Preparation and Analysis of Complex Barrier Layer of Heterocyclic and Long-Chain Organosilane on Copper Alloy Surface
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Preparation of Complex Film on Copper Alloy Surface
2.3. Characterization
3. Results and Discussion
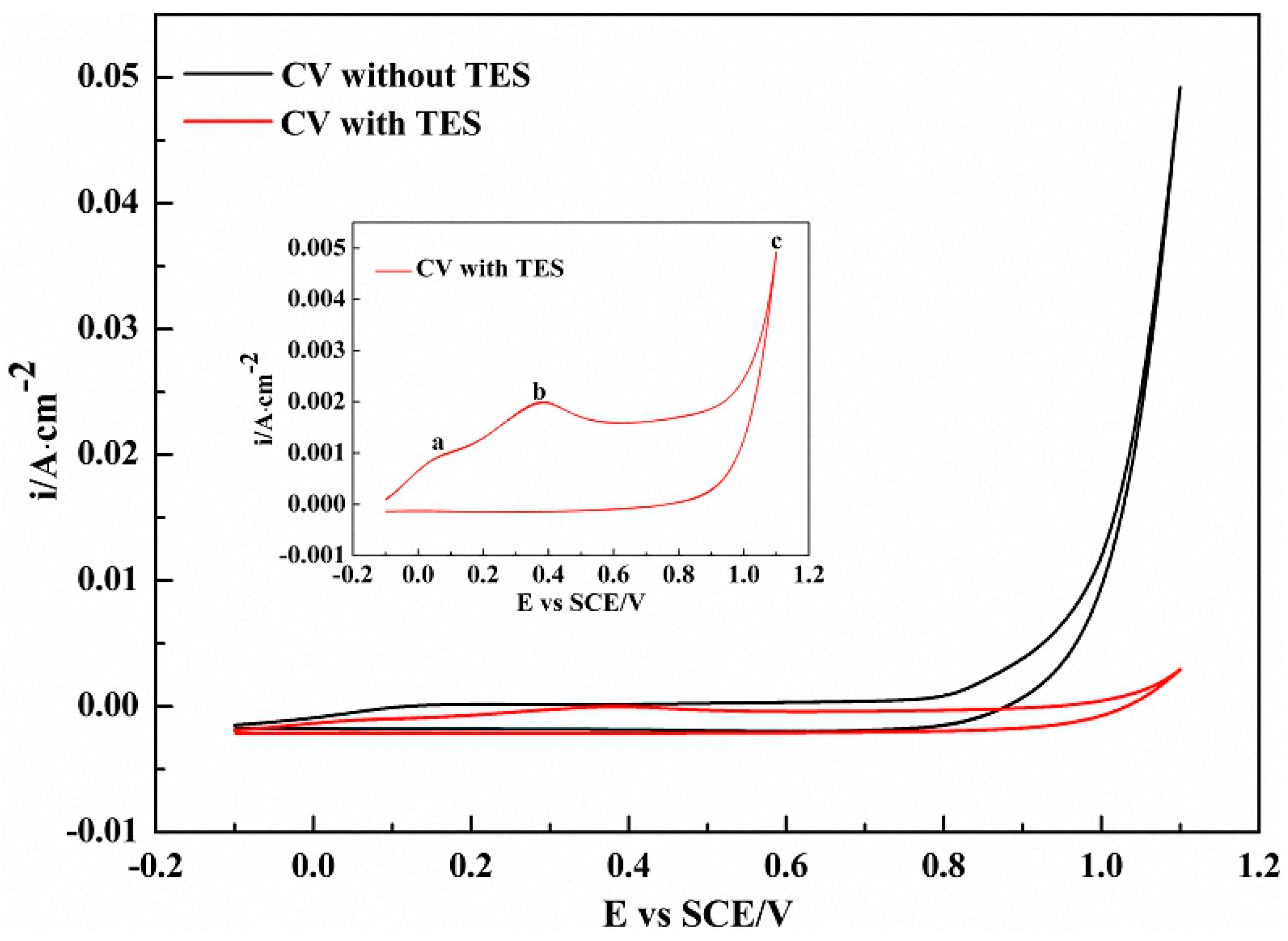
3.1. Electrodeposition of TES Monomer
3.2. FT-IR Spectra Analysis
3.3. Contact Angle
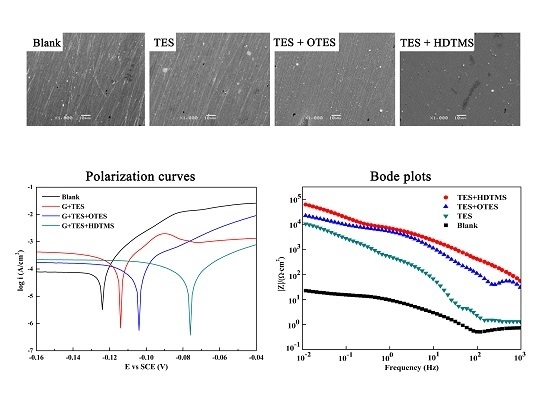
3.4. Surface Morphology
3.5. Cyclic Voltammetry Measurement for the Bare and Modified Copper Surface
3.6. Potentiodynamic Polarization
3.7. EIS
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nunez, L.; Reguera, E.; Corvo, F.; Gonzalez, E.; Vazquez, C. Corrosion of copper in seawater and its aerosols in a tropical island. Corros. Sci. 2005, 47, 461–484. [Google Scholar] [CrossRef]
- Gopi, D.; Govindaraju, K.M.; Prakash, V.C.A.; Sakila, D.M.A.; Kavitha, L. A study on new benzotriazole derivatives as inhibitors on copper corrosion in ground water. Corros. Sci. 2009, 51, 2259–2265. [Google Scholar] [CrossRef]
- Meysam, S.; Abdollah, O.; Abbas, A.R.; Maryam, K. Electrodeposition and characterization of polypyrrole films on copper. J. Electroanal. Chem. 2010, 645, 149–158. [Google Scholar]
- Zhang, Z.; Chen, S.H.; Li, Y.H.; Li, S.H.; Wang, L.H. A study of the inhibition of iron corrosion by imidazole and its derivatives self-assembled films. Corros. Sci. 2009, 51, 291–300. [Google Scholar] [CrossRef]
- Sherif, E.M.; Park, S.M. Inhibition of copper corrosion in 3.0% NaCl solution by N-Phenyl-1,4-phenylenediamine. J. Electrochem. Soc. 2005, 152, B428–B433. [Google Scholar] [CrossRef]
