Melt Protection of Mg-Al Based Alloys
Abstract
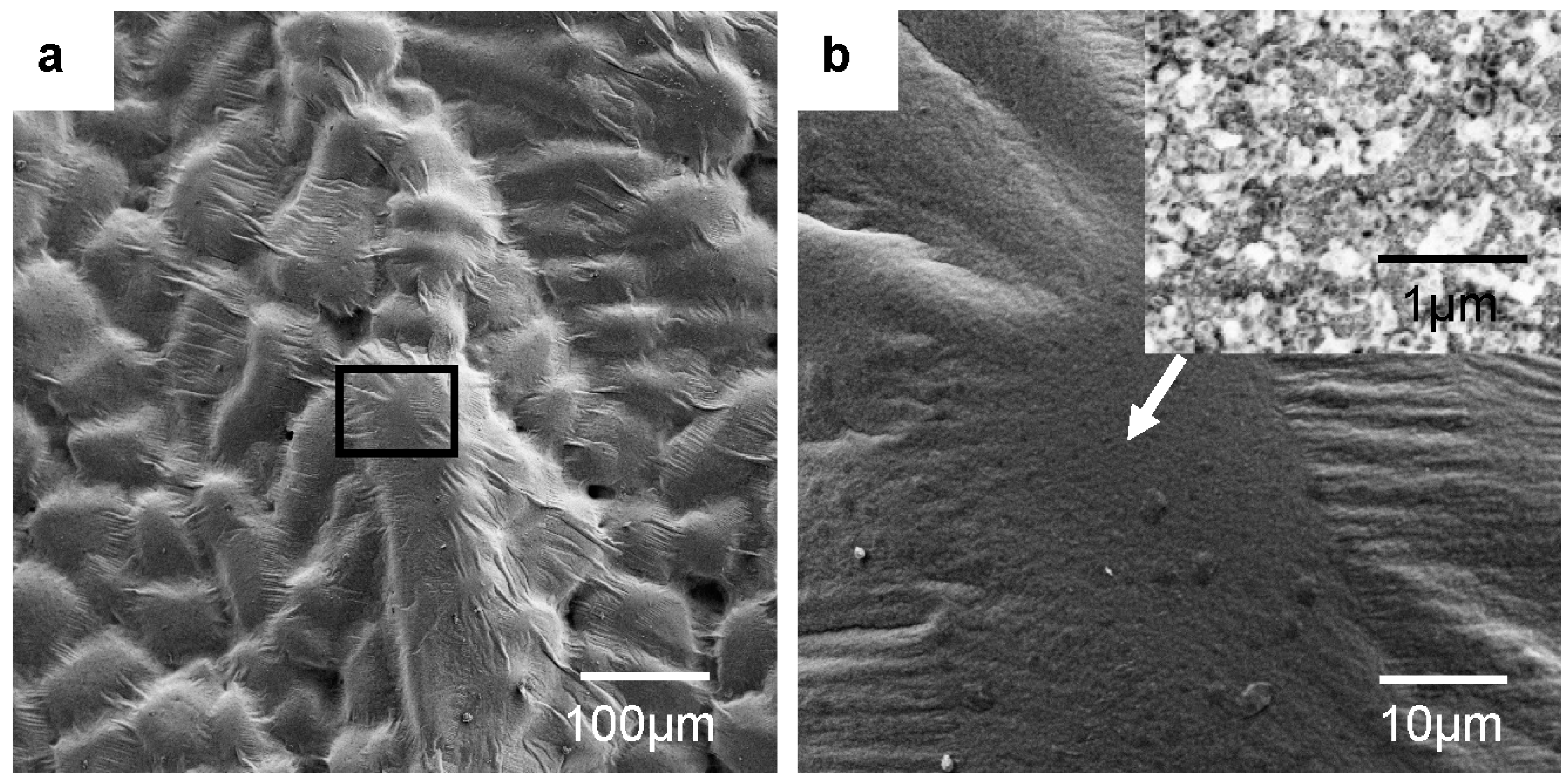
:1. Introduction
2. Reactive Element Effect
2.1. Ca Additions
2.2. Be Additions
2.3. Sr Additions
2.4. Ti Additions
3. Flux Additions
4. Alternatives to SF6 for Mg Melt Protection
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Emley, E.F. Principles of Magnesium Technology; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Kim, S.K.; Lee, J.K.; Yoon, Y.O.; Jo, H.H. Development of AZ31 Mg alloy wrought process route without protective gas. J. Mater. Process. Technol. 2007, 187–188, 757–760. [Google Scholar] [CrossRef]
- Foerster, G. HiLoN: A new approach to magnesium die casting. Adv. Mater. Process. 1998, 154, 79. [Google Scholar]
- Kim, S.K.; Hoseo, J. Magnesium-Based Alloy with Superior Fluidity and Hot-Tearing Resistance and Manufacturing Method Thereof. US Patent 20110236249 A1, 29 September 2011. [Google Scholar]
- Dervos, C.T.; Vassiliou, P. Sulfur hexafluoride (SF6): Global environmental effects and toxic byproduct formation. J. Air Waste Manag. Assoc. 2000, 50, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Aghion, E.; Gurion, B.; Bartos, S.C. Comparative Review of Primary Magnesium Production Technologies as Related to Global climate Change. In Proceedings of the 65th Annual World Magnesium Conference, Warsaw, Poland, 18–20 May 2008; Available online: http://www.citysat.com.pl/~omen/2008_World_Mg_Conference_Papers (accessed on 17 January 2016).
