Effect of BaO Addition on Densification and Mechanical Properties of Al2O3-MgO-CaO Refractories
Abstract
:1. Introduction
2. Experimental Process
3. Results and Discussion
3.1. Densification and Phase Composition
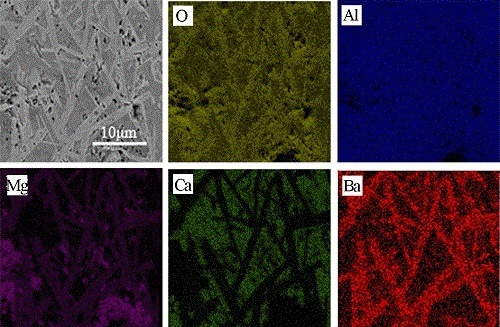
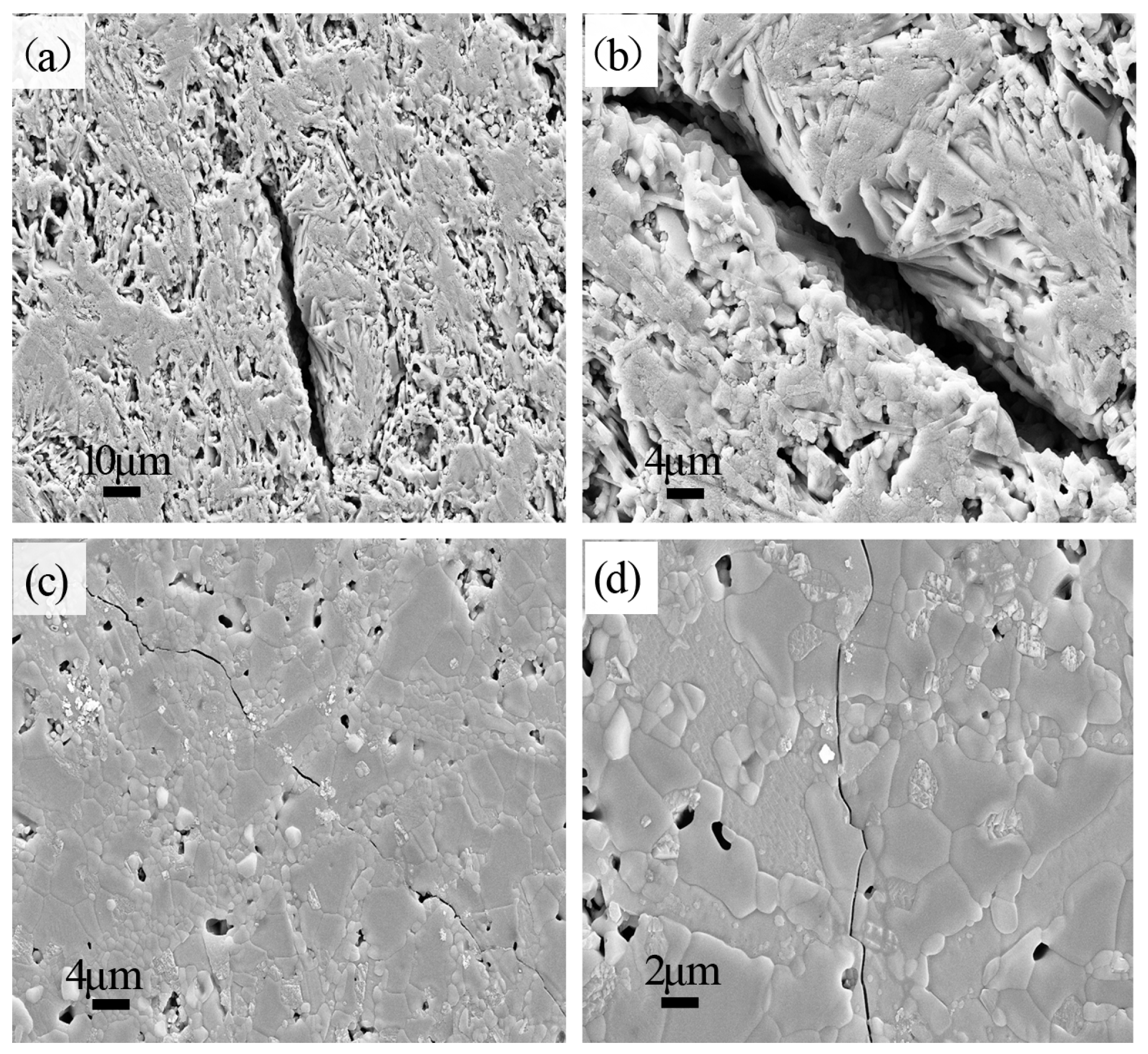
3.2. Microstructure
- (1)
- The platelet morphology of BAM shows relatively low aspect ratio and significant growth in the thickness direction, which is considered to mean higher lattice diffusion rate and faster mass transfer along the thickness direction, leading to sintering shrinkage and decrease of porosity.
