1. Introduction
Magnesium (Mg) is the sixth most abundant element in the earth’s crust comprising about 2.7% of its composition [
1]. Magnesium based materials are preferred when targeting weight-sensitive applications, as they are the lightest structural material available with a density of only 1.74 g/cc which is ~33%, ~61% and ~77% lower than that of aluminium, titanium, and iron, respectively [
2]. Increasing demand for light weighting drives the interest for magnesium to be used in various engineering applications to achieve higher fuel economy, emission reduction etc. Other than its low density, magnesium based materials also exhibit high specific mechanical properties, excellent castability and machinability, high damping characteristics, high thermal stability, high thermal and electrical conductivity, and resistance to electromagnetic radiation [
3,
4,
5,
6,
7]. However, the application areas of magnesium have been limited by its low corrosion resistance and relatively poor mechanical properties, such as low elastic modulus, low strength, limited room temperature ductility and toughness, rapid loss of strength with temperature, and poor creep resistance [
8,
9].
Even though magnesium based materials are not suitable where a high modulus is required, a range of applications has been evaluated in the biomaterials area in the recent past. The combination of superior biocompatibility, biodegradability, elastic modulus closer to human bone, and favourable mechanical properties makes magnesium one of the most sought after materials for orthopaedic applications like implants and fixation devices [
10]. In the recent past, many magnesium alloys have been developed targeting biomedical applications ranging from maxillofacial reconstruction, to paediatric orthopaedics, vascular stents, surgical clips, screws, plates, and bone-interfacing implants [
11,
12,
13]. Magnesium based materials have lower Young’s modulus (41–45 GPa) than commonly used metallic biomaterials such as titanium (55–110 GPa), 316L stainless steel (210 GPa), and cobalt chromium alloys (240 GPa) and show no indications of local or systemic toxicity [
14,
15,
16]. In addition, they are also osteo-conductive, facilitate bone cell in-growth, and have a role in cell attachment [
17]. Due to these advantages, new types of magnesium implant materials have been developed which effectively aid in the mitigation of stress-shielding effects and have potential for use as a bioresorbable material for degradable bone replacement, eliminating the need of secondary surgical procedures [
10]. Although magnesium has many advantages, suitable for hard tissue implant and tissue engineering scaffold material, usage of magnesium is still limited in clinical applications due to its poor formability, rapid degradation in a high chloride physiological environment and hydrogen evolution [
18]. Therefore, continuous efforts are being made by researchers to develop new types of magnesium alloys and composites to meet specific property requirements and explore new processing technologies to fabricate patient-specific implant components that can be provided with additional functions to further broaden the horizon of magnesium utilization in bio-medical applications.
Magnesium based materials are usually fabricated by conventional manufacturing methods such as deformation processing, casting, and powder metallurgy (P/M) techniques. Usually lightweight engineering parts with high performance can be obtained from deformation processing of magnesium based materials. However, due to the hexagonal closed packing (HCP) structure of magnesium, magnesium alloys exhibit poor cold workability at room temperature. Deformation processing of magnesium therefore needs to be performed at elevated forming temperatures to activate more slip systems and to allow better formability, which leads to poor surface quality and oxidation of parts and limits efficiency [
19]. As a result, consumption of wrought magnesium products only represent a small fraction, merely about 1.5% of total magnesium consumption [
20]. Presently, casting is the most conventional and dominant synthesis route used for the manufacture of magnesium alloys and composites. Although, casting techniques ensure great efficiency with higher precision, it is difficult to fabricate near-net shape structures of complex shapes and intricate internal architectures. Moreover, it is often the case that product quality is degraded by the thermodynamically stable phases that are formed during solidification from the melt and strong oxidising tendency of magnesium [
21]. It is not possible to control the morphology and/or distribution of these phases during cooling. Therefore, several P/M routes are being explored to target unique microstructures, novel alloy compositions, and high performance in magnesium alloys [
22]. Promising results were obtained by reinforcing magnesium with nanocrystalline and amorphous alloy powders. For example, Mg-Zn-Y alloys having very high tensile yield strengths in the range of 480–610 MPa with an elongation between 5% and 16% were developed using a rapidly solidified P/M approach [
23]. Also, advanced powder based manufacturing processes such as additive manufacturing (AM), cold spray, metal injection moulding, and friction stir processing are being developed to fabricate magnesium alloys having non-equilibrium compositions and limited defects [
22]. These techniques can be successfully employed to design intricate and near net shaped structures. In a recent study, Tandon et al. [
24] showed that magnesium alloy powders can be potentially used to manufacture and repair lightweight components for aerospace applications by using cold spray and laser assisted deposition processes.
As magnesium is expanding into a more promising lightweight regime and medical technology applications, there is a great need for intelligent selection of manufacturing processes to provide unique functional properties, crash performance, and corrosion resistance. Customised components and implants with improved mechanical and physical properties can be manufactured by additive manufacturing (AM) techniques. AM includes a whole host of “bottom up” approaches, wherein the processes involve creating three-dimensional objects fabricated directly from computer aided design (CAD) models by gradually building them up, layer-by-layer within a powder bed. Among the AM methods, laser-based AM has an immense potential for producing fully dense metallic structures using a variety of available metal powders and has attracted more and more attention [
25]. Selective laser melting (SLM) is one such process that uses high intensity laser as an energy source to directly fuse the high-temperature metallic powder layers successively deposited one over the other as ultrathin two-dimensional cross-sections [
26]. Generally, the geometrically complex components have been fabricated by SLM with a high dimensional precision and good surface integrity without other subsequent process requirements, which the conventional techniques (e.g., casting and machining) cannot keep pace with easily [
27]. SLM is an efficient approach that can significantly shorten the lead time and the costs involved in the manufacturing of high-value components and offers advantages of design freedom with a wide choice of materials, reduced material wastage, and elimination of expensive tooling. Over the years, the process of SLM has become very well established for titanium alloys [
28], steel and iron alloys [
29], nickel base super alloys [
30,
31], cobalt base alloys [
32], and aluminium alloys [
33] and is providing an ideal platform to fabricate complex products, novel shapes, hollow and functionally graded structures to exact dimensions for the aerospace, medical, and military industries.
Despite the continuing advancement, there are very few examples that have fully utilized the potential of SLM to produce fully dense near-net shape components of magnesium and to gain an understanding of laser processability. Accordingly, this review aims to identify the advancements to date in SLM processing of magnesium and magnesium alloy powders and outlines the trend for future research to expand the application range of magnesium based materials further.
2. Selective Laser Melting (SLM) Technology
SLM is a powder bed fusion process, wherein selective regions of the pre-spread powder particles are melted and fused by a high intensity laser energy source in a layer by layer manner according to computer aided design (CAD) data. The term “laser” implies that a laser is used for processing, “melting” refers to the particular situation in which powders are completely melted and the term “selective” specifies that only partial powder is processed [
28]. The SLM system generally is comprised of a processing laser, an automatic powder delivery system, a building platform, a controlling computer system, and main accessorial parts (e.g., inert gas system protection, roller/scrapper for powder deposition, and an overflow container) [
34]. The focus and the movement of the laser beam on the build table is controlled by using a beam deflection system consisting of galvano-mirrors and a flat field-focusing lens. The entire process including the powder feeding, deposition system, scanning, temperature, atmosphere, and build are controlled by a manufacturing software. The overall processing stages of SLM include (i) designing a 3D object model of the component to be fabricated using CAD software and then slicing the designed part into many layers with every layer defined by a layer thickness (typically 20–100 µm); (ii) a substrate is fixed and levelled for part fabrication on the build platform; (iii) a protective atmosphere (e.g., argon or nitrogen) is fed into the build chamber to minimize possible surface oxidation and hydrogen pickup; (iv) spreading of a thin layer of metal powder by powder re-coater on a substrate plate with a thickness as the aforementioned sliced layer thickness; (v) scanning/processing the powder bed in a predefined pattern in order to produce layer wise shape based on the geometry defined by the CAD model; and (vi) lowering of the building platform by a predetermined distance and repeating the last two stages for successive layers of powder until the required components are completely built. In SLM, each layer is fabricated by first generating an outline of the key component features which is referred to as contouring and subsequently the powder within the contour is melted using an appropriate scanning strategy. Once the laser scanning process is completed, specimens from the substrate plate are separated either manually or by electrical discharge machining (EDM).