- Cashion, S.P.; Ricketts, N.J.; Hayes, P.C. Characterisation of protective surface films formed on molten magnesium protected by air/SF6 atmospheres. J. Light Metals 2002, 2, 37–42. [Google Scholar] [CrossRef]
- Cashion, S.P.; Ricketts, N.J.; Hayes, P.C. The mechanism of protection of molten magnesium by cover gas mixtures containing sulphur hexafluoride. J. Light Metals 2002, 2, 43–47. [Google Scholar] [CrossRef]
- Aarstad, K. Protective Films on Molten Magnesium. Ph.D. Thesis, Norwegian University of Science and Technology, Norway, May 2004. Available online: http://www.diva-portal.org/smash/get/diva2:126206/FULLTEXT01.pdf (accessed on 19 March 2016). [Google Scholar]
- Aarstad, K.; Tranell, G.; Engh, T.A. Various Techniques to Study the Surface of Magnesium Protected by SF6. Magnes. Technol. 2003, 5–10. Available online: http://www.academia.edu/24051179/Various_techniques_to_study_the_surface_of_magnesium_protected_by_SF6 (accessed on 26 May 2016). [Google Scholar]
- Xiong, S.M.; Liu, X.L. Microstructure, composition, and depth analysis of surface films formed on molten AZ91D alloy under protection of SF6 mixtures. Metall. Mater. Trans. A: Phys. Metall. Mater. Sci. 2007, 38A, 428–434. [Google Scholar] [CrossRef]
- Wang, X.F.; Xiong, S.M. Oxidation behavior of molten magnesium in atmospheres containing SO2. Corros. Sci. 2011, 53, 4050–4057. [Google Scholar] [CrossRef]
- Pettersen, G.; Øvrelid, E.; Tranell, G.; Fenstad, J.; Gjestland, H. Characterisation of the surface films formed on molten magnesium in different protective atmospheres. Mater. Sci. Eng. A 2002, 332, 285–294. [Google Scholar] [CrossRef]
- Liu, J.R.; Chen, H.K.; Zhao, L.; Huang, W.D. Oxidation behaviour of molten magnesium and AZ91D magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres. Corros. Sci. 2009, 51, 129–134. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Huang, W. The protective surface film formed on molten ZK60 magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres. Corros. Sci. 2010, 52, 3639–3645. [Google Scholar] [CrossRef]
- Mirak, A.; Davidson, C.J.; Taylor, J.A. Characterisation of fresh surface oxidation films formed on pure molten magnesium in different atmospheres. Corros. Sci. 2010, 52, 1992–2000. [Google Scholar] [CrossRef]
- Chen, H. Effect of melt temperature on the oxidation behaviour of AZ91D magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres. Mater. Charact. 2010, 61, 894–898. [Google Scholar] [CrossRef]
- Ha, W.; Kim, Y.J. Effects of cover gases on melt protection of Mg alloys. J. Alloy. Compd. 2006, 422, 208–213. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, J.R.; Chen, H.K.; Huang, W.D. The characterization of surface films formed on molten magnesium and AZ91D alloy in air/1,1,1,2-tetrafluoroethane atmospheres. J. Alloy. Compd. 2009, 480, 711–716. [Google Scholar] [CrossRef]
- Milbrath, D.S. Development of 3MTMNovecTM 612 Magnesium Protection Fluid as a Substitute for SF6 over Molten Magnesium. In Proceedings of the 2nd International Conference on SF6 and the Environment, San Diego, CA, USA, 22 November 2002; Available online: https://www.epa.gov/sites/production/files/2016-02/documents/conf02_milbrath_paper.pdf (accessed on 20 May 2016).