- Ismail, K.M. Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions. Electrochim. Acta 2007, 52, 7811–7819. [Google Scholar] [CrossRef]
- Li, W.H.; Zhao, X.; Liu, F.Q.; Hou, B. Investigation on inhibition behavior of S-triazole–triazole derivatives in acidic solution. Corros. Sci. 2008, 50, 3261–3266. [Google Scholar] [CrossRef]
- Shukla, S.K.; Singh, A.K.; Quraishi, M.A. Triazines: Efficient corrosion inhibitors for mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 2012, 7, 3371–3389. [Google Scholar]
- Ashry, E.S.H.E.; Nemr, A.E.; Esawy, S.A.; Ragab, S. Corrosion inhibitors part II: Quantum chemical studies on the corrosion inhibitions of steel in acidic medium by some triazole, oxadiazole and thiadiazole derivatives. Electrochim. Acta 2006, 51, 3957–3968. [Google Scholar]
- Montemor, M.F.; Trabelsi, W.; Zheludevich, M.; Ferreira, M.G.S. Modification of bis-silane solutions with rare-earth cations for improved corrosion protection of galvanized steel substrates. Prog. Org. Coat. 2006, 57, 67–77. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yu, Q.; Zhang, Y.; Guo, Z.; Gu, N.; Wesche, K.-D. Self-assembled monolayers of 3-mpt and its mixed-monolayers with alkanethiol on silver: Studies by xps and electrochemical methods. Appl. Surf. Sci. 2004, 229, 377–386. [Google Scholar] [CrossRef]
- Maayta, A.K.; Fares, M.M.; Al-Shawabkeh, A.F. Influence of linear alkyl benzene sulphonate on corrosion of iron in presence of magnetic field: Kinetic and thermodynamic parameters. Int. J. Corros. 2010, 2010, 1687–9325. [Google Scholar] [CrossRef]