- (2)
- With greater BaO addition, the amount of CA6, which is difficult to densify, decreased sharply and even disappeared when 6 wt. % BaO was added. A greater number of CA2 grains, which have better sintering ability and a low coefficient of thermal expansion, were formed. Thus, the densification of the sample was promoted.
- (3)
- With part of MA reacting with BaAl12O19 to form the new phase BAM, the amount of MA with poor sintering ability decreased and thus promoted the densification of Al2O3-MgO-CaO system materials.
3.3. Mechanical Properties
- (1)
- The BaO-containing sample was a dense and homogeneous structure, which is attributed to the decrease in size of microcracks.
- (2)
- The new phase BAM presented higher bonding strength between MA and CA2 phases, leading to the increase of thermal shock resistance.
- (3)
- The increase in the amount of CA2 with a very low coefficient of thermal expansion improved the thermal shock resistance.
4. Conclusions
- (1)
- When BaO was introduced to Al2O3-MgO-CaO system materials, the new phase BAM was formed and the amount of MA decreased slightly. The amount of CA6 decreased sharply and almost disappeared when 6 wt. % of BaO was added. Besides, more CA2 remained by inhibiting the formation of CA6.
- (2)
- With BaO content increasing, the new phase BAM with a lower aspect ratio and thicker grains substituted for CA6 that exhibits a small grain size and high aspect ratio. In addition, the crystal of CA2 formed many dense areas. All of the above structural changes efficiently promoted the densification of the Al2O3-MgO-CaO system materials, with apparent porosity dramatically decreasing from 21.2% to 5.52% when adding 6 wt. % BaO after heating at 1600 °C for 2 h.
- (3)
- Attributed to their dense and homogeneous microstructure, as well as good bonding, the cold compressive strength and flexural strength of the sintered samples were greatly enhanced from 365 MPa and 178 MPa to 569 MPa and 243 MPa, respectively. Moreover, the thermal shock resistance also improved by the addition of 6 wt. % BaO.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Aza, A.H.; Pena, P.; de Aza, S. Ternary system Al2O3-MgO-CaO: I, Primary phase field of crystallization of spinel in the subsystem MgAl2O4-CaAl4O7-CaO-MgO. J. Am. Ceram. Soc. 1999, 82, 2193–2203. [Google Scholar] [CrossRef]
- Tchamba, A.B.; Elimbi, A.; Mbessa, M.; Melo, U.C.; Nzegge, O.M. Thermoelastic properties evolution and damping phenomena of Cameroonian calcined bauxite stabilized with calcium dialuminate refractory cement. Ceram. Int. 2015, 41, 53–59. [Google Scholar] [CrossRef]
- Altay, A.; Carter, C.B.; Arslan, I.; Gülgün, M.A. Crystallization of CaAl4O7 and CaAl12O19 powders. Philos. Mag. 2009, 89, 605–621. [Google Scholar] [CrossRef]
- Naghizadeh, R.; Rezaie, H.R.; Golestani-Fard, F. Effect of TiO2 on phase evolution and microstructure of MgAl2O4 spinel in different atmospheres. Ceram. Int. 2011, 37, 349–354. [Google Scholar] [CrossRef]