- Emami, S.; Sohn, H.Y.; Kim, H.G. Formation and evaluation of protective layer over magnesium melt under SF6/Air atmospheres. Metall. Mater. Trans. B 2014, 45, 1370–1379. [Google Scholar] [CrossRef]
- Regulation (EU) No 517/2014 of the European Parliament and of the Council of 16 April 2014 of fluorinated greenhouse gases and repealing.
- Wiese, B.; Mendis, C.L.; Ovri, H.; Reichel, H.-P.; Lorenz, U.; Kainer, K.U.; Hort, N. Role of CaO and Cover Gases on Protecting the Cast Surface of Mg. In Proceedings of the 10th International Conference on Magnesium Alloys and Their Applications, Jeju, Korea, 11–16 October 2015; pp. 814–819.
- Dishon, A. Financial Benefits from SF6 Emission Reductions. In Proceedings of the 65th Annual World Magnesium Conference, Warsaw, Poland, 18–20 May 2008; Available online: http://www.citysat.com.pl/~omen/2008_World_Mg_Conference_Papers (accessed on 17 January 2016).
- Alternatives to SF6 for Magnesium Melt Protection. Available online: https://www.epa.gov/sites/production/files/2016-02/documents/magbrochure_english.pdf (accessed on 20 May 2016).
- Hillis, J.E. The International Program to Identify Alternatives to SF6 for Magnesium Melt Protection. In Proceedings of the International Conference on SF6 and the Environment: Emission Reduction Strategies, San Diego, CA, USA, 21–22 November 2002; Available online: https://www.epa.gov/sites/production/files/2016-02/documents/conf02_hillis_paper.pdf (accessed on 20 May 2016).
- Czerwinski, F. The reactive element effect on high-temperature oxidation of magnesium. Int. Mater. Rev. 2015, 60, 264–296. [Google Scholar] [CrossRef]
- Pfeil, L.B. Improvements relating to hear-resisting alloys containing chromium. UK Patent No. 574088, 20 December 1945. [Google Scholar]
- Beranger, G.; Armanet, F.; Lambertin, M. Active Elements in Oxidation and Their Properties. In The Role of Active Elements in the Oxidation Behaviour of High Temperature Metals and Alloys, Proceedings of the European Colloquium Organised by: Commission of the European Communities, Directorate General: Science, Research and Development, Petten, The Netherlands, 12–13 December 1988; Lang, E., Ed.; Elsevier Applied Science: London, UK; New York, NY, USA, 1988; pp. 33–51. [Google Scholar]
- Czerwinski, F. Oxidation characteristics of magnesium alloys. JOM 2012, 64, 1477–1483. [Google Scholar] [CrossRef]
- Aydin, D.S.; Bayindir, Z.; Hoseini, M.; Pekguleryuz, M.O. The high temperature oxidation and ignition behaviour of Mg-Nd alloys part I: The oxidation of dilute alloys. J. Alloy. Compd. 2013, 569, 35–44. [Google Scholar] [CrossRef]
- Pint, B.A. Experimental observations in support of the dynamic-segregation theory to explain the reactive-element effect. Oxid. Metals 1996, 45, 1–37. [Google Scholar] [CrossRef]
- Ellingham, H.J.T. Reducibility of oxides and sulfides in metallurgical processes. J. Soc. Chem. Ind. (Lond.) 1944, 63, 125–133. [Google Scholar]
- Howard, S.M. Ellingham Diagrams, Internet Resource for MET 320—Metallurgical Thermodynamics, South Dakota School of Mines and Technology, Rapid City, SD, USA. Available online: http://showard.sdsmt.edu/MET320/Handouts/EllinghamDiagrams/Ellingham_v22_Macro.pdf (accessed on 12 May 2016).
- Sakamoto, M.; Akiyama, S.; Ogi, K. Suppression of ignition and burning of molten Mg alloys by Ca bearing stable film. J. Mater. Sci. Lett. 1997, 16, 1048–1050. [Google Scholar] [CrossRef]
- Nayeb-Hashemi, A.A.; Clark, J.B. The Ca-Mg (Calcium-Magnesium) system. Bull. Alloy Phase Diagr. 1987, 8, 58–65. [Google Scholar] [CrossRef]
- Wiese, B.; Mendis, C.L.; Tolnai, D.; Stark, A.; Schell, N.; Reichel, H.-P.; Brückner, R.; Kainer, K.U.; Hort, N. CaO dissolution during melting and solidification of a Mg–10 wt.% CaO alloy detected with in situ synchrotron radiation diffraction. J. Alloy. Compd. 2015, 618, 64–66. [Google Scholar] [CrossRef]
- Wiese, B.; Tolnai, D.; Mendis, C.L.; Eckerlebe, H.; Hort, N. In situ Diffraction of the Melting and the Solidification of Magnesium Alloys Containing CaO. Available online: http://photon-science.desy.de/annual_report/files/2013/20132901.pdf (accessed on 14 January 2016).
