Formation of Light-Weight Ferroalloys in the Fe2O3-Al2O3-C System at 1550 °C: Influence of Silica Impurities
Abstract
:1. Introduction
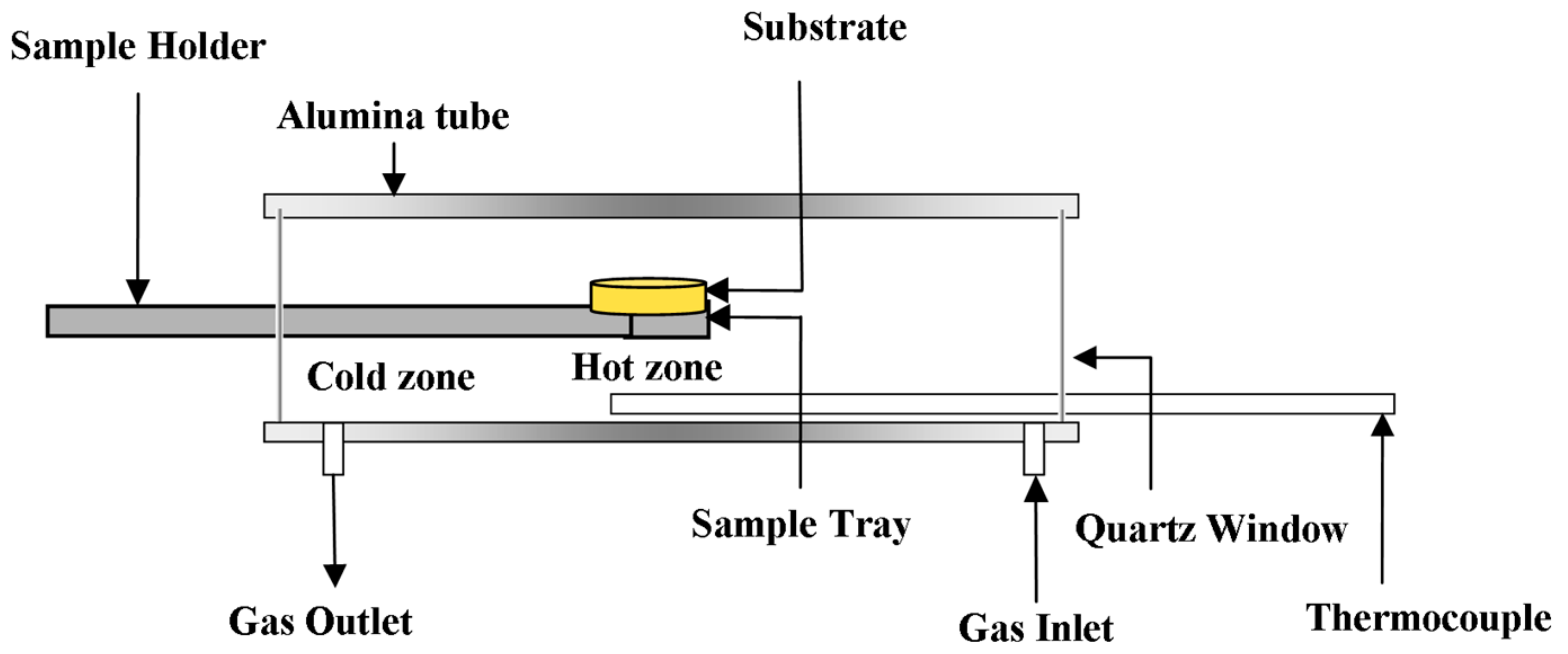
2. Materials and Methods
3. Results and Discussion
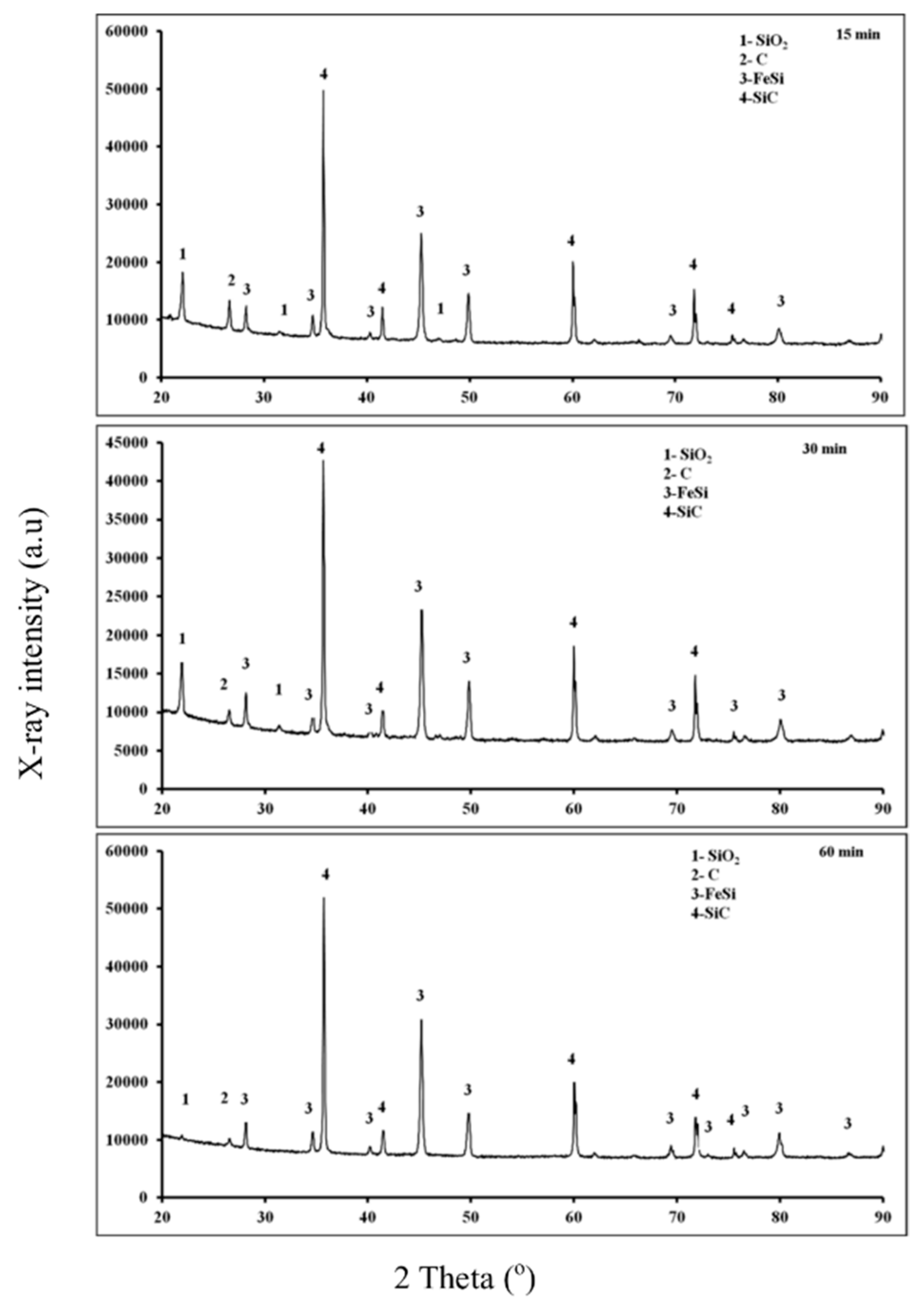
