Vanadium Effect on a Medium Carbon Forging Steel
Abstract
:1. Introduction
2. Materials and Methods
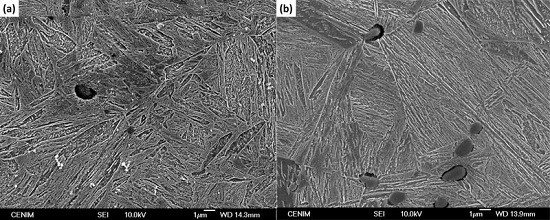
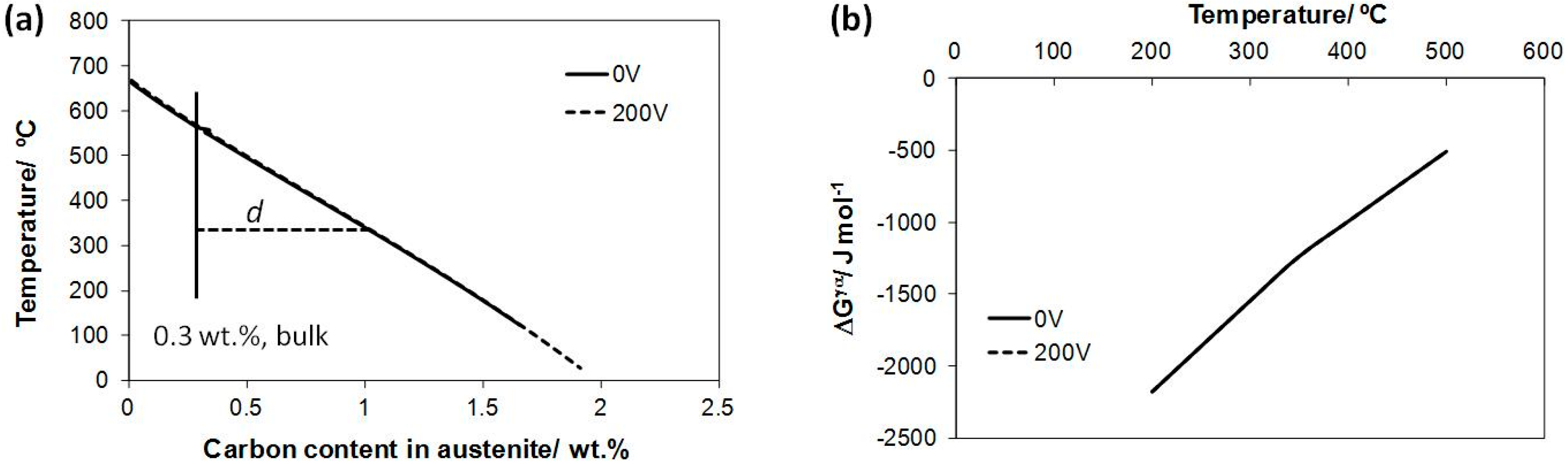
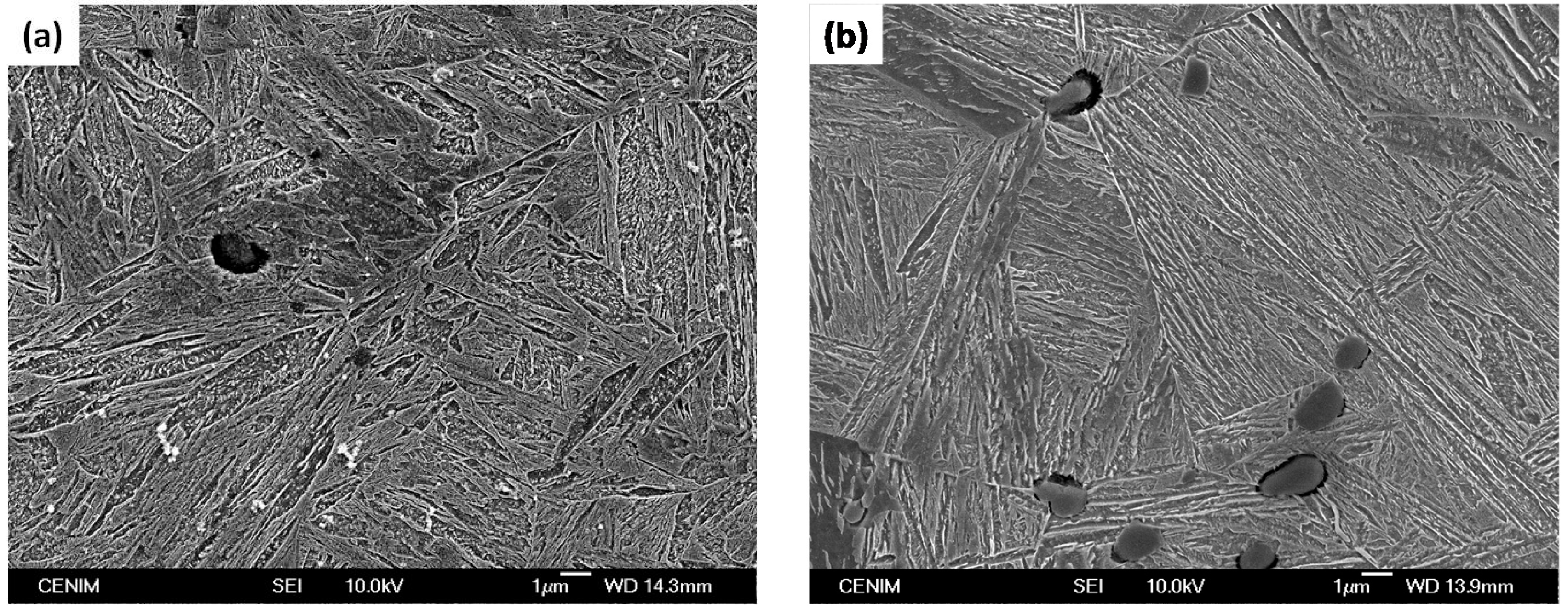
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gladman, T. The Physical Metallurgy of Microalloyed Steels; Institute of Materials: London, UK, 1997. [Google Scholar]
- Bhadeshia, H.K.D.H. Bainite in Steels: Theory and Practice, 3rd ed.; Maney Publishing: Leeds, UK, 2015; p. 616. [Google Scholar]
- Bhadeshia, H.K.D.H.; Honeycombe, R.W.K. Steels: Microstructure and Properties; Butterworths-Heinemann (Elsevier): Oxford, UK, 2006. [Google Scholar]
- Takahashi, M.; Bhadeshia, H.K.D.H. Model for transition from upper to lower bainite. Mater. Sci. Technol. 1990, 6, 592–603. [Google Scholar] [CrossRef]
- Takahashi, M.; Bhadeshia, H.K.D.H. A model for the microstructure of some advanced bainitic steels. Mater. Trans. JIM 1991, 32, 689–696. [Google Scholar] [CrossRef]
- Gomez, G.; Perez, T.; Bhadeshia, H.K.D.H. Air cooled bainitic steels for strong, seamless pipes part 2—Properties and microstructure of rolled material. Mater. Sci. Technol. 2009, 25, 1508–1512. [Google Scholar] [CrossRef]
- Gomez, G.; Perez, T.; Bhadeshia, H.K.D.H. Air cooled bainitic steels for strong, seamless pipes part 1—Alloy design, kinetics and microstructure. Mater. Sci. Technol. 2009, 25, 1501–1507. [Google Scholar] [CrossRef]
- Sugimoto, K.-I.; Yoshikawa, N. Advanced high-strength trip-aided steels for ultra high pressure DI-diesel engine common rail. In 3rd International SCT Conference Steels for Cars and Trucks SCT2011; Wieland, H.J., TEMA Technologie Marketing AG, Eds.; Stahleisen: Salzburg, Austria, 2011; pp. 460–467. [Google Scholar]
- Sourmail, T.; Michaud, H.; d’Eramo, E.; Baudry, G. Microalloyed bainitic steels for high performance forged mechanical components. In 2nd International Conference Super-High Strength Steels; AIM, Ed.; Associazione Italiana di Metallurgia: Verona, Italy, 2010. [Google Scholar]
- Hasler, S.; Roelofs, H.; Lembke, M.; Caballero, F.G. New air cooled steels with outstanding impact toughness. In 3rd International SCT Conference Steels for Cars and Trucks SCT2011; Wieland, H.J., TEMA Technologie Marketing AG, Eds.; Stahleisen: Salzburg, Austria, 2011; pp. 330–337. [Google Scholar]
- Woodhead, J.H. The physical metallurgy of vanadium steels. In Proc. Semin. Vanadium‘79; Vanitec: Chicago, IL, USA, 1979; pp. 3–10. [Google Scholar]