- Mori, K.; Okai, Y.; Horie, H.; Yamada, H. The corrosion and inhibition of copper powders. Corros. Sci. 1991, 32, 1237–1252. [Google Scholar] [CrossRef]
- Mori, K.; Sasaki, H.; Kobayashi, I.; Sai, S.; Hirahara, H.; Oishi, Y. Adhesion of nylon-6 to triazine trithiol-treated metals during injection molding. J. Adhes. Sci. Technol. 2000, 14, 791–803. [Google Scholar] [CrossRef]
- Wang, F.; Mori, K.; Oishi, Y. Electrochemical polymerization of 6-(N-Allyl-1,1,2,2-tetrahydroperfluorodecyl)amino-1,3,5-triazine-2,4-dithiol monosodium on aluminum. Polym. J. 2006, 38, 484–489. [Google Scholar] [CrossRef]
- Baba, H.; Kodama, T.; Mori, K.; Hirahara, H. The corrosion inhibition of copper by potentiostatic anodization in triazinedithiol solutions. Corros. Sci. 1997, 41, 555–564. [Google Scholar] [CrossRef]
- Li, Y.N.; Wang, D.; Zhang, H.N.; Wang, F. Study on triazinethiol electropolymerized films prepared by cyclic voltammetry and galvanostatic on copper alloy surface. Int. J. Electrochem. Sci. 2011, 6, 4404–4410. [Google Scholar]
- Kang, Z.X.; Ye, Q.; Sang, J. Fabrication of super-hydrophobic surface on copper surface by polymer plating. J. Mater. Process. Technol. 2009, 209, 4543–4547. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.N.; Wang, Y.B.; Cao, Z. Self-assembled monolayer of designed and synthesized triazinedithiolsilane molecule as interfacial adhesion enhancer for integrated circuit. Nanoscale. Res. Lett. 2011, 6, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.H. Corrosion behaviour of zinc in sodium perchlorate solutions. Appl. Sur. Sci. 2001, 174, 201–209. [Google Scholar] [CrossRef]