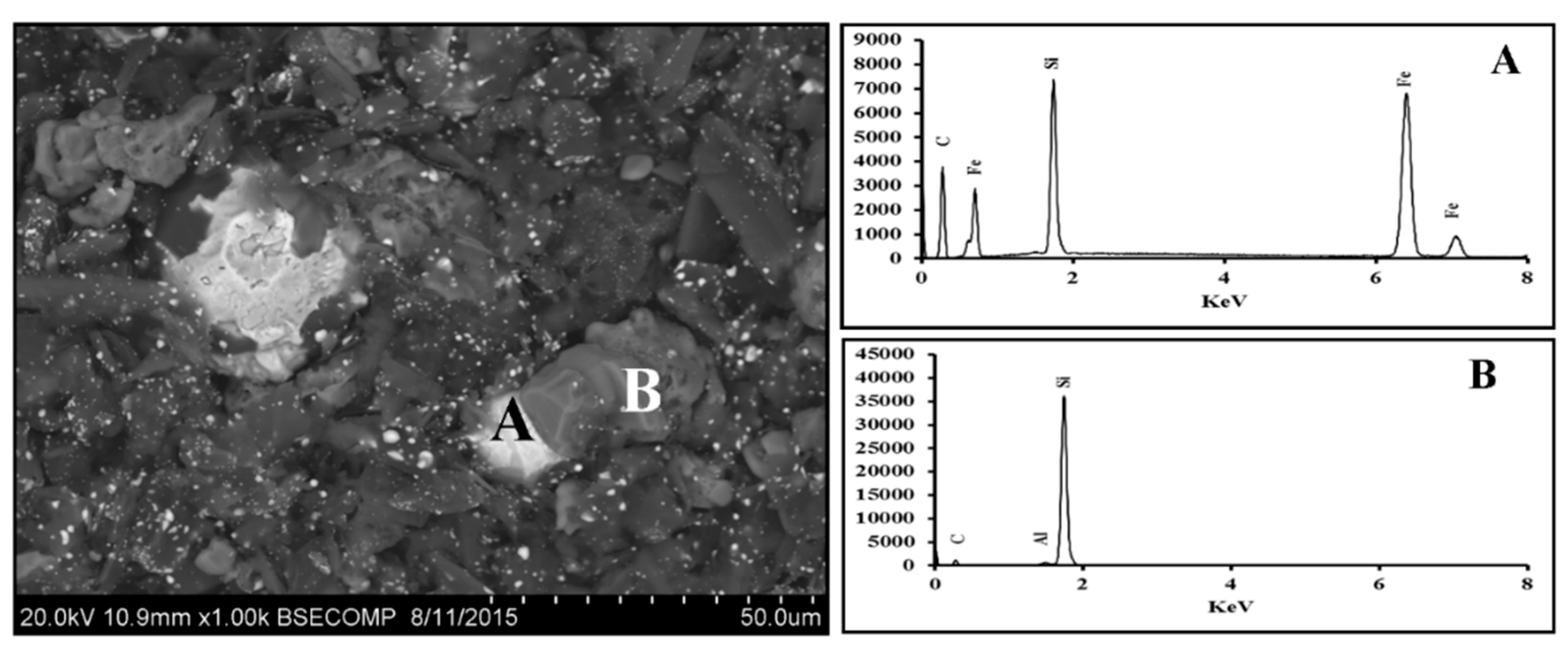
3.1. Fe2O3-SiO2-C System
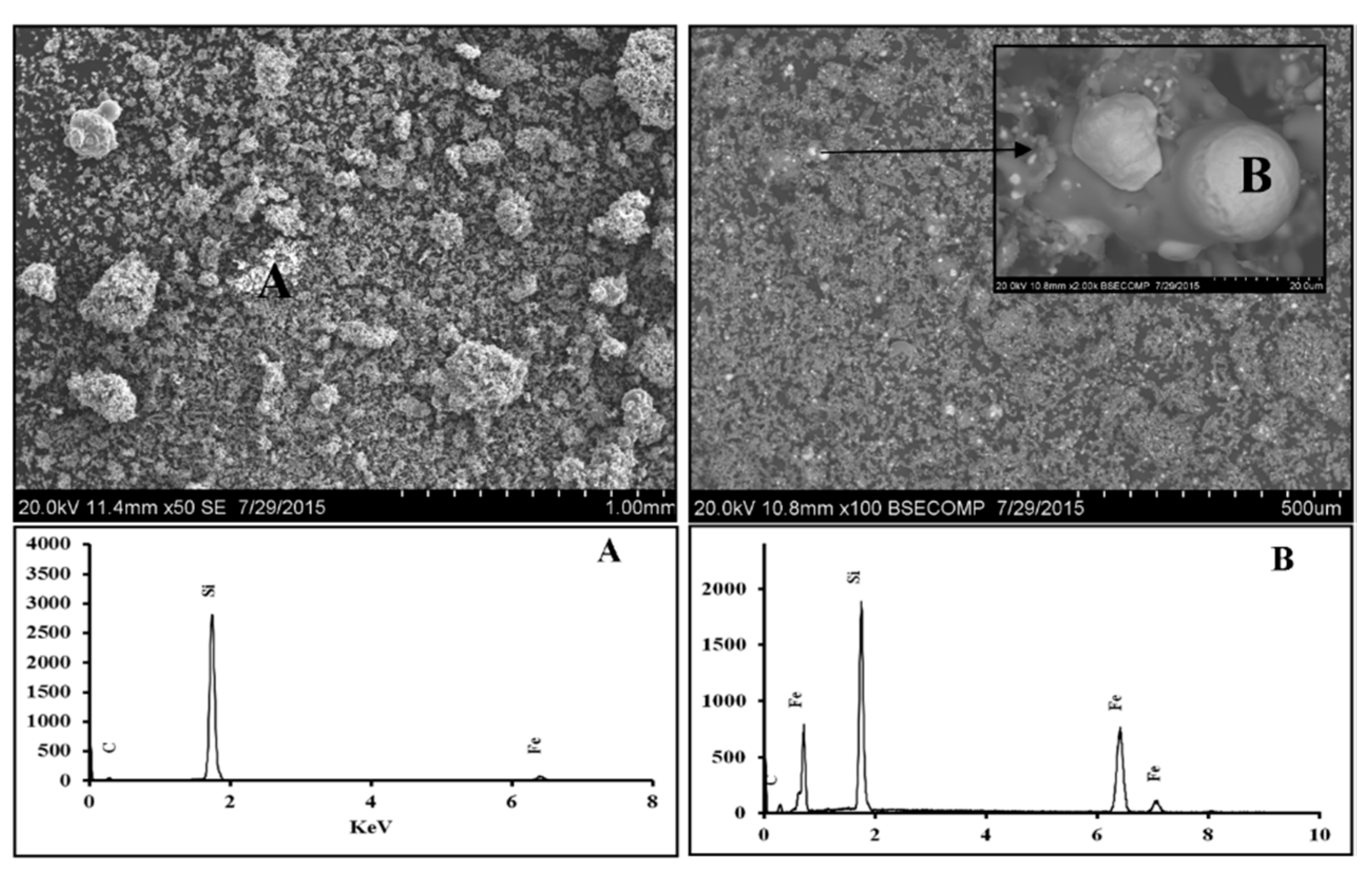
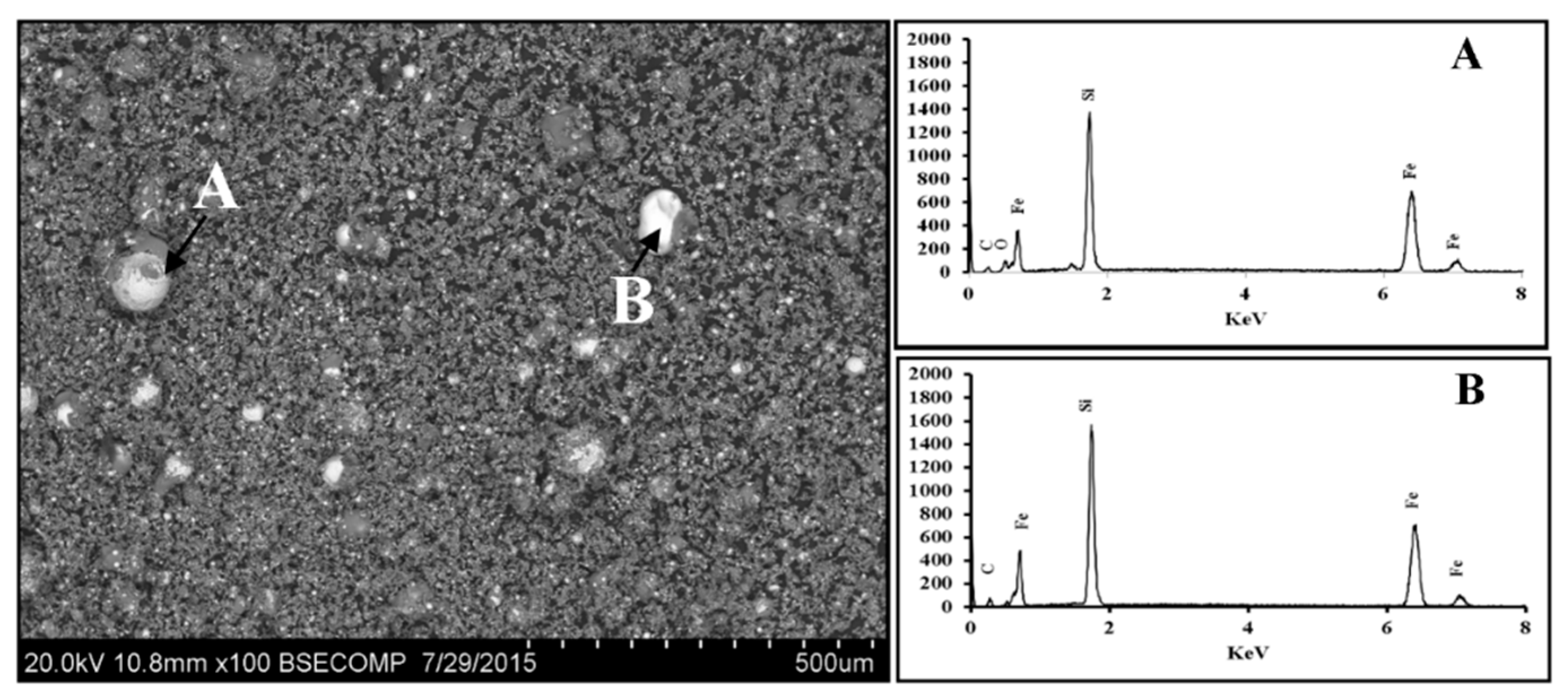
3.1.1. Scanning Electron Microscopy
3.1.2. X-ray Diffraction
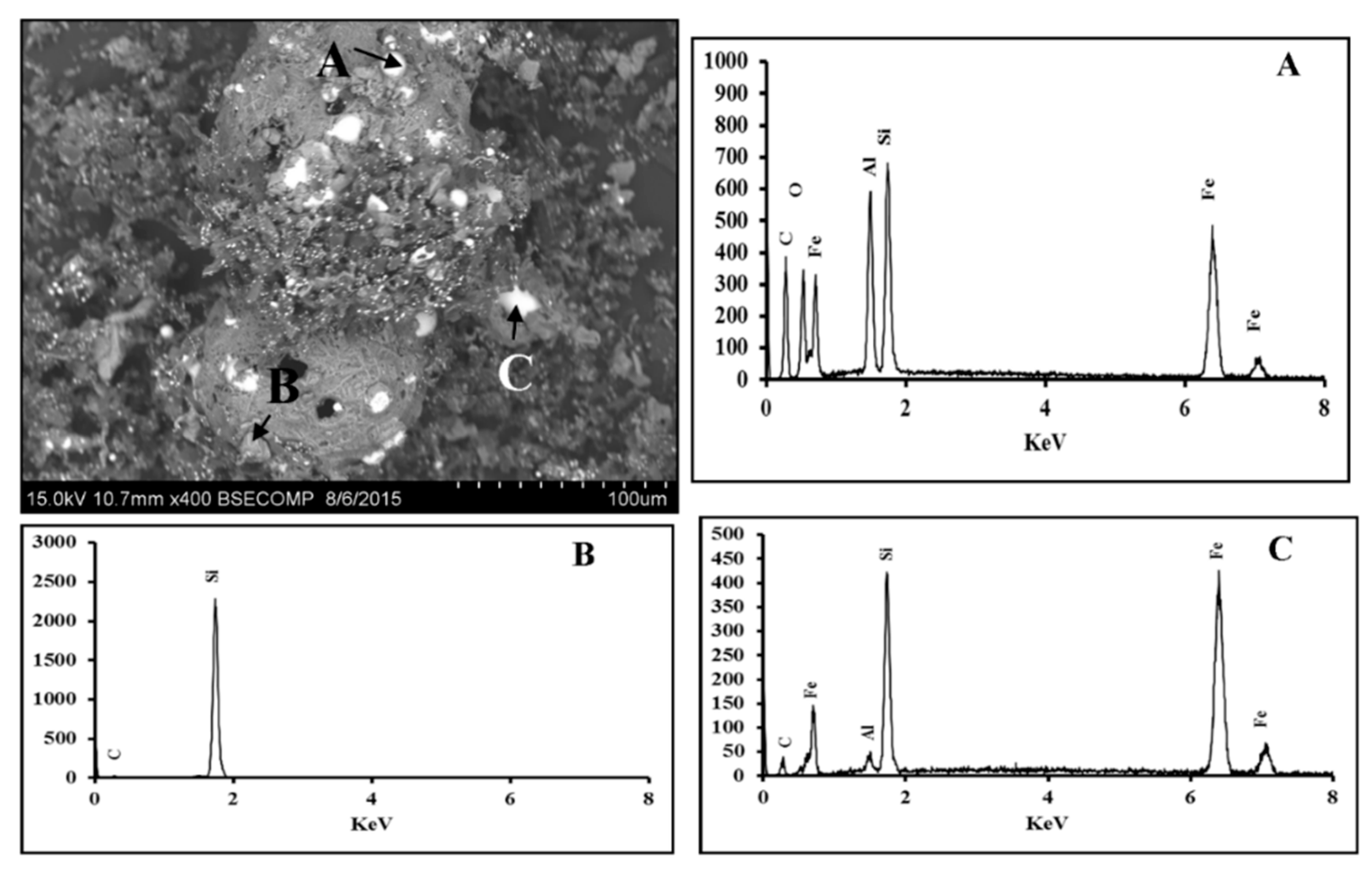
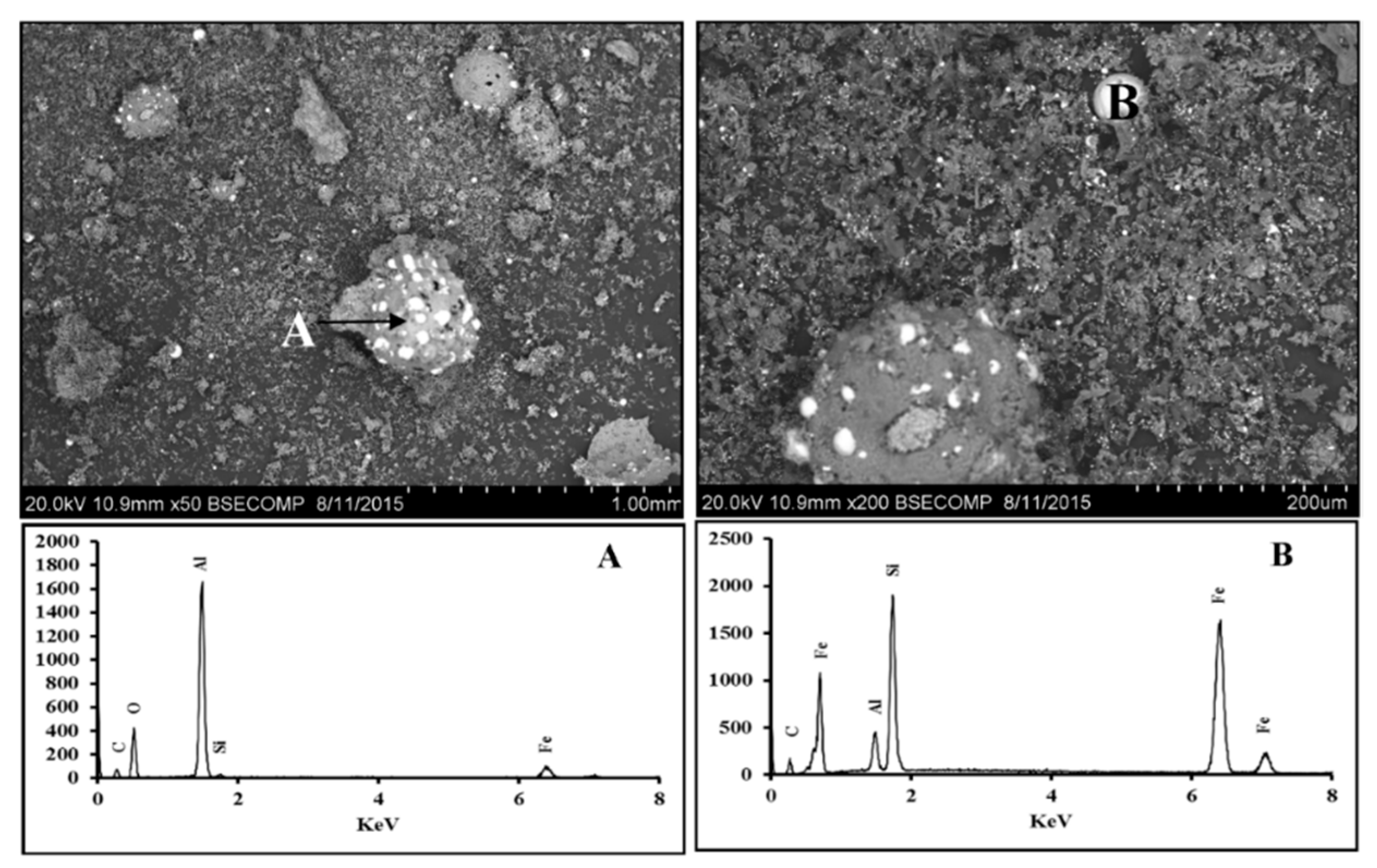