- Grange, R.A. Estimating the hardenability of carbon steels. Metall. Trans. 1973, 4, 2231–2244. [Google Scholar] [CrossRef]
- Adrian, H. A mechanism for effect of vanadium on hardenability of medium carbon manganese steel. Mater. Sci. Technol. 1999, 15, 366–378. [Google Scholar] [CrossRef]
- Gol’dshtein, M.I.; Guterman, S.G. Effect of small amounts of vanadium on the hardenability of structural carbon steel. Met. Sci. Heat Treat. 1965, 6, 440–442. [Google Scholar] [CrossRef]
- Garbarz, B.; Pickering, F.B. Effect of austenite grain boundary mobility on hardenability of steels containing vanadium. Mater. Sci. Technol. 1988, 4, 967–975. [Google Scholar] [CrossRef]
- Garbarz, B.; Pickering, F.B. Effect of vanadium and austenitising temperature on hardenability of (0.2–0.3)c–1.6 mn steels with and without additions of titanium, aluminium, and molybdenum. Mater. Sci. Technol. 1988, 4, 117–126. [Google Scholar] [CrossRef]
- Tsunekage, N.; Kobayashi, K.; Tsubakino, H. Influence of sulphur and vanadium additions on toughness of bainitic steels. Mater. Sci. Technol. 2001, 17, 847–855. [Google Scholar] [CrossRef]
- Kim, K.S.; Du, L.X.; Gao, C.R. Influence of vanadium content on bainitic transformation of a low-carbon boron steel during continuous cooling. Acta Metall. Sin. 2015, 28, 692–698. [Google Scholar] [CrossRef]
- He, K.; Edmonds, D.V. Formation of acicular ferrite and influence of vanadium alloying. Mater. Sci. Technol. 2002, 18, 289–296. [Google Scholar] [CrossRef]
- Siwecki, T.; Eliasson, J.; Lagneborg, R.; Hutchinson, B. Vanadium microalloyed bainitic hot strip steels. ISIJ Int. 2010, 50, 760–767. [Google Scholar] [CrossRef]
- Li, Y.; Milbourn, D. Vanadium in bainitic steels: A review of recent developments. In Advanced Steels: The Recent Scenario in Steel Science and Technology; Weng, Y., Dong, H., Gan, Y., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2011; pp. 303–308. [Google Scholar]
- Hutchinson, B.; Siwecki, T.; Komenda, J.; Hagström, J.; Lagneborg, R.; Hedin, J.E.; Gladh, M. New vanadium-microalloyed bainitic 700 MPa strip steel product. Ironmak. Steelmak. 2014, 41, 1–6. [Google Scholar] [CrossRef]
- San Martín, D.; Palizdar, Y.; Cochrane, R.C.; Brydson, R.; Scott, A.J. Application of nomarski differential interference contrast microscopy to highlight the prior austenite grain boundaries revealed by thermal etching. Mater. Charact. 2010, 61, 584–588. [Google Scholar] [CrossRef] [Green Version]