SLM is a deposition welding process wherein the laser beam melts the powder particles in welding beads and shows phenomena usually observed for welding processes [
26]. The physical behaviour of the SLM process includes absorption, reflection, radiation, and heat transfer, melting and coalescence of powder particles, phase transformations, a moving interface between solid phase and liquid phase, fluid flow caused by the surface tension gradient and mass transportation within the molten pool followed by solidification, and chemical reactions [
26,
35]. Because of the use of high-intensity laser, the powder particles are heated at a faster rate as the energy is absorbed via both bulk-coupling and powder-coupling mechanisms [
36]. The energy is converted into heat and eventually the powder particles melt, coalesce, and form an agitated melt pool for some milliseconds (typically 0.5 to 25 ms). The molten pool formed, acquires the shape of a circular or segmental cylinder under the effect of surface tension [
37]. Extremely short interaction time between the laser beam and the powder bed results in the formation of a transient temperature field with a high temperature up to 10
5 °C and a significant rapid quenching effect with very high cooling rates up to 10
6–8 °C/s [
38]. Rapid solidification may cause development of non-equilibrium metallurgical phenomena such as microstructural refinement, solid solution hardening and the formation of metastable phases, which can have a substantial effect in improving the resultant mechanical properties and corrosion resistance of the laser processed materials [
39,
40].
Literature has shown that components produced by SLM are completely dense and homogeneous without microscopic pores or voids and do not require any post-processing (such as infiltration with other materials or heat treatment) usually needed to improve the SLS (Selective Laser Sintered) components, other than the removal of parts and supports from the substrate plate. Another major advantage of SLM lies in its high feasibility in processing non-ferrous pure metals like Ti, Al, Cu, Mg, etc., which to date cannot be well processed using SLS [
39]. Some common materials that have been investigated for SLM include: ferrous alloys, titanium, cobalt-chrome, nickel, aluminium, magnesium, copper, zinc, tungsten, and gold [
41]. SLM also has the potential to produce components of very complex geometries with a gradient porosity which in turn allows the choice of property distribution to achieve required functions [
42]. SLM is unique in that it can be used for the additive manufacturing (AM) of functionally graded and pure-metal parts, as well as for laser cladding/repair. Additive repair of damaged turbine engine hot-section components [
43,
44] made from nickel base super alloys is one example of such repairs. SLM is also capable of multi-material processing, i.e., utilizing different feedstock materials simultaneously to produce various alloys and functionally graded materials (FGMs) [
45,
46].
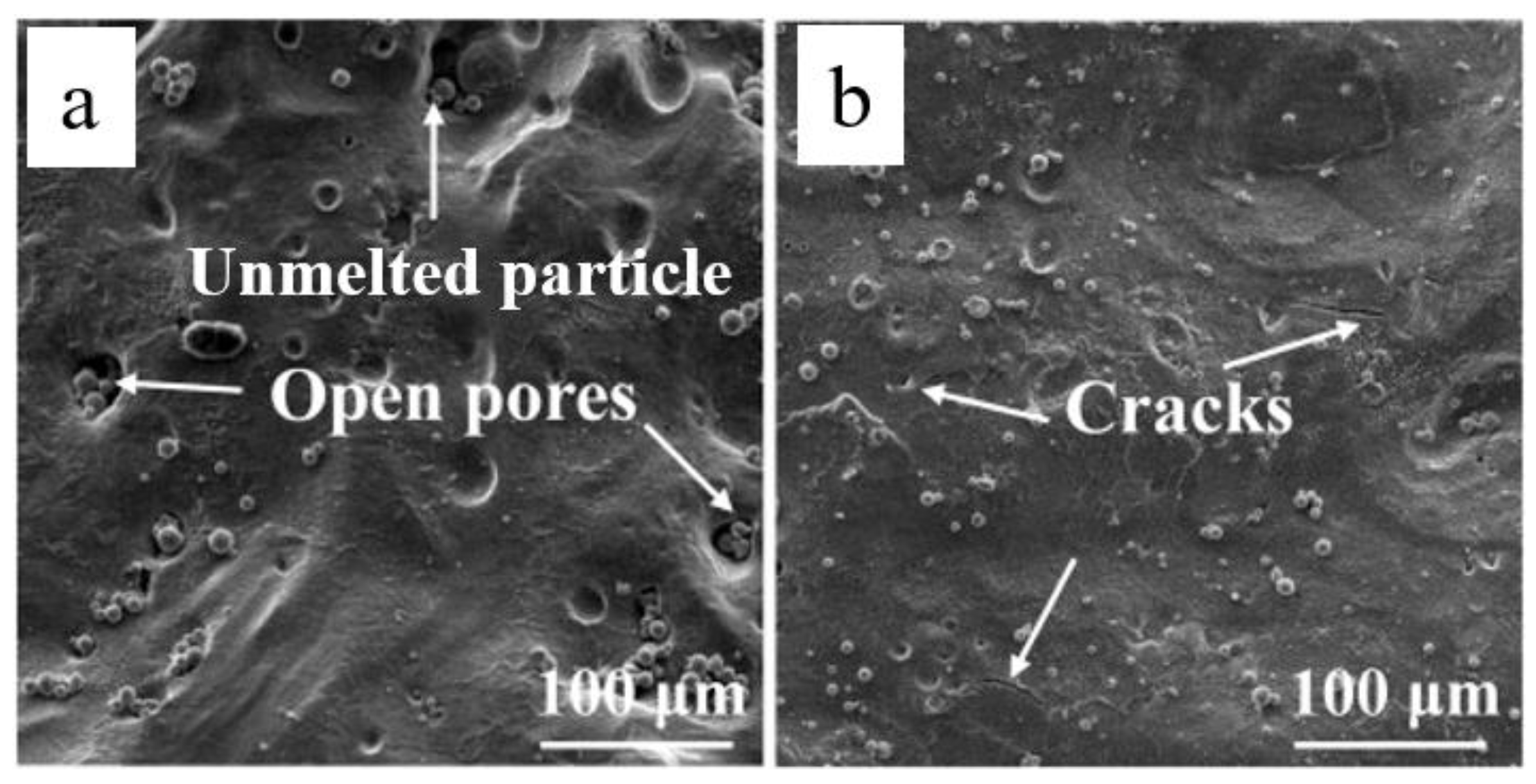
The main goal in SLM is to produce metallic parts with 100% density. Obtaining this objective is not easy because there is no mechanical pressure during SLM, and fluid dynamics in SLM is mainly driven by gravity and capillary forces along with thermal effects. Also, the absence of mechanical pressure during the processing may lead to reduced solubility of some elements in the melt during solidification causing discontinuous melting of the tracks and formation of pores resulting in an uneven surface [
47,
48] as shown in
Figure 1a, where distribution of porosity and unmelted area within ZK60 sample produced by SLM is revealed. The materials also experience varying degrees of thermal fluctuation during the SLM process which may induce residual stresses in the laser melted layer undergoing rapid solidification [
41]. This can lead to formation of hot cracks and delamination of parts as shown in
Figure 1b. High heating/cooling rates during SLM can also lead to the formation of narrow heat affected zone (HAZ) around the melt pool. Presence of HAZ can alter the composition and/or microstructure of material influencing the quality and properties of the SLM-processed sample [
28]. The transient thermal behaviour during the SLM process can be controlled considerably by processing parameters, such as laser power, scan speed, hatch spacing, layer thickness, and scanning pattern.