- Pekguleryuz, M.O. Alloying behaviour of magnesium and alloy design. In Fundamentals of Magnesium Alloy Metallurgy; Pekguleryuz, M.O., Kainer, K.U., Kaya, A.A., Eds.; Woodhead Publishing Ltd.: Oxford, UK; Cambridge, PA, USA; New Delhi, India, 2003; p. 169. [Google Scholar]
- Friedrich, H.E.; Mordike, B.L. Magnesium Technology: Metallurgy, Design Data, Applications; Springer-Verlag: Berlin, Germany, 2006; p. 119. [Google Scholar]
- Czerwinski, F.; Zielinska-Lipiec, A. The microstructure evolution during semisolid molding of a creep-resistant Mg-5Al-2Sr alloy. Acta Mater. 2005, 53, 3433–3444. [Google Scholar] [CrossRef]
- Balart, M.J.; Fan, Z. Surface oxidation of molten AZ31, AM60B and AJ62 magnesium alloys in air. Int. J. Cast Metals Res. 2014, 27, 301–311. [Google Scholar] [CrossRef]
- Nayeb-Hashemi, A.A.; Clark, J.B. The Mg-Sr (Magnesium-Strontium) system. Bull. Alloy Phase Diagr. 1986, 7, 149–156. [Google Scholar] [CrossRef]
- Balart, M.J.; Fan, Z. Surface oxidation of molten AZ91D magnesium alloy in air. Int. J. Cast Metals Res. 2014, 27, 167–175. [Google Scholar] [CrossRef]
- Kang, H.; Kang, S.; Park, S.; Bae, D. An Interstitial Magnesium Alloy Containing Oxygen Atoms. In Proceedings of the 10th International Conference on Magnesium Alloys and Their Applications, Jeju, Korea, 11–16 October 2015.
- Payyapilly, J.J. Formation and Growth Mechanisms of a High Temperature Interfacial Layer Between Al and TiO2. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 19 November 2008; pp. 77, 79. Available online: https://vtechworks.lib.vt.edu/bitstream/handle/10919/29733/Jairaj.pdf (accessed on 19 March 2016). [Google Scholar]
- Umakoshi, Y.; Yamaguchi, M.; Sakagami, T.; Yamane, T. Oxidation resistance of intermetallic compounds Al3Ti and TiAl. J. Mater. Sci. 1989, 24, 1599–1603. [Google Scholar] [CrossRef]
- Huang, Z.; Sirong, Y. Microstructure characterization on the formation of in situ Mg2Si and MgO reinforcements in AZ91D/Flyash composites. J. Alloy. Compd. 2011, 509, 311–315. [Google Scholar] [CrossRef]
- Lun Sin, S.; Elsayed, A.; Ravindran, C. Inclusions in magnesium and its alloys: A review. Int. Mater. Rev. 2013, 58, 419–436. [Google Scholar] [CrossRef]
- Fruehling, J.W. Protective Atmospheres for Molten Magnesium. Ph.D. Thesis, The University of Michigan, Ann Arbor, MI, USA, 1970. [Google Scholar]
- Norbert, H.; Wiese, B.; Dieringa, H.; Ulrich Kainer, K. Magnesium melt protection. Mater. Sci. Forum 2015, 828–829, 78–81. [Google Scholar]
- Aarstad, K.; Syvertsen, M.; Engh, T.A. Solubility of Fluorine in Molten Magnesium. Magnes. Technol. 2002. [Google Scholar] [CrossRef]
- Shih, T.S.; Liu, J.B.; Wei, P.S. Oxide films on magnesium and magnesium alloys. Mater. Chem. Phys. 2007, 104, 497–504. [Google Scholar] [CrossRef]
- Salas, O.; Ni, H.; Jayaram, V.; Vlach, K.C.; Levi, C.G.; Mehrabian, R. Nucleation and growth of Al2O3/metal composites by oxidation of aluminium alloys. J. Mater. Res. 1991, 6, 1964–1981. [Google Scholar] [CrossRef]
- Wagner, C. Mechanism of counterdiffusion through reaction in the solid state. Z. Anorg. Allg. Chem. 1938, 236, 320–338. [Google Scholar] [CrossRef]
- Carter, R.E. Mechanism of solid state reaction between MgO and Al2O3 and MgO and Fe2O3. J. Am. Ceram. Soc. 1961, 44, 116–120. [Google Scholar] [CrossRef]
- Hesse, D.; Senz, S. Interfacial reaction mechanisms and the structure of moving heterophase boundaries during pyrochlore- and spinel-forming solid state reactions. Z. Metallkunde 2004, 95, 252–257. [Google Scholar] [CrossRef]
- Gaskell, D.R. Reaction equilibria in systems containing components in condensed solution. In Introduction to the Thermodynamics of Materials, 5th ed.; Taylor & Francis Group: New York, NY, USA; London, UK, 2012; p. 430. Available online: https://books.google.co.uk/books?id=3xfcBQAAQBAJ&pg=PA430&lpg=PA430&dq=free+energy+MgAl2O4&source=bl&ots=wGdalbSfqk&sig=bpZG2u3G_ElsbBet6uJOnfBP-oY&hl=en&sa=X&ved=0ahUKEwibgLqn5L3MAhULKx4KHWUKA-kQ6AEIKzAE#v=onepage&q=free%20energy%20MgAl2O4&f=false (accessed on 5 May 2016).