- ASTM E975-13. Standard Practice for X-ray Determination of Retained Austenite in Steel with Near Random Crystallographic Orientation; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Dickson, M.J. Significance of texture parameters in phase analysis by X-ray diffraction. J. Appl. Crystallogr. 1969, 2, 176–180. [Google Scholar] [CrossRef]
- Mtdata, version 4.73; National Physical Laboratory: Teddington, UK, 2003.
- Cornide, J.; Garcia-Mateo, C.; Capdevila, C.; Caballero, F.G. An assessment of the contributing factors to the nanoscale structural refinement of advanced bainitic steels. J. Alloy. Compd. 2013, 577, S43–S47. [Google Scholar] [CrossRef]
- Staśko, R.; Adrian, H.; Adrian, A. Effect of nitrogen and vanadium on austenite grain growth kinetics of a low alloy steel. Mater. Charact. 2006, 56, 340–347. [Google Scholar] [CrossRef]
- Glodowski, R.J. A review of vanadium microalloying in hot rolled steel sheet products. In International Seminar 2005 on Application Technologies of Vanadium in Flat—Rolled Steels; Vanitec: Suzhou, China, 2005; pp. 43–51. [Google Scholar]
- Garcia-Mateo, C.; Lopez, B.; Rodriguez-Ibabe, J. Static recrystallization kinetics in warm worked vanadium microalloyed steels. Mater. Sci. Eng. A 2001, 303, 216–225. [Google Scholar] [CrossRef]
- Lagneborg, R.; Siwecki, T.; Zajac, S.; Hutchinson, B. Role of vanadium in microalloyed steels. Scand. J. Metall. 1999, 28, 186–241. [Google Scholar]
- Garcia-Mateo, C.; Caballero, F.G. Advanced high strength bainitic steels. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Tyne, C.J.V., Yilbas, B., Eds.; Elsevier Ltd.: Oxford, UK, 2014; Volume 1, pp. 165–190. [Google Scholar]
- Mittemeijer, E.J. Fundamentals of Materials Science: The Microstructure–Property Relationship Using Metals as Model Systems; Springer Berlin Heidelberg: Berlin, Germany, 2010. [Google Scholar]
- Takahashi, M.; Bhadeshia, H.K.D.H. The interpretation of dilatometric data for transformations in steels. J. Mater. Sci. Lett. 1989, 8, 477–478. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.; Bhadeshia, H. Development of hard bainite. ISIJ Int. 2003, 43, 1238–1243. [Google Scholar] [CrossRef]
- Matsuzaki, A.; Bhadeshia, H.K.D.H. Effect of austenite grain size and bainite morphology on overall kinetics of bainite transformation in steels. Mater. Sci. Technol. 1999, 15, 518–522. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.; Bhadeshia, H. Acceleration of low-temperature bainite. ISIJ Int. 2003, 43, 1821–1825. [Google Scholar] [CrossRef]