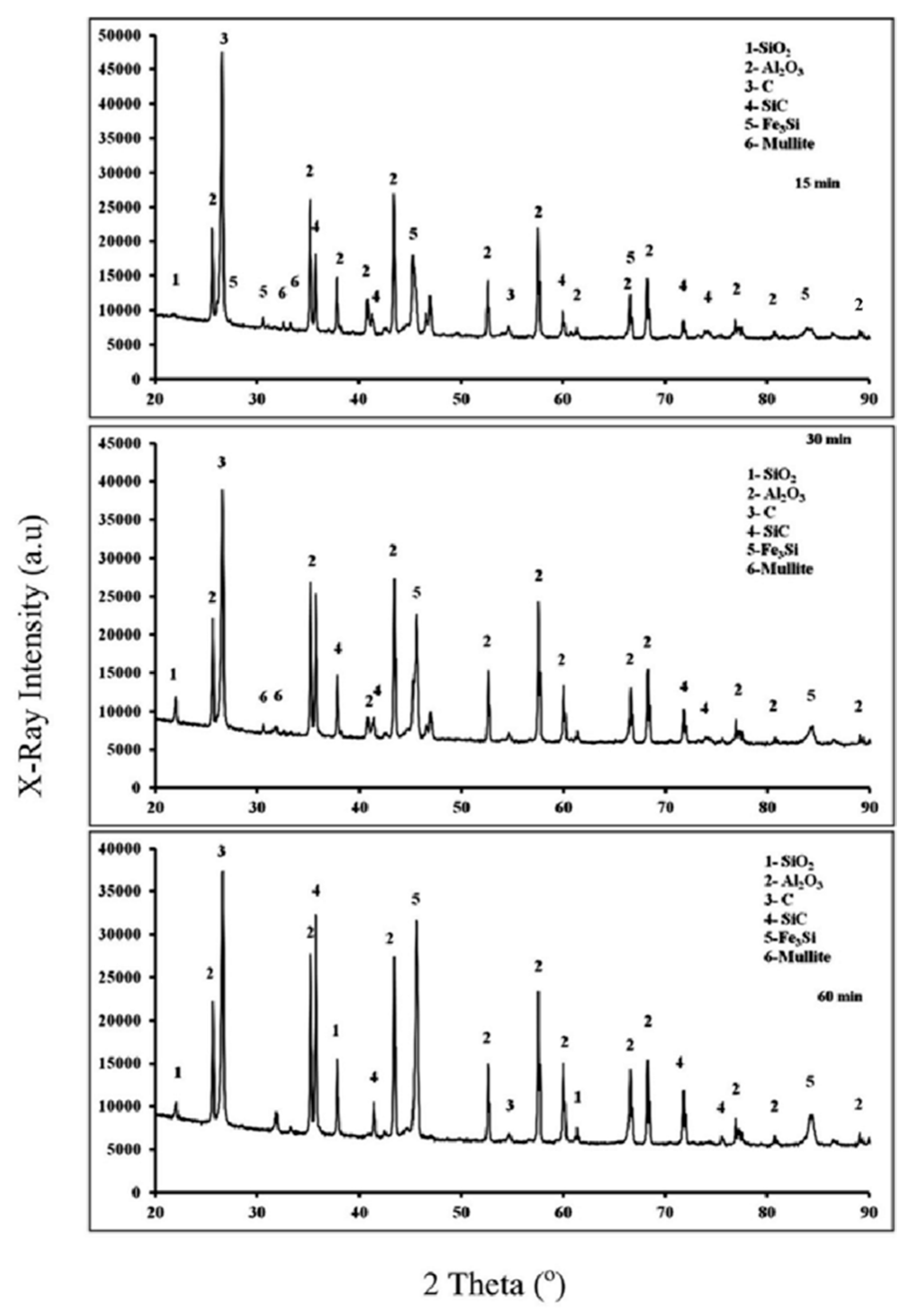
3.2. Fe2O3-Al2O3-SiO2-C System
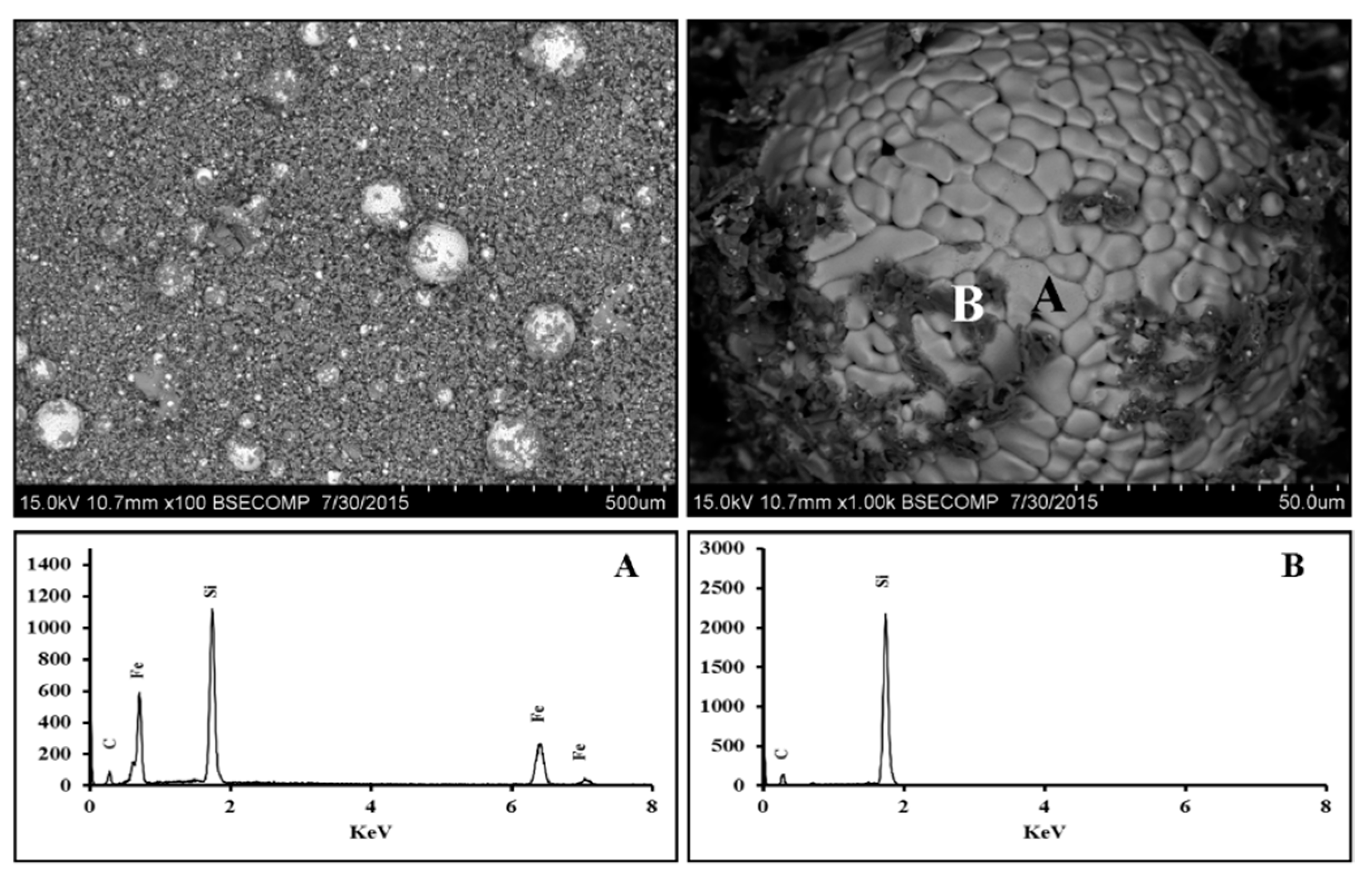
3.2.1. Scanning Electron Microscopy
3.2.2. X-ray Diffraction
3.3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steels for the Cars of Tomorrow. Available online: https://www.mpg.de/938220/Steel_for_the_Cars_of_Tomorrow (accessed on 26 July 2017).
- Chatterjee, S. Transformations in TRIP-Assisted Steels: Microstructure and Properties. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2006. [Google Scholar]
- World Motor Vehicle Production by Country and Type. Available online: http://www.oica.net/wp-content/uploads//Total-2016.pdf (accessed on 28 July 2017).
- Sutou, Y.; Kamiya, N.; Umino, R.; Ohnuma, R.; Ishida, I. High strength Fe-20Mn-Al-C alloys with low density. ISIJ Int. 2010, 50, 893–899. [Google Scholar] [CrossRef]
- Energy Trends in Selected Manufacturing Sectors: Opportunities and Challenges for Environmentally Preferable Energy Outcomes. Available online: https://archive.epa.gov/sectors/web/pdf/ch3-1.pdf (accessed on 26 July 2017).
- Grjotheim, K.; Krohan, C.; Malinovsky, M.; Malinovsky, K.; Thonstad, J. Aluminium Electrolysis: Fundamentals of the Hall-Heroult Process, 2nd ed.; Aluminium-Verlag: Dusseldorf, Germany, 1982. [Google Scholar]
- Mitigating Emissions from Aluminum. Available online: http://climate.columbia.edu/files/2012/04/GNCS-Aluminum-Factsheet.pdf (accessed on 28 July 2017).