- Sarkar, R.; Das, S.K.; Banerjee, G. Effect of addition of Cr2O3 on the properties of reaction sintered MgO-Al2O3 spinels. J. Eur. Ceram. Soc. 2002, 22, 1243–1250. [Google Scholar] [CrossRef]
- Chen, M.; Wang, N.; Liu, W. Preparation and properties of alumina-magnesia precast block for ladle lining. Mater. Lett. 2007, 61, 3388–3390. [Google Scholar] [CrossRef]
- Xu, L.; Chen, M.; Huang, W.J.; Wang, N.; Dong, J.H.; Yin, X.-L. Effects of CaO content on sintering and lightweight of Al2O3-MgO-CaO refractories. Mater. Res. Innov. 2015, 19, 212–217. [Google Scholar] [CrossRef]
- Dominguez, C.; Chevalier, J.; Torrecillas, R.; Fantozzi, G. Microstructure development in calcium hexaluminate. J. Eur. Ceram. Soc. 2001, 21, 381–387. [Google Scholar] [CrossRef]
- Asmi, D.; Low, I.M. Physical and mechanical characteristics of in-situ alumina/calcium hexaluminate composites. J. Mater. Sci. Lett. 1998, 17, 1735–1738. [Google Scholar] [CrossRef]
- Dominguez, C.; Torrecillas, R. Influence of Fe3+ in the sintering and microstructural evolution of reaction sintered calcium hexaluminate. J. Eur. Ceram. Soc. 1998, 18, 1373–1379. [Google Scholar] [CrossRef]
- Luz, A.P.; Braulio, M.A.L.; Martinez, A.G.T.; Pandolfelli, V.C. Slag attack evaluation of in situ spinel-containing refractory castables via experimental tests and thermodynamic simulations. Ceram. Int. 2012, 38, 1497–1505. [Google Scholar] [CrossRef]
- Braulio, M.A.L.; Martinez, A.G.T.; Luz, A.P.; Liebske, C.; Pandolfelli, V.C. Basic slag attack of spinel-containing refractory castables. Ceram. Int. 2011, 37, 1935–1945. [Google Scholar] [CrossRef]
- Göbbels, M.; Kimura, S.; Woermann, E. The aluminum-rich part of the system BaO-Al2O3-MgO-I: Phase relationships. J. Solid State Chem. 1998, 136, 253–257. [Google Scholar] [CrossRef]
- Göbbels, M.; Kimura, S.; Woermann, E. The aluminum-rich part of the system BaO-Al2O3-MgO-II: Crystal structure of the β-alumina-related compound, Ba2Mg6Al28O50. J. Solid State Chem. 1998, 136, 258–262. [Google Scholar] [CrossRef]
- Sako, E.Y.; Braulio, M.A.L.; Zinngrebe, E.; van der Laan, S.R.; Pandolfelli, V.C. In-depth microstructural evolution analyses of cement-bonded spinel refractory castables: Novel insights regarding spinel and CA6 formation. J. Am. Ceram. Soc. 2012, 95, 1732–1740. [Google Scholar] [CrossRef]
- Braulio, M.A.L.; Pandolfelli, V.C. Tailoring the Microstructure of Cement-Bonded Alumina-Magnesia Refractory Castables. J. Am. Ceram. Soc. 2010, 93, 2981–2985. [Google Scholar] [CrossRef]
- Asmi, D.; Low, I.M. Self-reinforced Ca-hexaluminate/alumina composites with graded microstructure. Ceram. Int. 2008, 34, 311–316. [Google Scholar]
- Auvray, J.M.; Gault, C.; Huger, M. Microstructural changes and evolutions of elastic properties versus temperature of alumina and alumina-magnesia refractory castables. J. Eur. Ceram. Soc. 2008, 28, 1953–1960. [Google Scholar] [CrossRef]
- Luz, A.P.; Martinez, A.G.T.; Braulio, M.A.L.; Pandolfelli, V.C. Thermodynamic evaluation of spinel containing refractory castables corrosion by secondary metallurgy slag. Ceram. Int. 2011, 37, 1191–1201. [Google Scholar] [CrossRef]