Figure 2 provides an illustration of these process parameters commonly studied in SLM. These process parameters are adjusted such that a single melt vector can fuse completely with the neighbouring melt vectors and the preceding layer. Application of inappropriate processing conditions can lead to various undesirable effects such as irregularities in the surface morphology, thermal cracks, and balling effects. Therefore, it is important to establish the relationships between the principal SLM parameters and surface morphology and to optimize the SLM processing parameters to produce metallic parts with 100% density without cracks and fusion defects.
4. Microstructure
Transient cooling patterns employed in SLM dictate the microstructures formed in a deposited layer, due to the rapid and directional solidification resulting in finer microstructures. SLM possesses the capability to control grain sizes and shapes, phase percentages, and phase compositions by manipulation of process parameters as per the design requirements to fabricate parts with tailored mechanical properties. The microstructural characteristics of the consolidated materials fabricated by SLM are strongly sensitive to their thermal history during the build, which may include high heating/cooling rates, significant temperature gradients, bulk temperature rises, and more. The resulting microstructures, which are very fine and far from equilibrium are a consequence of very high solidification rates observed in the SLM process, ranging between 10
6 and 10
11 °C/s [
96]. Since many process variables/parameters impact the thermal history, predicting the microstructural features of SLM parts, and the degree of their dependence on the process parameters, is still a major challenge. However, overcoming this challenge is vital for establishing the effective control mechanisms for fabricating SLM parts with superior mechanical properties. Various authors have investigated the effects of certain parameters on the microstructural characteristics and material properties of SLMed magnesium parts [
58,
59,
60,
61,
62,
63,
64,
65]. However, it is still unclear how to apply these findings to fabricate complex parts with various shapes since their microstructures will have a unique dependence on thermal history. The solidified microstructure obtained when the SLM processing parameters are varied is dependent on: local solidification rates within the melt pool, the ratio of cooling rate to thermal gradient,
R, and the temperature gradient at the solid-liquid interface,
G. Two critical solidification parameters are the ratio,
G/
R, which affects the solid-liquid interface shape controlling the type of microstructure, and the cooling rate,
G ×
R, which affects the undercooling controlling the scale of microstructure [
99,
100]. Different G and R values may result in three major structure morphologies within SLM parts: columnar (elongated grain morphology), columnar-plus-equiaxed, and equiaxed (isotropic grain morphology). It has been found that a higher solidification rate promotes the transition from columnar to equiaxed grain morphologies [
101] and that increasing the cooling rate,
G ×
R, leads to a finer microstructure. The tendency to form a columnar structure increases by increasing the ratio
G/
R, while decreasing
G/
R is favourable for equiaxed structures [
99].
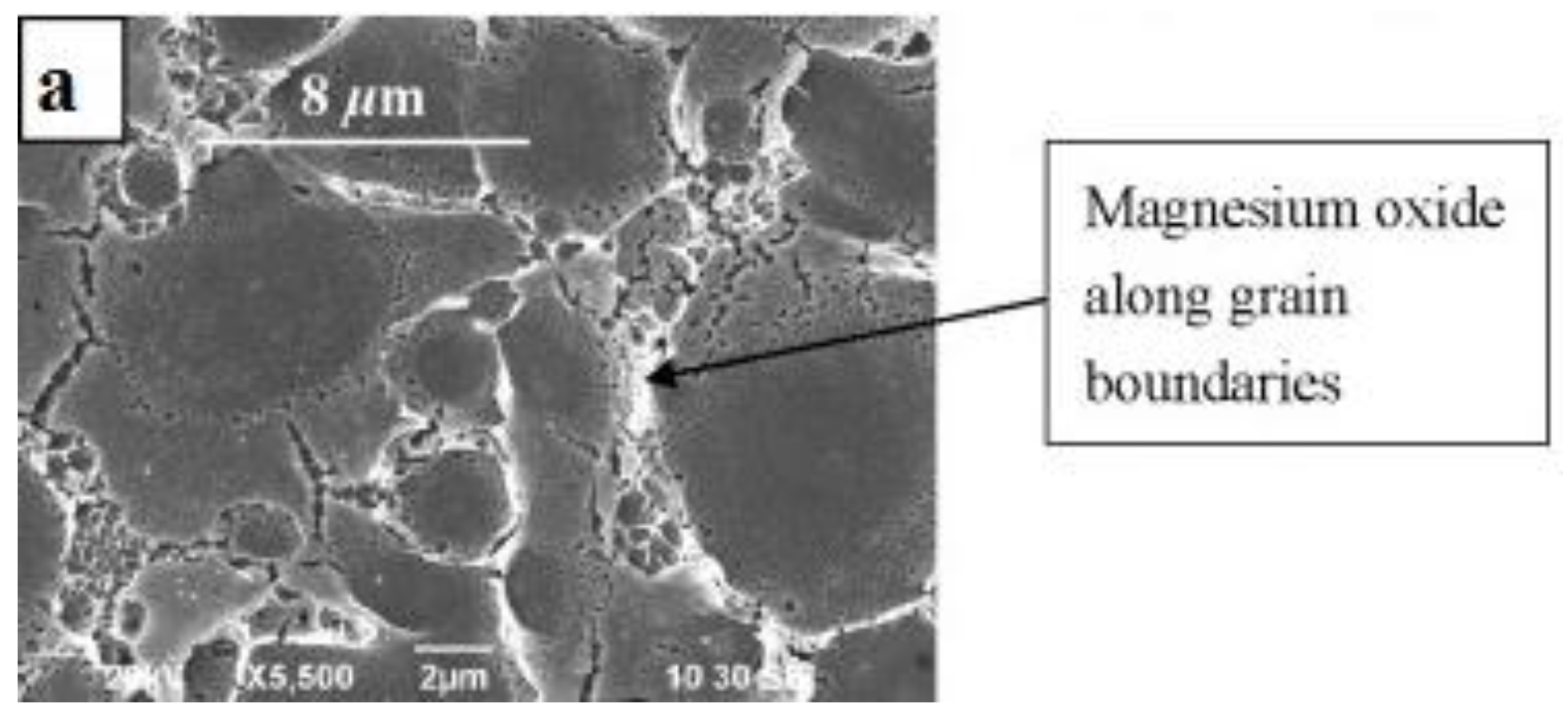
Microstructural features along with resulting properties observed for different SLMed magnesium alloy powders are compiled and tabulated for ease of comparison in
Table 6. Conventionally cast magnesium alloys are generally characterized by a coarse microstructure consisting of primary α-Mg and lamellar eutectic (α-Mg + intermetallic) phases with an average grain size in the range of 50–150 µm. However, rapid cooling rates associated along with epitaxial solidification in the SLM process, results in a highly refined microstructure in magnesium alloys with grain sizes of α-Mg matrix in the range of 1–20 µm and often favour the formation of partially or fully divorced eutectic (separation of eutectic phases) homogenously distributed along the grain boundaries of dendritic/columnar primary α-Mg (
Figure 8) [
49,
61]. SLM, being a non-equilibrium process, can extend the solubility of alloying elements in Mg and obtain single-phase Mg alloys with wider composition range [
61]. SLM also results in compositional and microstructural changes caused by the combined effects of selective evaporation of elements like Mg and Zn having very high vapour pressures, and consequent enrichment in the relative content of elements like Al and Zr at the surface by “solute capture” phenomenon. In the process of laser rapid melting, very high temperature gradients generated in the melt pool contribute to the formation of a strong Marangoni convection and result in improved homogenous dispersion of alloying elements in the melt pool [
62]. Then a subsequent high rate growth of the solid/liquid interface contributes towards “solute capture” phenomenon in α-Mg matrix, resulting in larger amounts of solute atoms to be captured, extending the solid solution limit of alloying elements in α-Mg and retarding the nucleation β-phases [
49,
61]. Such compositional changes can influence the microstructure, mechanical properties, and corrosion behaviour of laser-melted magnesium alloys.