- Jafari, H.; Idris, M.H.; Ourdjini, A. High temperature oxidation of AZ91D magnesium alloy granule during in situ melting. Corros. Sci. 2011, 53, 655–663. [Google Scholar] [CrossRef]
- Czerwinski, F. The oxidation behaviour of an AZ91D magnesium alloy at high temperatures. Acta Mater. 2002, 50, 2639–2654. [Google Scholar] [CrossRef]
- Fan, Z.; Ji, S.; Fang, X.; Liu, G.; Patel, J.B.; Das, A. Development of Rheo-diecasting (RDC) Process for Production of High Integrity Components. In Proceedings of the Shape Casting: 2nd International Symposium, Orlando, FL, USA, 1 May 2007; Crepeau, P.N., Tiryakioglu, M., Campbell, J., Eds.;
- Wang, Y.; Fan, Z.; Thompson, G. Characterization of magnesium oxide and its interface with alpha Mg in Mg-Al based alloys. Philos. Mag. Lett. 2011, 91, 516–529. [Google Scholar] [CrossRef]
- Lee, Y.C.; Dahle, A.K.; StJohn, D.H. The role of solute in grain refinement of magnesium. Metall. Mater. Trans. A 2013, 31A, 2895–2906. [Google Scholar] [CrossRef]
- Fukuta, T.; Obunai, K.; Ozaki, K.; Shibata, K. Improvement of mechanical properties of injection molded AZ91D alloy by mixing carbon black as a grain refinement additive. In Proceedings of the 10th International Conference on Magnesium Alloys and Their Applications, Jeju, Korea, 11–16 October 2015; pp. 551–557.
- Emami, S. Formation and Evaluation of Protective Layer Over Magnesium Melt Under Various Gaseous Atmospheres. Ph.D. Thesis, The University of Utah, Salt Lake City, UT, USA, December 2013. Available online: http://content.lib.utah.edu/utils/getfile/collection/etd3/id/2640/filename/2637.pdf (accessed on 19 March 2016). [Google Scholar]
- Emami, S.; Sohn, H.Y. Formation and evaluation of protective layer over magnesium melt under CO2/air mixtures. Metall. Mater. Trans. B 2015, 46, 226–234. [Google Scholar] [CrossRef]
- Shih, T.S.; Chung, C.B.; Chong, K.Z. Combustion of AZ61A under different gases. Mater. Chem. Phys. 2002, 74, 66–73. [Google Scholar] [CrossRef]
- Shih, T.S.; Wang, J.H.; Chong, K.Z. Combustion of magnesium alloys in air. Mater. Chem. Phys. 2004, 85, 302–309. [Google Scholar] [CrossRef]
- Chen, H.L.; Schmid-Fetzer, R. The Mg-C phase equilibria and their thermodynamic basis. Int. J. Mater. Res. 2012, 103, 1294–1301. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balart, M.J.; Patel, J.B.; Fan, Z. Melt Protection of Mg-Al Based Alloys. Metals 2016, 6, 131. https://doi.org/10.3390/met6060131
Balart MJ, Patel JB, Fan Z. Melt Protection of Mg-Al Based Alloys. Metals. 2016; 6(6):131. https://doi.org/10.3390/met6060131
Chicago/Turabian StyleBalart, María J., Jayesh B. Patel, and Zhongyun Fan. 2016. "Melt Protection of Mg-Al Based Alloys" Metals 6, no. 6: 131. https://doi.org/10.3390/met6060131