- Dominguez, C.; Chevalier, J.; Torrecillas, R.; Gremillard, L.; Fantozzi, G. Thermomechanical properties and fracture mechanisms of calcium hexaluminate. J. Eur. Ceram. Soc. 2001, 21, 907–917. [Google Scholar] [CrossRef]
- Díaz, L.A.; Torrecillas, R.; de Aza, A.H.; Pena, P. Effect of spinel content on slag attack resistance of high alumina refractory castables. J. Eur. Ceram. Soc. 2007, 27, 4623–4631. [Google Scholar] [CrossRef]
- Gehre, P.; Aneziris, C.G.; Berek, H.; Parr, C.; Reinmöller, M. Corrosion of magnesium aluminate spinel-rich refractories by sulphur-containing slag. J. Eur. Ceram. Soc. 2015, 35, 1613–1620. [Google Scholar] [CrossRef]
- Xu, L.; Chen, M.; Jin, L.Y.; Wang, N.; Yin, X.-L.; Liu, L. Effect of ZrO2 addition on densification and mechanical properties of MgAl2O4-CaAl4O7-CaAl12O19 composite. J. Am. Ceram. Soc. 2015, 98, 4117–4123. [Google Scholar] [CrossRef]
- Sánchez-Herencia, A.J.; Moreno, R.; Baudín, C. Fracture behaviour of alumina-calcium hexaluminate composites obtained by colloidal processing. J. Eur. Ceram. Soc. 2000, 20, 2575–2583. [Google Scholar] [CrossRef]
- Chen, M.; Wang, N.; Yu, J.K.; Yamaguchi, A. Effect of porosity on carbonation and hydration resistance of CaO materials. J. Eur. Ceram. Soc. 2007, 27, 1953–1959. [Google Scholar] [CrossRef]
- Cinibulk, M.K. Hexaluminates as a cleavable fiber-matrix interphase: Synthesis, texture development, and phase compatibility. J. Eur. Ceram. Soc. 2000, 20, 569–582. [Google Scholar] [CrossRef]
- Arai, H.; Eguchi, K.; Machida, M. Cation Substituted Layered Hexaluminates for a High-Temperature Combustion Catalyst. In Proceedings of the MRS International Meeting on Advanced Materials, Tokyo, Japan, 31 May–3 June 1988.
- Nugroho, S.; Chen, Z.C.; Kawasakia, A.; Jarligo, M.O.D. Solid-state synthesis and formation mechanism of barium hexaaluminate from mechanically activated Al2O3-BaCO3 powder mixtures. J. Alloy. Compd. 2010, 502, 466–471. [Google Scholar] [CrossRef]






| Batch | Al2O3 | MgO | CaO | BaO |
|---|---|---|---|---|
| MB0 | 82 | 10 | 8 | 0 |
| MB2 | 82 | 10 | 8 | 2 |
| MB4 | 82 | 10 | 8 | 4 |
| MB6 | 82 | 10 | 8 | 6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Chen, M.; Xu, L.; Yin, X.; Sun, W. Effect of BaO Addition on Densification and Mechanical Properties of Al2O3-MgO-CaO Refractories. Metals 2016, 6, 84. https://doi.org/10.3390/met6040084
Liu L, Chen M, Xu L, Yin X, Sun W. Effect of BaO Addition on Densification and Mechanical Properties of Al2O3-MgO-CaO Refractories. Metals. 2016; 6(4):84. https://doi.org/10.3390/met6040084
Chicago/Turabian StyleLiu, Lei, Min Chen, Lei Xu, Xueliang Yin, and Wenjie Sun. 2016. "Effect of BaO Addition on Densification and Mechanical Properties of Al2O3-MgO-CaO Refractories" Metals 6, no. 4: 84. https://doi.org/10.3390/met6040084
APA StyleLiu, L., Chen, M., Xu, L., Yin, X., & Sun, W. (2016). Effect of BaO Addition on Densification and Mechanical Properties of Al2O3-MgO-CaO Refractories. Metals, 6(4), 84. https://doi.org/10.3390/met6040084