Microstructures obtained in magnesium alloys with SLM processing are comparable to that achieved by other laser processing techniques such as laser surface melting (LSM) and selective laser surface melting (SLSM).
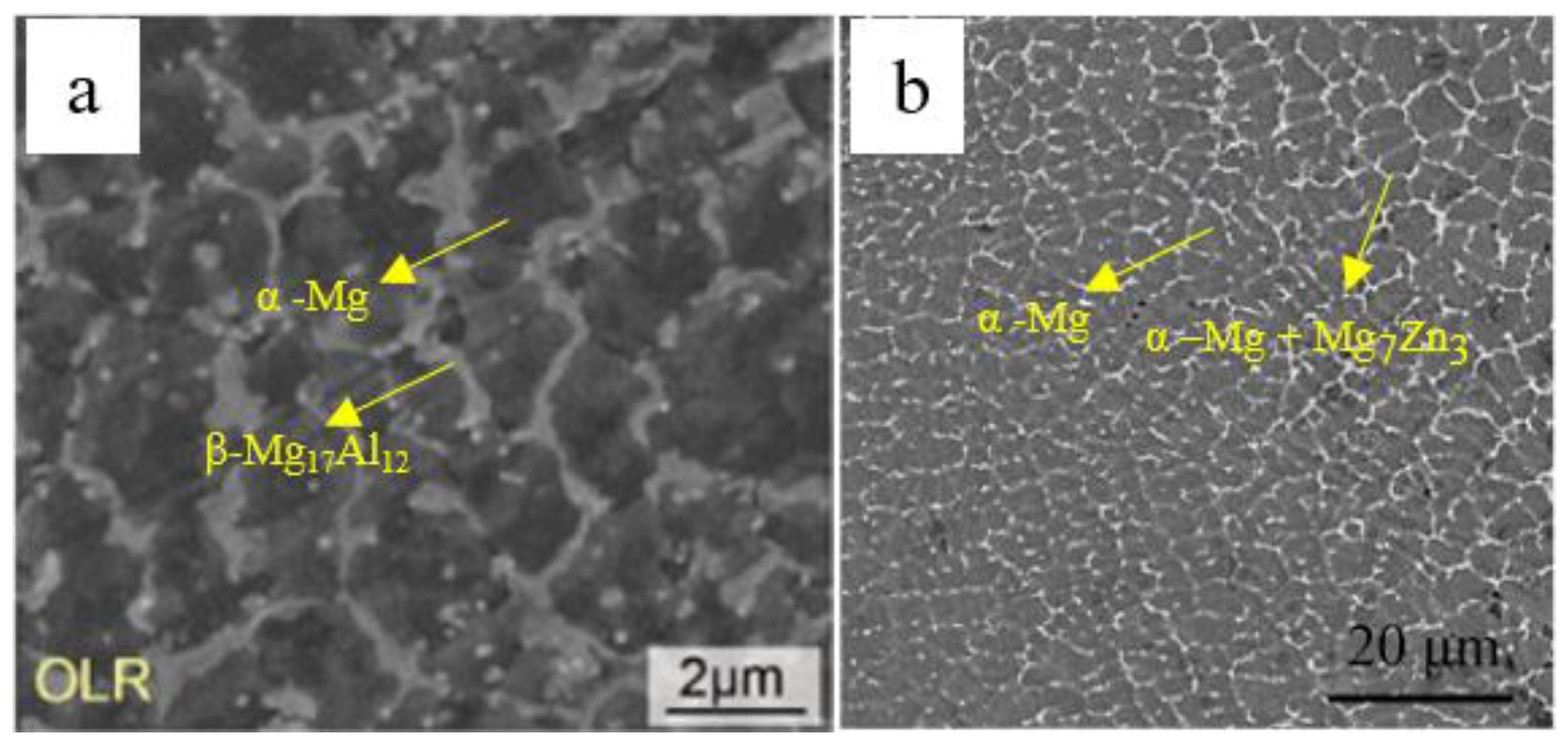
Figure 9 shows the different types of microstructure that can be achieved by laser processing of AZ91D alloy. The commercial die cast AZ91 alloy (
Figure 9a) is composed of α-Mg solid solution and β-Mg
17Al
12 lamellar eutectic phase distributed along the grain boundaries and its microstructure presents large grains due to the slow cooling rate of the casting process used.
Figure 9b,c present the typical appearance of the surface of AZ91 alloy, after modification by LSM and SLSM, respectively. Surface modification by LSM (with a laser power of 600 W and scanning speed of 45 mm/s) revealed that the microstructure was characterized by very small isolated β-Mg
17Al
12 phase particles immersed in a continuous supersaturated solution of Al in α-Mg matrix, producing a homogeneous and continuous modified layer. SLSM, which is a modification of the LSM process, can be achieved by the application of lower laser energy input (375 W and 90 mm/s) causing modification of the β-Mg
17Al
12 phase only, without any change in the α-Mg matrix. SLSM also resulting in a eutectic microstructure based on fine plates of β-Mg
17Al
12 and α-Mg phase. Modification β-phase and its surrounding led to a reduction in its hardness and its dispersion, resulting in a more homogenous and continuous material.
Figure 9d presents the microstructure of SLMed AZ91 (at a laser energy input of 166.7 J/mm
3), which also presents a feature of equiaxed α-Mg with fully divorced eutectic β-Mg
17Al
12 distributed reticularly along the grain boundaries. As the morphology of the eutectic in the hypo eutectic Mg-Al alloys depends on the cooling rate [
102], change in the form of β phase between LSM, SLSM, SLM, and die-castAZ91D is induced by the high cooling rates inherent to laser melting processes.