- Cai, M.; Park, S.M. Oxidation of zinc in alkaline solutions studied by electrochemical impedance spectroscopy. J. Electrochem. Soc. 1996, 143, 2125. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Yu, F.; Sun, W.W.; Zhu, H.Y.; Yin, Y.S. Fabrication and anti-corrosion property of superhydrophobic hybrid film on copper surface and its formation mechanism. Surf. Interface Anal. 2009, 41, 872–877. [Google Scholar] [CrossRef]




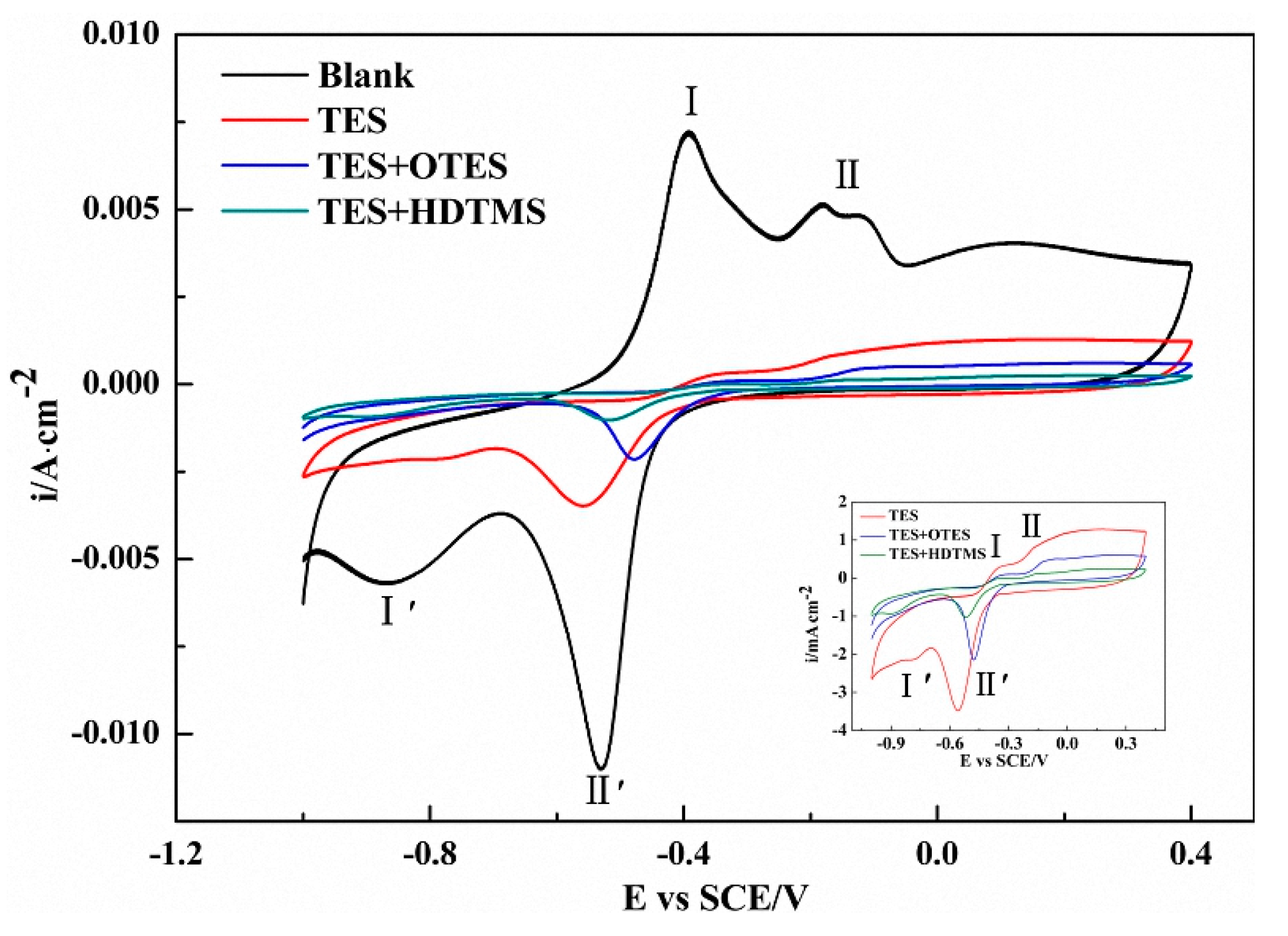


| Sample | Anodic Peak I Potential (V) | Anodic Peak II Potential (V) | Oxidative Current Density (mA/cm2) | Area of CV Curves (10−4) |
|---|---|---|---|---|
| Blank | −0.39 | −0.18/−0.13 | 8.416 | 62.8 |
| TES | −0.33 | −0.12 | 0.312 | 18.0 |
| TES/OTES | −0.31 | −0.07 | 0.196 | 4.77 |
| TES/HDTMS | −0.29 | −0.06 | 0.173 | 2.51 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Tang, T.; Dang, P.; Zhang, Z.; Wang, F. Preparation and Analysis of Complex Barrier Layer of Heterocyclic and Long-Chain Organosilane on Copper Alloy Surface. Metals 2016, 6, 162. https://doi.org/10.3390/met6070162
Zhao Q, Tang T, Dang P, Zhang Z, Wang F. Preparation and Analysis of Complex Barrier Layer of Heterocyclic and Long-Chain Organosilane on Copper Alloy Surface. Metals. 2016; 6(7):162. https://doi.org/10.3390/met6070162
Chicago/Turabian StyleZhao, Qian, Tiantian Tang, Peilin Dang, Zhiyi Zhang, and Fang Wang. 2016. "Preparation and Analysis of Complex Barrier Layer of Heterocyclic and Long-Chain Organosilane on Copper Alloy Surface" Metals 6, no. 7: 162. https://doi.org/10.3390/met6070162