- Barford, J.; Owen, W.S. The effect of austenite grain size and temperature on the rate of bainite transformation. Met. Sci. Heat Treat. Met. 1961, 4, 359–360. [Google Scholar]
- Umemoto, M.; Horiuchi, K.; Tamura, I. Transformation kinetics of bainite during isothermal holding and continuous cooling. Trans. Iron Steel Inst. Jpn. 1982, 22, 854–861. [Google Scholar] [CrossRef]
- Hu, F.; Hodgson, P.D.; Wu, K.M. Acceleration of the super bainite transformation through a coarse austenite grain size. Mater. Lett. 2014, 122, 240–243. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Capdevila, C.; Caballero, F.G.; de Andres, C.G. Influence of V precipitates on acicular ferrite transformation part 1: The role of nitrogen. ISIJ Int. 2008, 48, 1270–1275. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Cornide, J.; Capidevila, C.; Caballero, F.G.; de Andres, C.G. Influence of V precipitates on acicular ferrite transformation part 2: Transformation kinetics. ISIJ Int. 2008, 48, 1276–1279. [Google Scholar] [CrossRef]







| Alloy | C | Si | Mn | Cr | Mo | V | Ti | B | N |
|---|---|---|---|---|---|---|---|---|---|
| 0 V | 0.24 | 0.70 | 1.53 | 0.78 | 0.08 | 0.01 | 0.025 | 30 ppm | 80 ppm |
| 200 V | 0.24 | 0.70 | 1.58 | 0.79 | 0.08 | 0.21 | 0.025 | 30 ppm | 80 ppm |
| Alloy | HV30 | Vγ (%) | PAGS (μm) |
|---|---|---|---|
| 0 V | 346 | 15 ± 3 | 12 ± 2 |
| 200 V | 344 | 15 ± 3 | 22 ± 3 |
| Alloy | Theoretical | Experimental | V Precipitate | ||
|---|---|---|---|---|---|
| Bs (°C) | Ms (°C) | Bs (°C) | ppm | % | |
| 0 V | 522 | 369 | 533 | 0 | 0 |
| 200 V | 514 | 361 | 515 | 7 | 1 |
| Alloy | 375 °C | 400 °C | 425 °C |
|---|---|---|---|
| 0 V | 79 | 118 | 168 |
| 200 V | 89 | 99 | 135 |
| Isothermal Temperature (°C) | 0 V | 200 V | ||
|---|---|---|---|---|
| Martensite (%) | HV | Martensite (%) | HV | |
| 375 °C | 0 | 439 | 0 | 451 |
| 400 °C | 5 | 379 | 5 | 438 |
| 425 °C | 10 | 380 | 15 | 400 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Mateo, C.; Morales-Rivas, L.; Caballero, F.G.; Milbourn, D.; Sourmail, T. Vanadium Effect on a Medium Carbon Forging Steel. Metals 2016, 6, 130. https://doi.org/10.3390/met6060130
Garcia-Mateo C, Morales-Rivas L, Caballero FG, Milbourn D, Sourmail T. Vanadium Effect on a Medium Carbon Forging Steel. Metals. 2016; 6(6):130. https://doi.org/10.3390/met6060130
Chicago/Turabian StyleGarcia-Mateo, Carlos, Lucia Morales-Rivas, Francisca G. Caballero, David Milbourn, and Thomas Sourmail. 2016. "Vanadium Effect on a Medium Carbon Forging Steel" Metals 6, no. 6: 130. https://doi.org/10.3390/met6060130