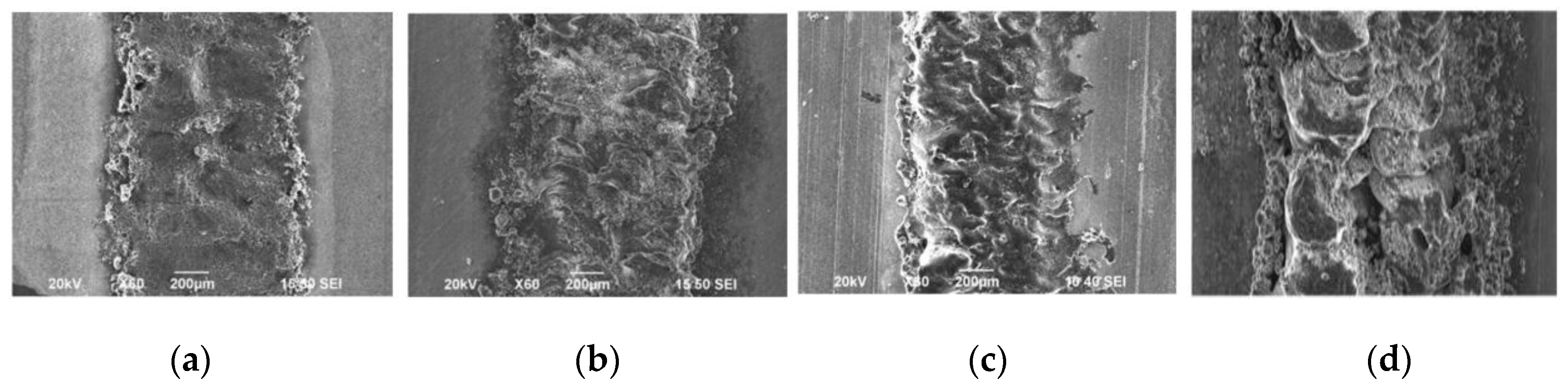
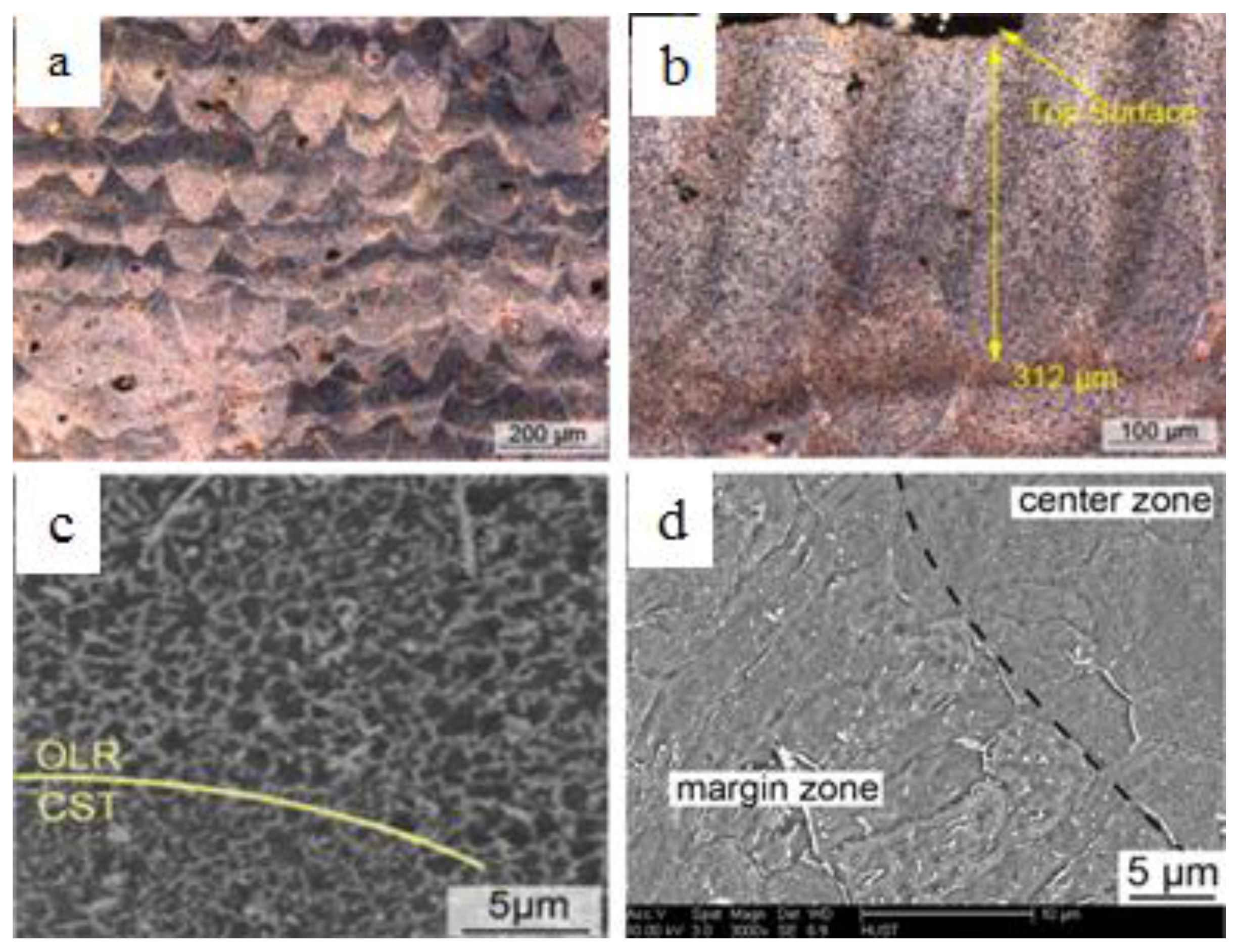
Under an optical microscope with lower magnifications, the SLM formation features are shown clearly. For example, the curve-like regular laser melted tracks on the cross-section correspond to laser scanning strategy, and the laminar material structure and columnar architecture throughout the vertical section are determined by the specimen building strategy, such as the scanning pattern, hatch spacing, and thickness of layers. The cut ends of melted tracks in the form of a series of arcs on the vertical section which are aligned layer by layer, are induced by the Gauss energy distribution of laser as shown in
Figure 10a [
61]. The penetration depth of the molten pools was observed to be up to 312 µm, which was approximately eight times the layer thickness (40 µm) used in the study for AZ91D alloys (fabricated at a laser energy density of 166.7 J/mm
3) indicating that each layer of the as deposited sample undergoes a remelting process more than once. The multiple remelting process plays a significant role in determining the microstructure of SLMed samples as different thermal histories experienced by different layers of the part, led to variation of microstructures along the height direction, as the conduction, convection, and radiation conditions change [
61]. The as processed microstructure contains at least two distinct regions: one significantly finer than the other, as shown in
Figure 10c,d for AZ91D and ZK60 alloys, respectively. At the edges of the melt pool, the material experiences more cycles of the remelting process caused by both overlapping of the scan lines and creation of subsequent layers to induce relatively lower cooling rates, resulting in localized coarsening of the microstructure. This difference in thermal history between the edges and centre of the melt pool can induce non-uniform distribution of microstructure in the scale of several microns. As can be seen from
Figure 10c in the case of AZ91D alloy, the grains on the centre of the scanning tracks (CST) were finer (~1 µm) than those near the overlapping edges (OLR) because of the decreasing cooling rate and multiple remelting cycles experienced at the edges of the melt pool. Also, decreasing the temperature gradient inside the melt pool, can lead to occurrence of columnar-to-equiaxed transition towards the centre of the melt pool. As can be seen from
Figure 10d, columnar α-Mg grains dominate the margin zone of the molten pool whereas α-Mg grains in the centre zone of the molten pool presented an equiaxed morphology in the case of ZK60 alloys.
