Meng, J.; Wu, R. Effect of Ce on microstructure, mechanical properties and corrosion behavior of high-pressure die-cast Mg-4Al-based alloy. J. Alloy. Compd. 2011, 509, 1069–1078. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Gupta, M. Increasing significantly the failure strain and work of fracture of solidification processed AZ31B using nano-Al2O3 particulates. J. Alloy. Compd. 2008, 459, 244–250. [Google Scholar] [CrossRef]
- Nayyeri, M.J.; Ganjkhanlou, Y.; Kolahi, A.; Jamili, A.M. Effect of Ca and Rare Earth Additions on the Texture, Microhardness, Microstructure and Structural Properties of As-Cast Mg–4Al–2Sn Alloys. Trans. Indian Inst. Met. 2014, 67, 469–475. [Google Scholar] [CrossRef]
- Yahya, N.; Puspitasari, P.; Latiff, N.R.A. Characterization and Development of Biosystems and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 9–32. [Google Scholar]
- Zhaoxu, D.; Daining, F.; Kah, S.A. On the effect of nano-particle clustering on toughening of nano-composite ceramics. Acta Mech. Sin. 2002, 18, 146–158. [Google Scholar] [CrossRef]
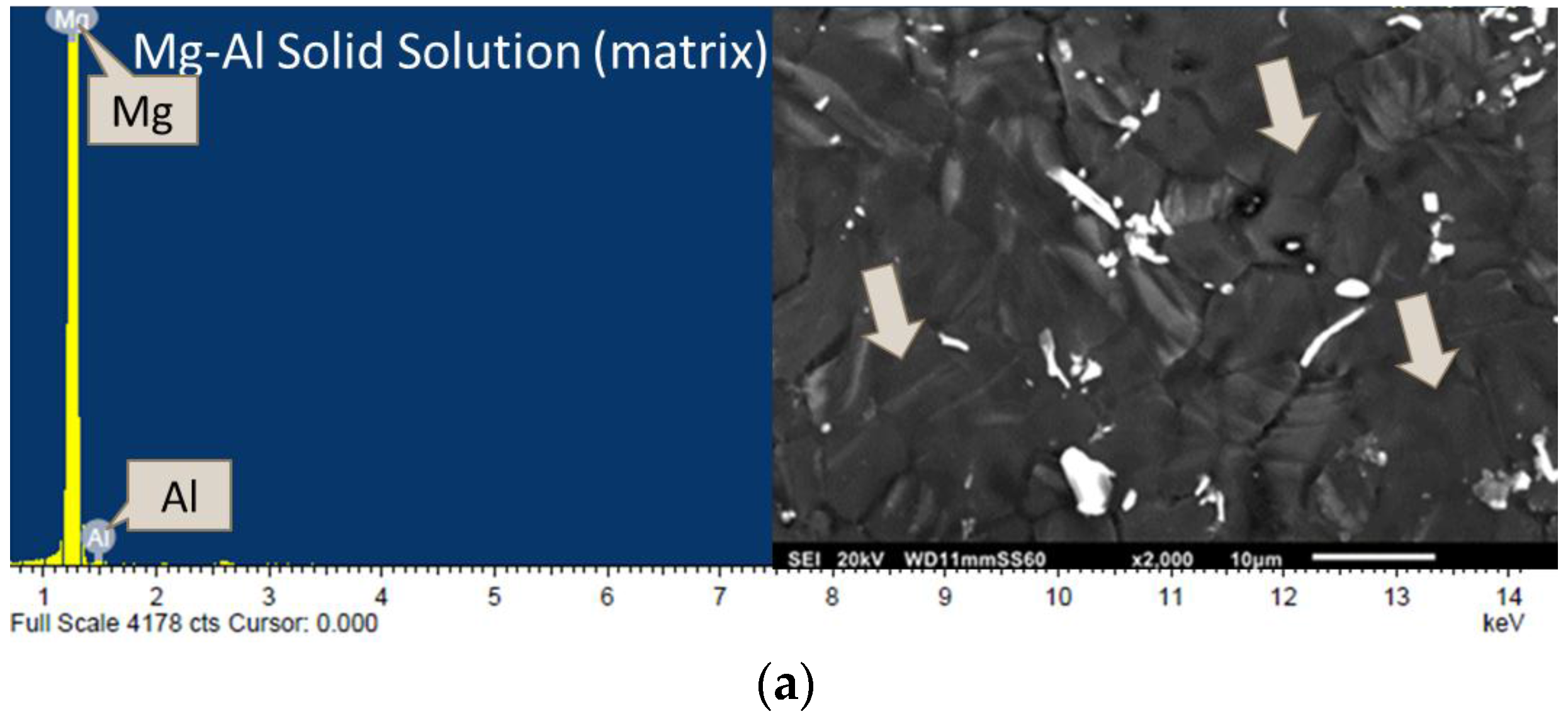
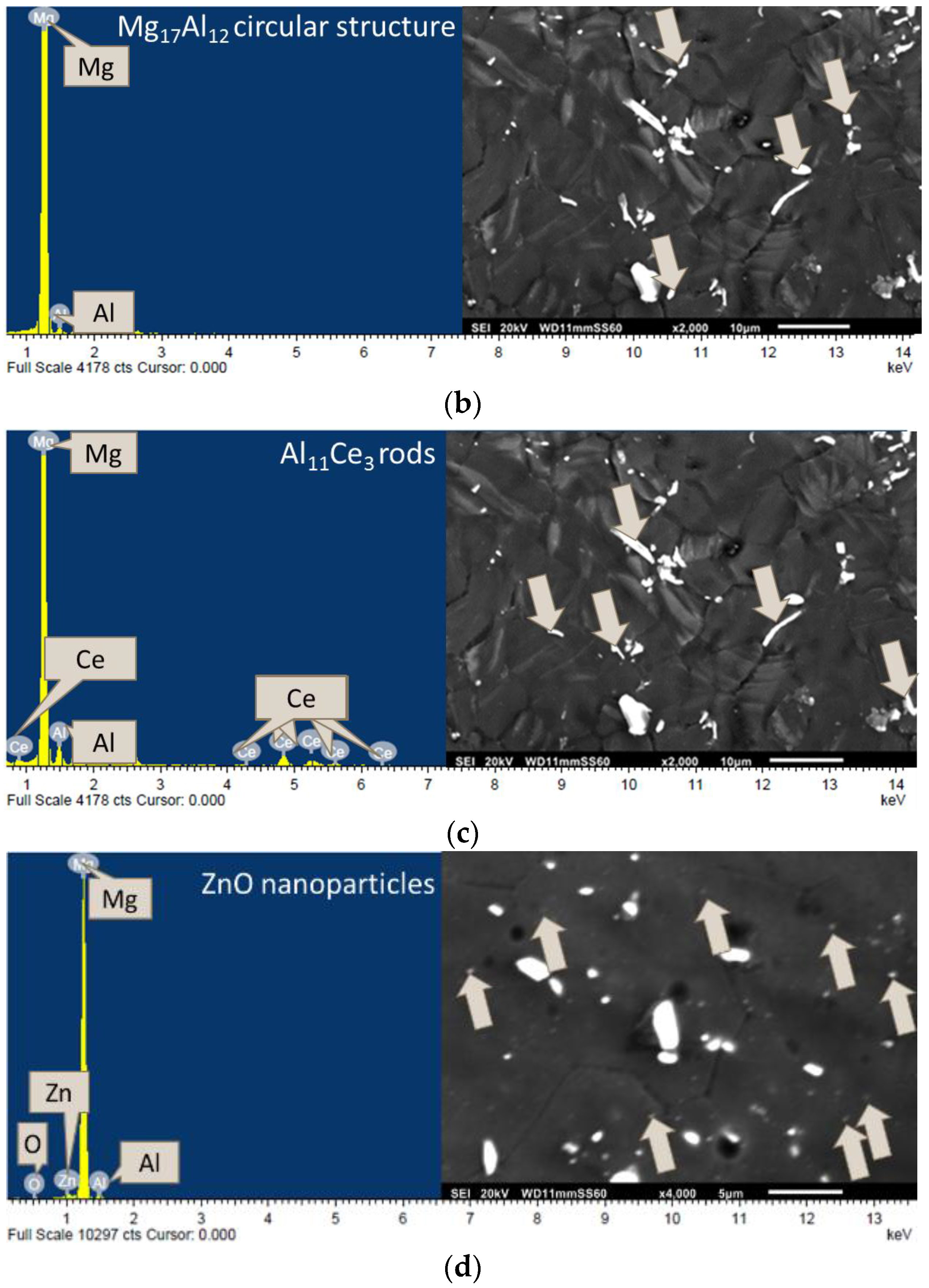
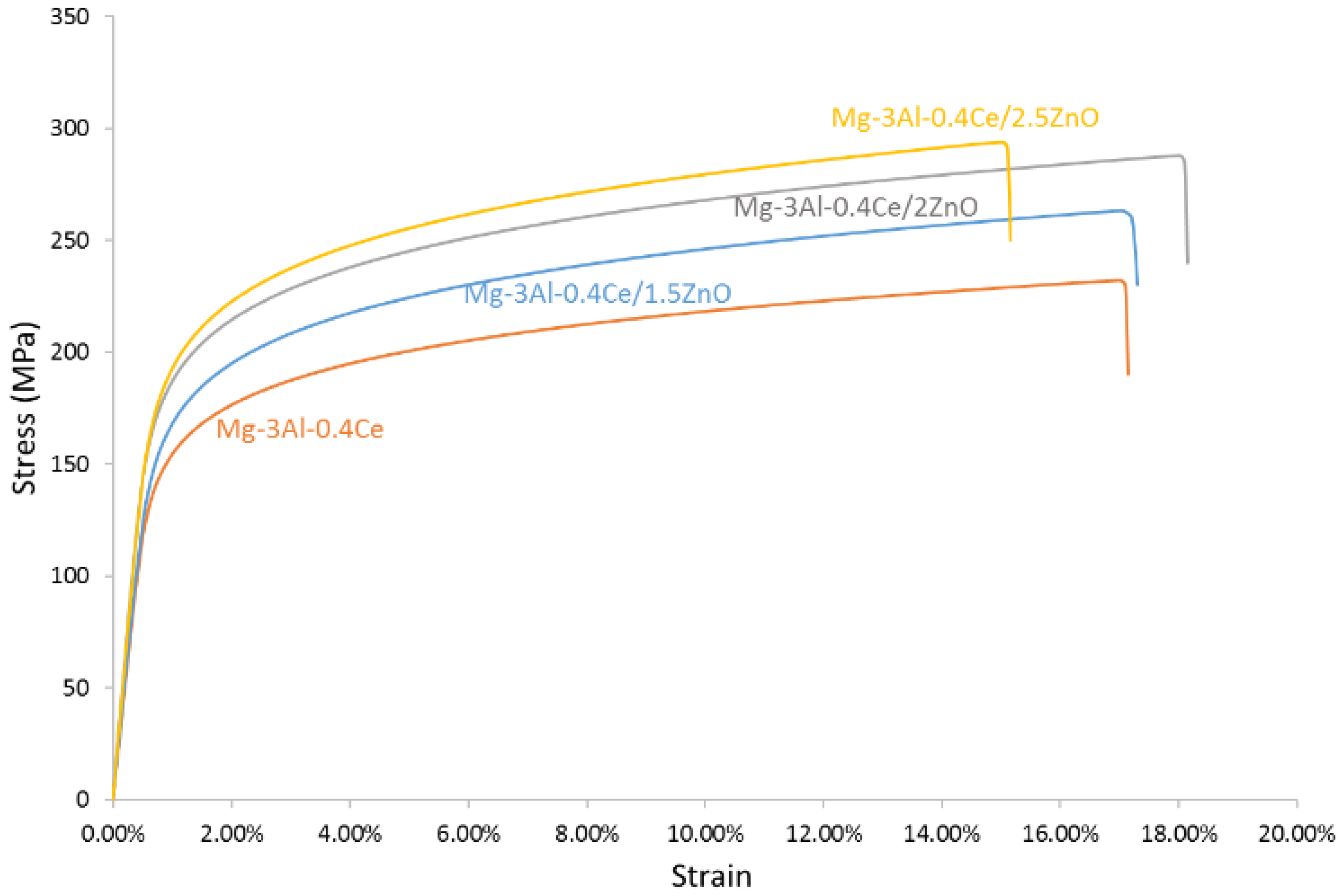
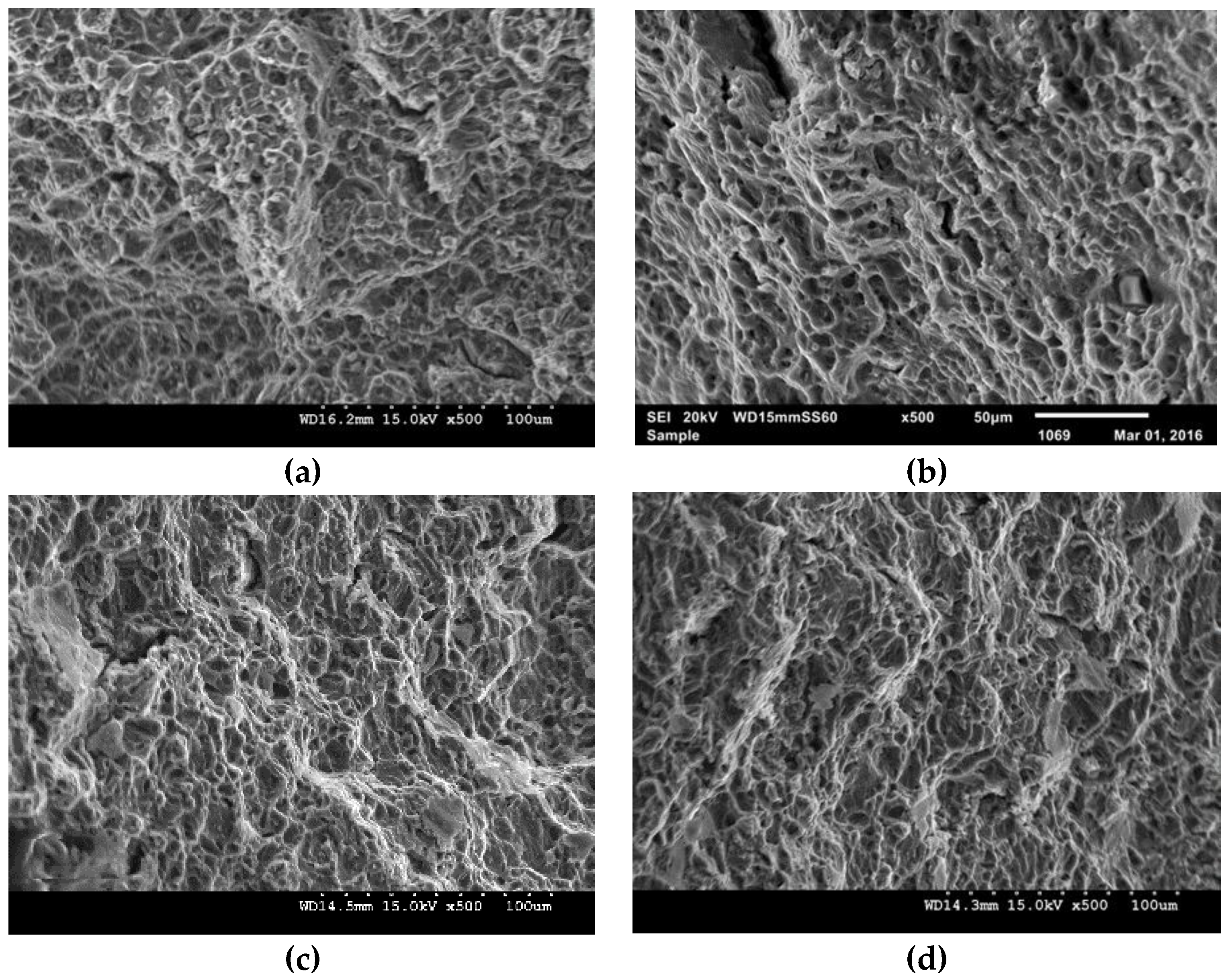