- Ikram-Ul-Haq, M.; Khanna, R.; Mukherjee, P.S.; Sahajwalla, V. Recent developments in lower temperature carbothermic reduction of alumina as alternative routes for aluminium production. Mater. Sci. 2017, 11, 1–18. [Google Scholar]
- Halmann, M.; Epstein, M.; Steinfeld, A. Vacuum carbothermic reduction of alumina. Miner. Process. Extr. Metall. Rev. 2011, 32, 247–254. [Google Scholar] [CrossRef]
- Vishnevetsky, I.; Epstein, M.; Rubin, R. Solar carboreduction of alumina under vacuum. Energy Procedia 2014, 49, 2059–2069. [Google Scholar] [CrossRef]
- Wai, C.; Hutchison, S. A thermodynamic study of the carbothermic reduction of alumina in plasma. Metall. Mater. Trans. B 1990, 21, 406–408. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Liu, D.; Ostrovski, O. Low-temperature Synthesis of Aluminium Carbide. ISIJ Int. 2011, 51, 870–877. [Google Scholar] [CrossRef]
- Ferguson, R.P.; Cranford, N.J. Producing Aluminium Halides by the Reaction of Alumina, Carbon and Free Halogen. U.S. Patent 2,446,221 A, 3 August 1948. [Google Scholar]
- Dewan, M.; Rhamdhani, M.; Brooks, G.; Monaghan, B.; Prentice, L. Alternative Al production methods: Part 2—Thermodynamic analyses of indirect carbothermal routes 2013. Miner. Process. Extr. Metall. 2013, 122, 113–121. [Google Scholar] [CrossRef]
- Balomanos, E.; Panias, D.; Paspalaris, I.; Friedrich, B.; Jaroni, B.; Steinfeld, A.; Gugliemini, E.; Halman, M.; Epstein, M.; Vishnevsky, I. Carbothermic Reduction of Alumina: A Review of Developed Processes and Novel Concepts. In Proceedings of the EMC 2011, European Metallurgical Conference, Berlin, Germany, 26–29 June 2011; pp. 729–744. [Google Scholar]
- Frank, R.A.; Finn, C.W.; Elliot, J.F. Physical chemistry of the carbothermic reduction of alumina in the presence of a metallic solvent: Part II. Measurements of kinetics of reaction. Metall. Mater. Trans. B 1989, 20, 161–173. [Google Scholar] [CrossRef]
- Khanna, R.; IKram-ul-Haq, M.; Wang, Y.; Seetharaman, S.; Sahajwalla, V. Chemical interactions of alumina–carbon refractories with molten steel at 1823 K: Implications for refractory degradation and steel quality. Metall. Mater. Trans. B 2011, 42, 677–684. [Google Scholar] [CrossRef]
- Ikram-ul-Haq, M.; Khanna, R.; Kongkarat, S.; Sahajwalla, V. Chemical interactions in Al2O3-C/Fe system at 1823 K: Implications for refractory recycling. ISIJ Int. 2012, 52, 1801–1808. [Google Scholar] [CrossRef]
- Khanna, R.; Ikram-ul-Haq, M.; Seetharaman, S.; Sahajwalla, V. Carbothermic reduction of alumina at 1 823 K: On the role of molten iron and reaction mechanisms. ISIJ Int. 2016, 56, 1300–1302. [Google Scholar] [CrossRef]
- Khanna, R.; Ikram-ul-Haq, M.; Sadi, S.F.; Sahajwalla, V.; Mukherjee, P.S.; Seetharaman, S. Reduction reactions in Al2O3-C-Fe and Al2O3-C-Fe2O3 systems at 1823 K. ISIJ Int. 2014, 54, 1485–1490. [Google Scholar] [CrossRef]
- Khanna, R.; McCarthy, F.; Sun, H.; Sahajwalla, V.; Simento, N. Dissolution of carbon from coal-chars into liquid iron at 1550 °C. Metall. Mater. Trans. B 2014, 36, 719–729. [Google Scholar] [CrossRef]
- Rahman, M.; Khanna, R.; Sahajwalla, V.; O’Kane, P. The influence of ash impurities on interfacial reactions between carbonaceous materials and EAF slag at 1550 °C. ISIJ Int. 2009, 49, 329–336. [Google Scholar] [CrossRef]
- Sahajwalla, V.; Mehta, A.S.; Khanna, R. Influence of slag composition and ash impurities on the phenomena occurring in the carbon/slag interfacial region. Metall. Mater. Trans. B 2004, 35, 75–84. [Google Scholar] [CrossRef]
- Filsinger, D.H.; Bourrie, D.B. Silica to silicon: Key carbothermic reactions and kinetics. J. Am. Ceram. Soc. 1990, 73, 1726–1732. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikram-Ul-Haq, M.; Mukherjee, P.S.; Khanna, R. Formation of Light-Weight Ferroalloys in the Fe2O3-Al2O3-C System at 1550 °C: Influence of Silica Impurities. Metals 2017, 7, 391. https://doi.org/10.3390/met7100391
Ikram-Ul-Haq M, Mukherjee PS, Khanna R. Formation of Light-Weight Ferroalloys in the Fe2O3-Al2O3-C System at 1550 °C: Influence of Silica Impurities. Metals. 2017; 7(10):391. https://doi.org/10.3390/met7100391
Chicago/Turabian StyleIkram-Ul-Haq, Muhammad, Partha Sarathi Mukherjee, and Rita Khanna. 2017. "Formation of Light-Weight Ferroalloys in the Fe2O3-Al2O3-C System at 1550 °C: Influence of Silica Impurities" Metals 7, no. 10: 391. https://doi.org/10.3390/met7100391
APA StyleIkram-Ul-Haq, M., Mukherjee, P. S., & Khanna, R. (2017). Formation of Light-Weight Ferroalloys in the Fe2O3-Al2O3-C System at 1550 °C: Influence of Silica Impurities. Metals, 7(10), 391. https://doi.org/10.3390/met7100391