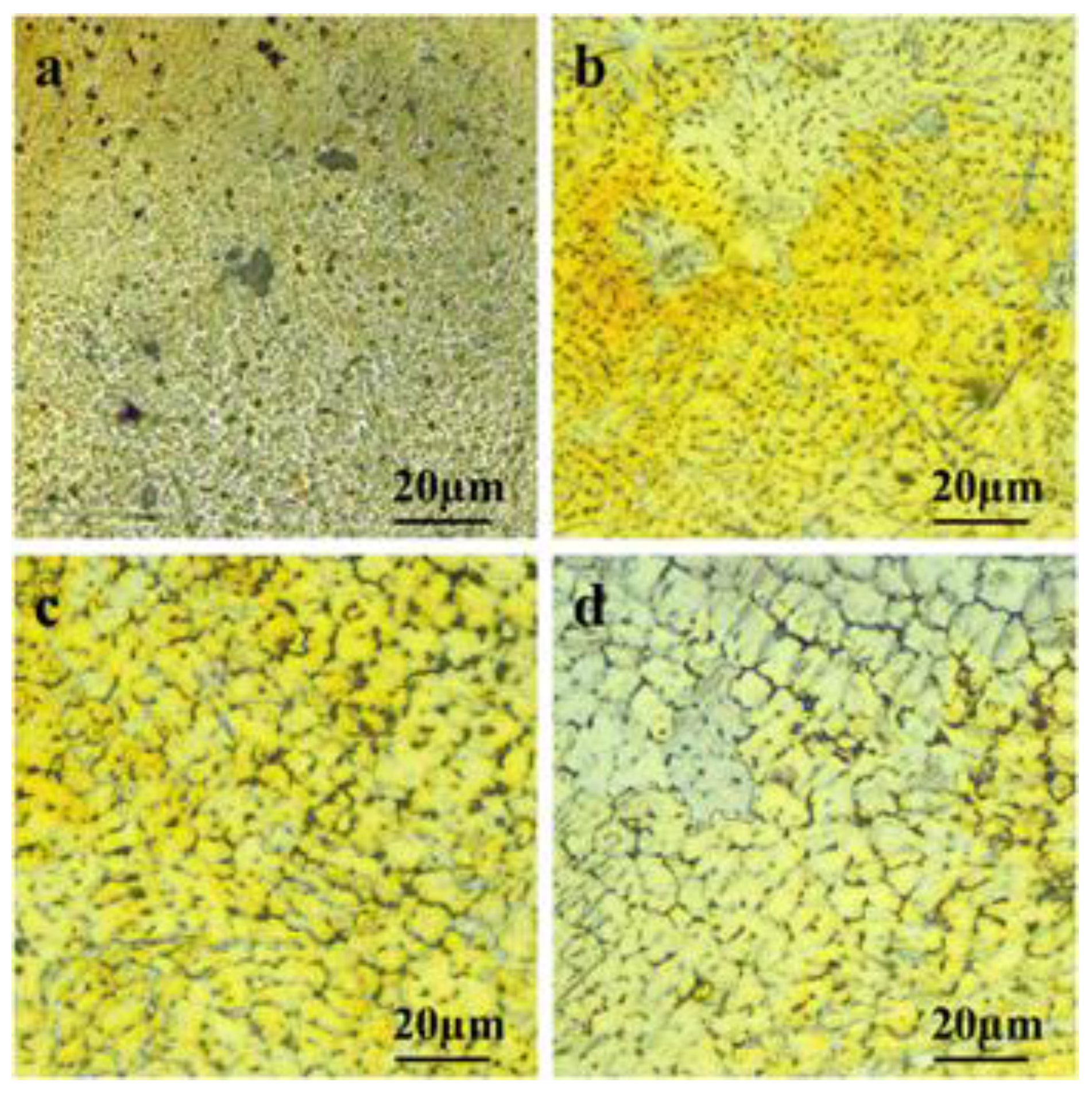
Microstructural features of SLM processed magnesium alloys can be significantly influenced by the processing parameters used. The combination of higher scanning speeds and lower laser power results in a lower incident energy at the top of the part, typically resulting in finer microstructures due to higher cooling rates. In contrast, lower cooling rates and coarser microstructures can be obtained by decreasing scanning speed and increasing laser power. At relatively lower scanning speeds, prolonged interaction of the laser beam with powders results in the restraining of heat dissipation in the melt pool. As a result, relatively equivalent cooling rates during solidification can be achieved due to larger heat accumulation and thus providing enhanced kinetic qualifications for epitaxial growth of the grains [
49]. With the increase of laser energy density, the crystalline structure of magnesium alloys experience successive changes in the order of clustered finer dendrites, uniform equi-axed grains to coarsened equi-axed grains. As can be seen from the microstructure of SLM processed ZK60 alloys, extremely fine dendrites (~2 µm) which clustered severely together, were observed at a relatively lower laser energy input of 420 J/mm
3 (
Figure 11a). The dendrites coarsened to some extent (~4 µm) and changed to a column shaped structure with an increase in laser energy input to 500 J/mm
3 (
Figure 11b), but still exhibited a disordered distribution. Further increase in the laser energy input to 600 J/mm
3 and 750 J/mm
3 resulted in orderly dispersed, equi-axed grains of ~6 µm (
Figure 11c) and ~8 µm (
Figure 11d), respectively. The dendritic crystalline structure was formed through the heterogenous nucleation of α-Mg and subsequent dendrite growth, whereas, the equi-axed crystalline structure was formed through the homogenous nucleation of α-Mg and subsequent equi-axed growth of grains [
49]. Similar results were observed in the investigation of SLM of Mg-9%Al alloy powders by Zhang et al. [
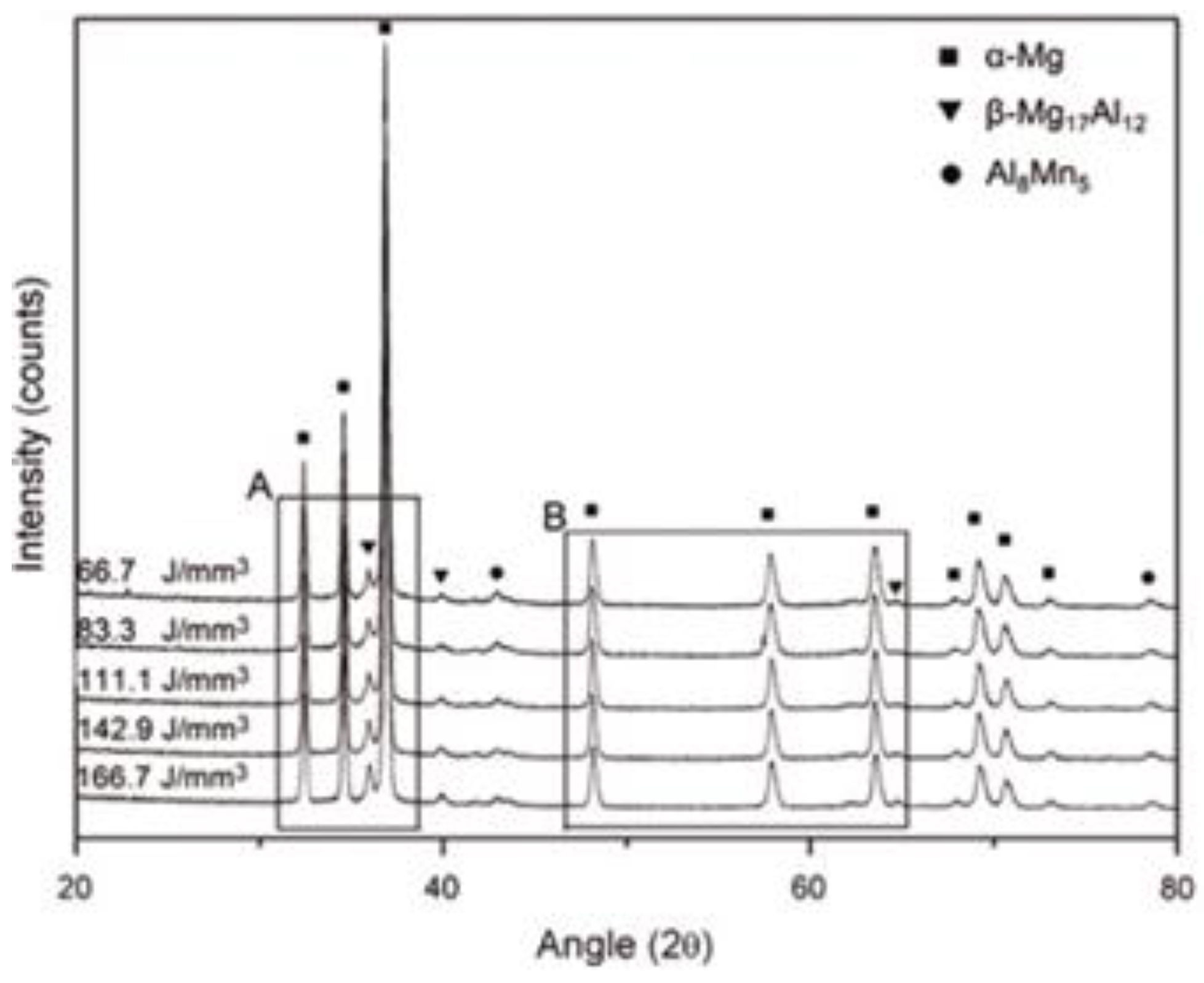
60] wherein significant grain refinement in the laser-melted region was observed with grain sizes in the range of 10–20 µm. The microstructure the Mg-Al alloy consisted of equi-axed grains, transformed from dendritic grains under a high temperature gradient. An XRD analysis of the laser-melted samples indicated the presence of phases like α-Mg, Mg
17Al
12, MgO, Al
2O
3. The Al
2O
3 phase was formed as a result of incomplete reaction between Mg and Al, only under a low energy density input of 93.75 J/mm
3. Further, it was also observed that the content of Mg decreased in the laser-melted region because of selective evaporation with the increase in laser energy density.