| Composition | Experimental Density (g/cm3) | Theoretical Density (g/cm3) | Porosity (%) | Grain Size (µm) |
|---|---|---|---|---|
| Mg-3Al-0.4Ce | 1.759 ± 0.013 | 1.762 | 0.201 | 6.5 ± 1.5 |
| Mg-3Al-0.4Ce-1.5ZnO | 1.779 ± 0.012 (↑ 1.13%) 1 | 1.781 | 0.120 | 6.4 ± 1.5 |
| Mg-3Al-0.4Ce-2ZnO | 1.783 ± 0.013 (↑ 1.36%) 1 | 1.787 | 0.235 | 6.1 ± 1.5 |
| Mg-3Al-0.4Ce-2.5ZnO | 1.774 ± 0.028 (↑ 0.85%) 1 | 1.794 | 1.080 | 6.0 ± 1.6 |
| Composition | CTE (µK−1) |
|---|---|
| Mg-3Al-0.4Ce | 25.52 ± 0.8 |
| Mg-3Al-0.4Ce-1.5ZnO | 22.03 ± 1.0 |
| Mg-3Al-0.4Ce-2ZnO | 24.16 ± 2.0 |
| Mg-3Al-0.4Ce-2.5ZnO | 24.36 ± 0.6 |
| Composition | Microhardness (Hv) | 0.2% Offset Tensile Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Fracture Strain (%) |
|---|---|---|---|---|
| Mg-3Al-0.4Ce | 105 ± 4 | 144 ± 8 | 232 ± 11 | 17 ± 1 |
| Mg-3Al-0.4Ce-1.5ZnO | 113 ± 3 (↑ 7.6%) 1 | 157 ± 8 (↑ 9%) 1 | 263 ± 8 (↑ 13%) 1 | 17 ± 5 |
| Mg-3Al-0.4Ce-2ZnO | 144 ± 4 (↑ 37.1%) 1 | 173 ± 11 (↑ 20%) 1 | 288 ± 11 (↑ 24%) 1 | 18 ± 2 |
| Mg-3Al-0.4Ce-2.5ZnO | 161 ± 5 (↑ 53.3%) 1 | 180 ± 7 (↑ 25%) 1 | 294 ± 9 (↑ 27%) 1 | 15 ± 2 |
| Mg-3Al-0.2Ce [8] | - | 120 | 235 | - |
| Mg-3Al-0.5Ce [8] | - | 125 | 230 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekumalla, S.; Farhan, N.; Srivatsan, T.S.; Gupta, M. Nano-ZnO Particles’ Effect in Improving the Mechanical Response of Mg-3Al-0.4Ce Alloy. Metals 2016, 6, 276. https://doi.org/10.3390/met6110276
Tekumalla S, Farhan N, Srivatsan TS, Gupta M. Nano-ZnO Particles’ Effect in Improving the Mechanical Response of Mg-3Al-0.4Ce Alloy. Metals. 2016; 6(11):276. https://doi.org/10.3390/met6110276
Chicago/Turabian StyleTekumalla, Sravya, Najib Farhan, Tirumalai S. Srivatsan, and Manoj Gupta. 2016. "Nano-ZnO Particles’ Effect in Improving the Mechanical Response of Mg-3Al-0.4Ce Alloy" Metals 6, no. 11: 276. https://doi.org/10.3390/met6110276