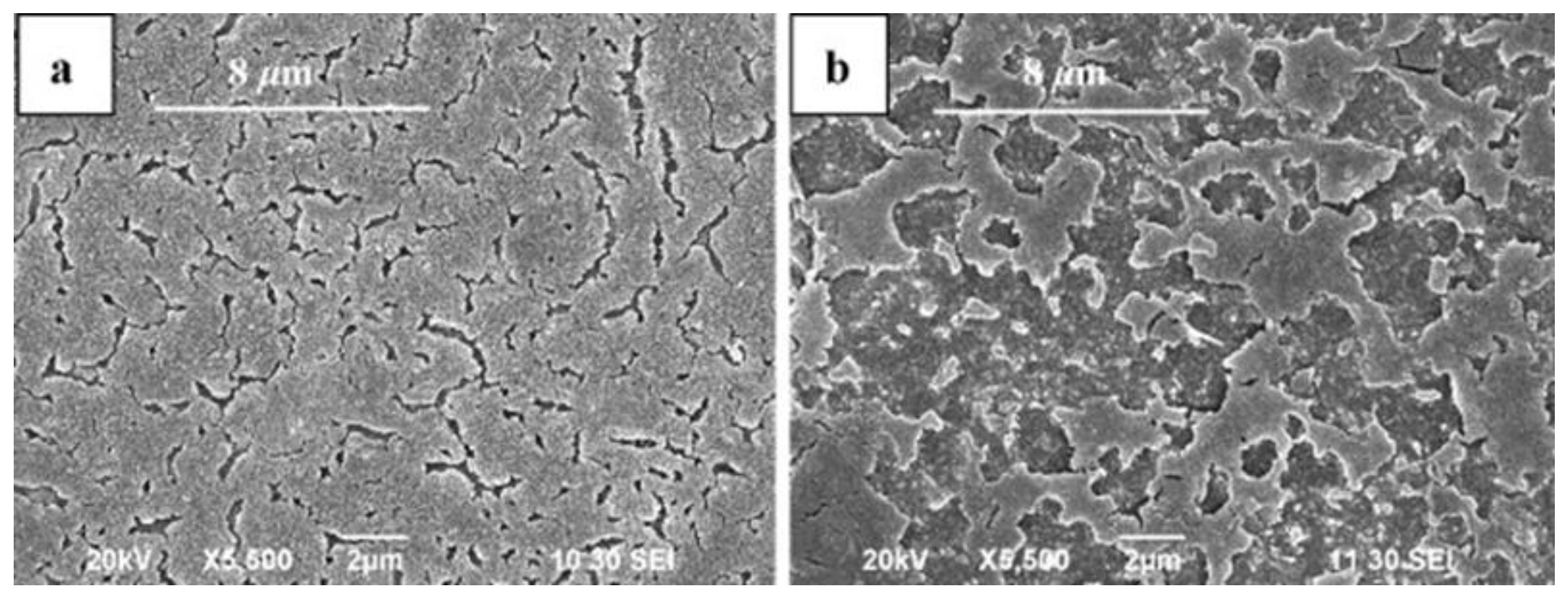
Reports also indicate that the type and mode of the laser beam used can affect the microstructures formed in SLM processed magnesium as the resultant consolidation mechanism of metallic powders is a function of energy density delivered [
39]. Ng et al. [
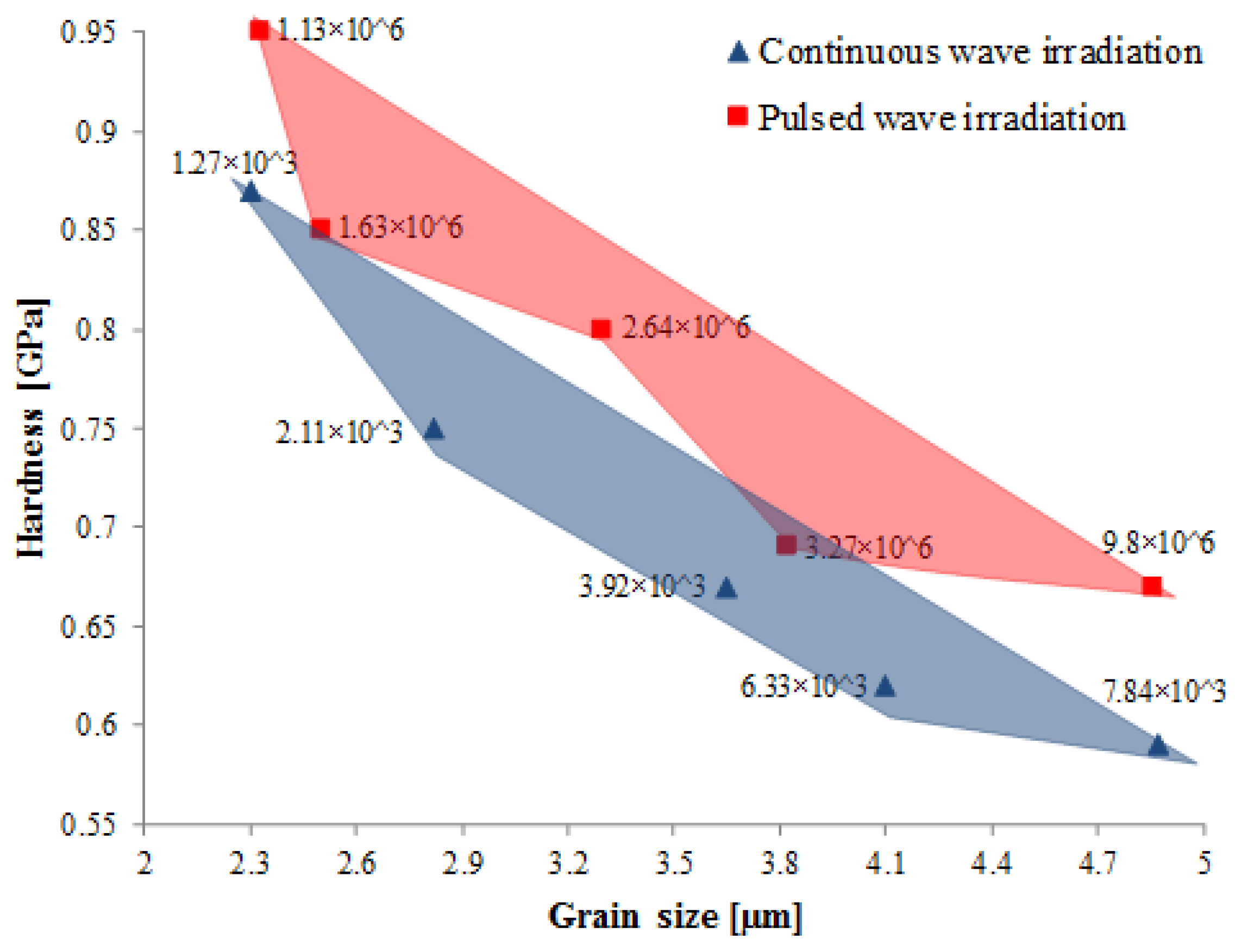
57] compared microstructures of the tracks formed in SLM processing of magnesium powders, processed under both continuous and pulsed mode of irradiation. Under continuous wave irradiation, laser melting led to the formation of fully recrystallized grains in the melted zones with grain sizes in the range of 2.3–4.87 µm (laser energy densities varied from 1.27 × 10
9 J/m
2 to 7.84 × 10
9 J/m
2). The α-Mg single phase solidified in the form of equi-axed crystals as seen in
Figure 12a. However, in the case of tracks melted under pulsed mode of irradiation, incomplete growth of the α-Mg phase was observed (
Figure 12b). Full growth of α-Mg was inhibited as the solidification rates achieved under pulsed mode was higher than continuous irradiation. Besides, due to the shorter interaction time in case of pulsed mode, there is insufficient time for the crystals to arrange themselves such that thermodynamic equilibrium prevails at the solid/liquid interface. The average size of grains obtained under pulsed mode were smaller than those obtained in the continuous mode laser melted tracks. Also, smaller laser spot size (50–180 µm) and layer thickness (typically 20–50 µm) used in the SLM process when compared to other laser processing technologies such as direct laser deposition (DLD), laser rapid forming (LRF) and laser net shape manufacturing (LNSM) led to the formation of a smaller melting pool, thereby resulting in the formation of a refined microstructure [
29]. Normally, layer thickness, alone, has little influence on the microstructure, but its influence is dependent on other parameters, such as laser power, scanning speed, specific energy density, and powder mass flow rate. For example, as the specific laser energy density is lowered, thinner layer thickness will be required, as the energy per unit area to melt the powder is reduced. However, it was observed by Savalani et al. [
58] that different layer thicknesses directly affect the oxygen content in the matrix material thereby resulting in phase and microstructural changes. Oxidation occurring during SLM processing of magnesium at different layer thicknesses ranged from approximately 9.1 to 11.7 at %. The level of oxidation was found to be inversely proportional to the layer thickness, as it decreased from 11.7% to 9.1% with the increase in layer thickness from 150 µm to 300 µm.
7. Corrosion Behaviour
Magnesium alloys, generally, reveal a poor corrosion resistance, which is mainly associated with their high chemical activity and the lack of a protective passive oxide film [
117,
118]. This disadvantage has been a major obstacle restricting their further application in automotive, aerospace, and electronics industries. Also, magnesium has a high negative standard electrode potential, which leads to the rapid corrosion of magnesium based alloys in chloride physiological conditions [
119]. This has delayed the introduction of magnesium based materials for therapeutic applications to date, as the hydrogen gas produced at a high rate from corrosion cannot be dealt with by the host tissue [
18]. Additionally, shift in alkaline pH in the region surrounding the corroding surface is also a concern for biomedical applications [
119]. Therefore, development of magnesium alloys with improved corrosion behaviour may help to resolve the current limitations of magnesium alloys for use in the aforementioned industries. Rapid solidification has been identified as an effective method to improve the strength and corrosion resistance of magnesium alloys for structural and corrosive media utilization [
40]. Laser melting is one such rapid solidification process involving cooling rates up to 10
6–8 °C/s and is capable of modifying surface properties as it can homogenize and refine the microstructure, and dissolve secondary phases [
120,
121]. However, so far, little effort has been made to examine the corrosion behaviour of the SLM-produced magnesium parts as most studies on the SLM of magnesium alloy powders have been focussed on the densification and mechanical properties of the SLM-produced samples.
Because of the paucity of literature dealing with corrosion behaviour and its associated mechanism during SLM of magnesium alloys, it would be useful to examine the effect of laser surface melting (LSM) treatment on the corrosion behaviour of magnesium alloys, as corrosion is essentially a surface degradation process. LSM treatment has been effective in improving the corrosion resistance of AZ and AM type of magnesium alloys, such as AZ31, AZ61, AZ91, and AM60 [
122,
123,
124,
125,
126,
127,
128]. Improved corrosion resistance of these alloys was mainly attributed to the pronounced refinement of α-Mg grains and uniform redistribution of inter metallic compounds after laser treatment. In the case of magnesium alloys, it has been observed that, grain refinement and homogenization of compositional distribution can decrease the volume fraction of the effective cathodes, thereby limiting the cell action caused by the accumulation of cathodic phases [
129]. In addition, the corrosion resistance also increased with the enrichment of aluminium content in the laser-melted zone because of the selective evaporation of magnesium, providing passive characteristics to the melted surface [
124]. Enrichment of alloying elements can shift the corrosion potential of α-Mg to the positive direction, lowering the corrosion susceptibility of α-Mg matrix [
130]. Furthermore, rapid laser-melting could increase the solid solubility of alloying elements, such as Mn, Al, and Cr, promoting the formation of more protective and self-healing films and thus limiting the occurrence of local galvanic corrosion [
129]. It is expected that insights gained from how LSM treatment can enhance the corrosion resistance of magnesium alloys, could be helpful in understanding the mechanisms associated with corrosion behaviour of SLM processed magnesium alloys.
In a recent study, Shuai et al. [
49] investigated the corrosion behaviour of SLM-processed ZK60 alloy by performing immersion tests in Hanks’s solution (pH 7.4 ± 0.1) at 37 ± 0.5 °C for 48 h. Hydrogen volume evolution rates observed were in the range of 0.006–0.0019 mL·cm
−2·h
−1 as the laser energy input varied from 420 J/mm
3 to 750 J/mm
3. The lowest hydrogen evolution volume rate of 0.006 mL·cm
−2·h
−1 was observed at a laser energy input of 600 J/mm
3, for which a maximum densification of 97.4% was achieved. Such low volume release rate of hydrogen achieved, is within the limit of 0.01 mL·cm
−2·h
−1 that can be tolerated by the body without posing any serious threat [
131]. SLM processed ZK60 alloy showed significant enhancement in corrosion resistance compared to casted ZK60, which had a hydrogen volume evolution rate of 0.154 mL·cm
−2·h
−1 (~80 times higher) during immersion in Hank’s solution [
132]. However, the outcome from Shuai et al. [
49] contradicts the findings from studies carried out by Dai et al. [
133,
134] who reported that SLM-produced Ti-6Al-4V has an unfavourable corrosion resistance compared to its traditional counterparts. The reported contradiction about the effects of laser melting on the corrosion behaviour of SLM processed metal powders might have been possibly engineered by different mechanisms of microstructure evolution and corrosion reactions, occurring during laser processing of different materials. The exact nature of the corrosion reactions and the associated mechanisms affecting the corrosion behaviour for various metallic powders need to be explored further in future studies.
When ZK60 alloy was soaked in Hank’s solution, the following reactions occurred [
49]:
The corrosion of ZK60 in Hank’s solution accompanied a release of H
2. The reactions were driven by the relative potential difference between the relative anode and cathode. As for the ZK60 alloy, homogenously distributed Mg
7Zn
3 secondary phase served as a cathode and formed a galvanic couple with the Mg matrix resulting in macroscopic homogenous corrosion characteristics. The corrosion was triggered by the dissolution of the α-Mg matrix adjacent to intermetallic compounds. The enhanced corrosion resistance observed in SLM processed ZK60 alloy can partly be ascribed to the increase in the corrosion potential caused by enrichment of the solid solution of Zn (caused by increased “solute capture” effect), which possessed a higher corrosion potential (−0.76 V) than that of Mg (−2.34 V). Thus, increase in the corrosion potential of Mg matrix, namely, decrease in the relative potential difference, had a beneficial effect on reducing the relative anodic and cathodic reaction supported by the Mg alloy. In addition, once the corrosion was initiated, the refined Mg
7Zn
3 phases distributed evenly and mainly served as a barrier to impede corrosion process. Similarly, Yang et al., during SLM processing of Mg-Mn alloys [
66], observed that corrosion resistance of pure magnesium was enhanced by the addition Mn of up to 2 wt % during immersion testing carried out in simulated body fluid (SBF) (pH 7.4) at 37 °C for 48 h. The hydrogen volume evolution observed for Mg-2Mn alloy (0.017 mL·cm
−2·h
−1) was significantly lower than that of pure Mg (0.068 mL·cm
−2·h
−1). The enhancement of Mg corrosion resistance was attributed to the increase in corrosion potential and grain refinement caused by the solid solution of Mn. Further, they suggested SLM processed Mg-2Mn alloy as a potential candidate for future bone implants. However, corrosion in body fluids is influenced by various factors such as pH, concentration and types of ions, protein adsorption on orthopedic implant, and influence of the biochemical activities of surrounding tissues [
135,
136]. Therefore, further investigations are still necessary to develop the reliability of SLM processed magnesium parts for biomedical applications.