Environmental Compatibility of Lightweight Aggregates from Mine Tailings and Industrial Byproducts
Abstract
:1. Introduction
2. Materials and Methods
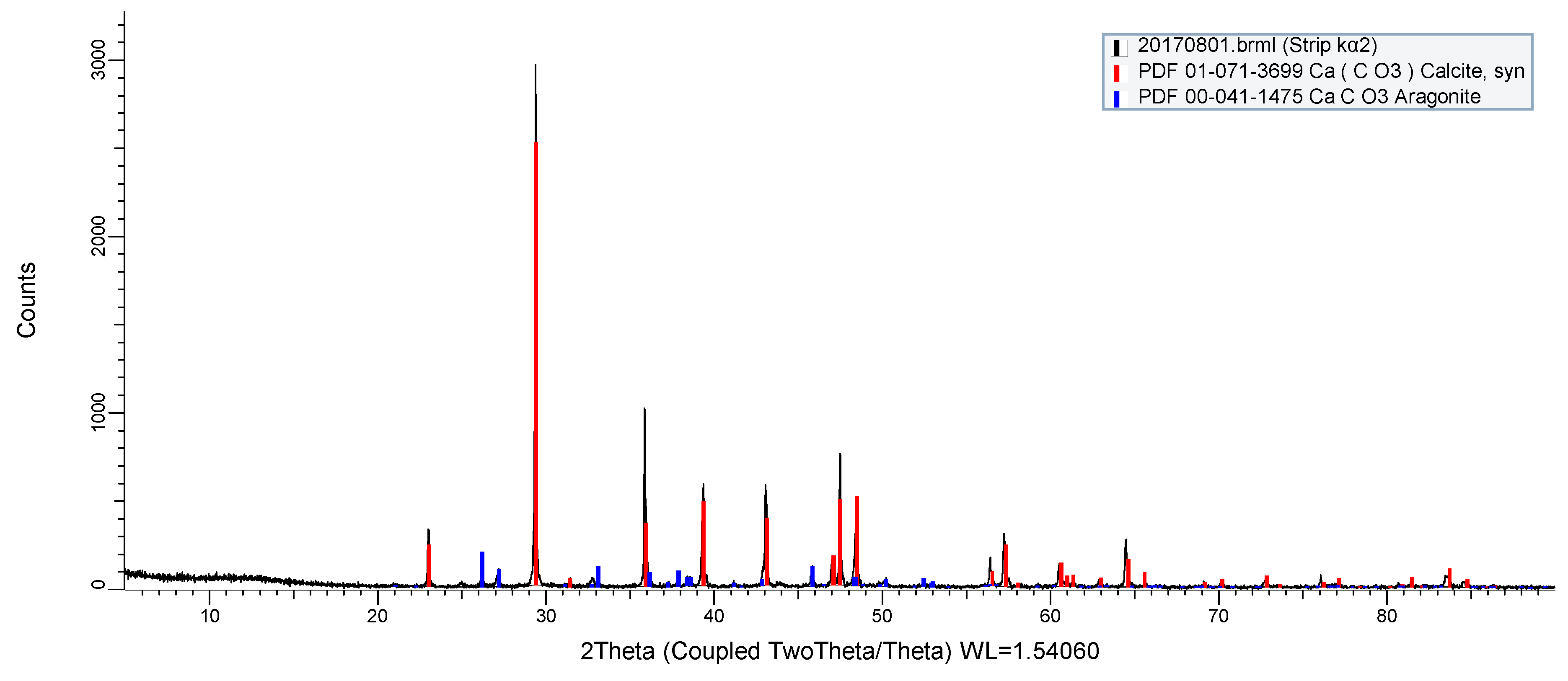
2.1. Sample Preparation and Characterization
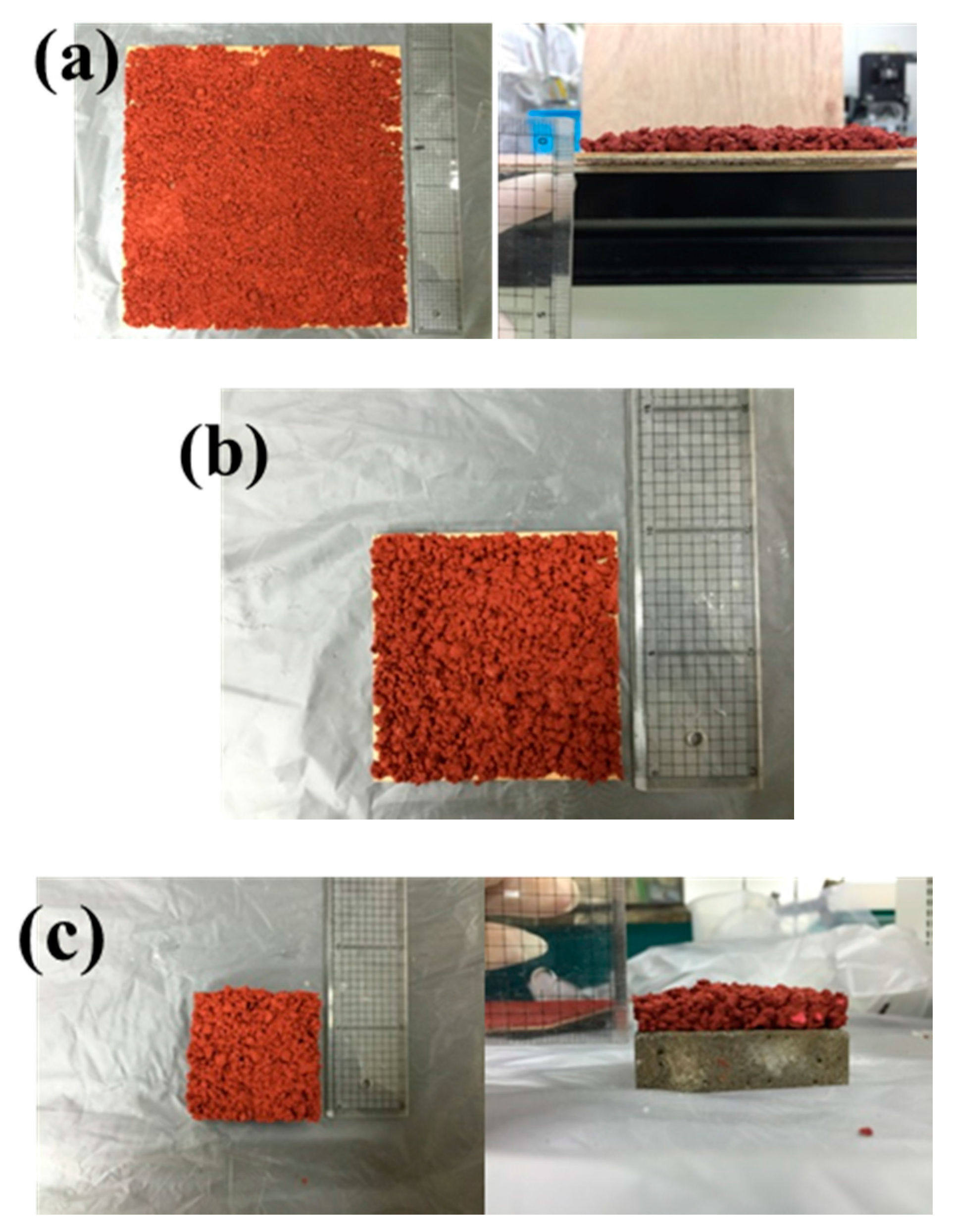
2.2. Physical Feasibility Tests
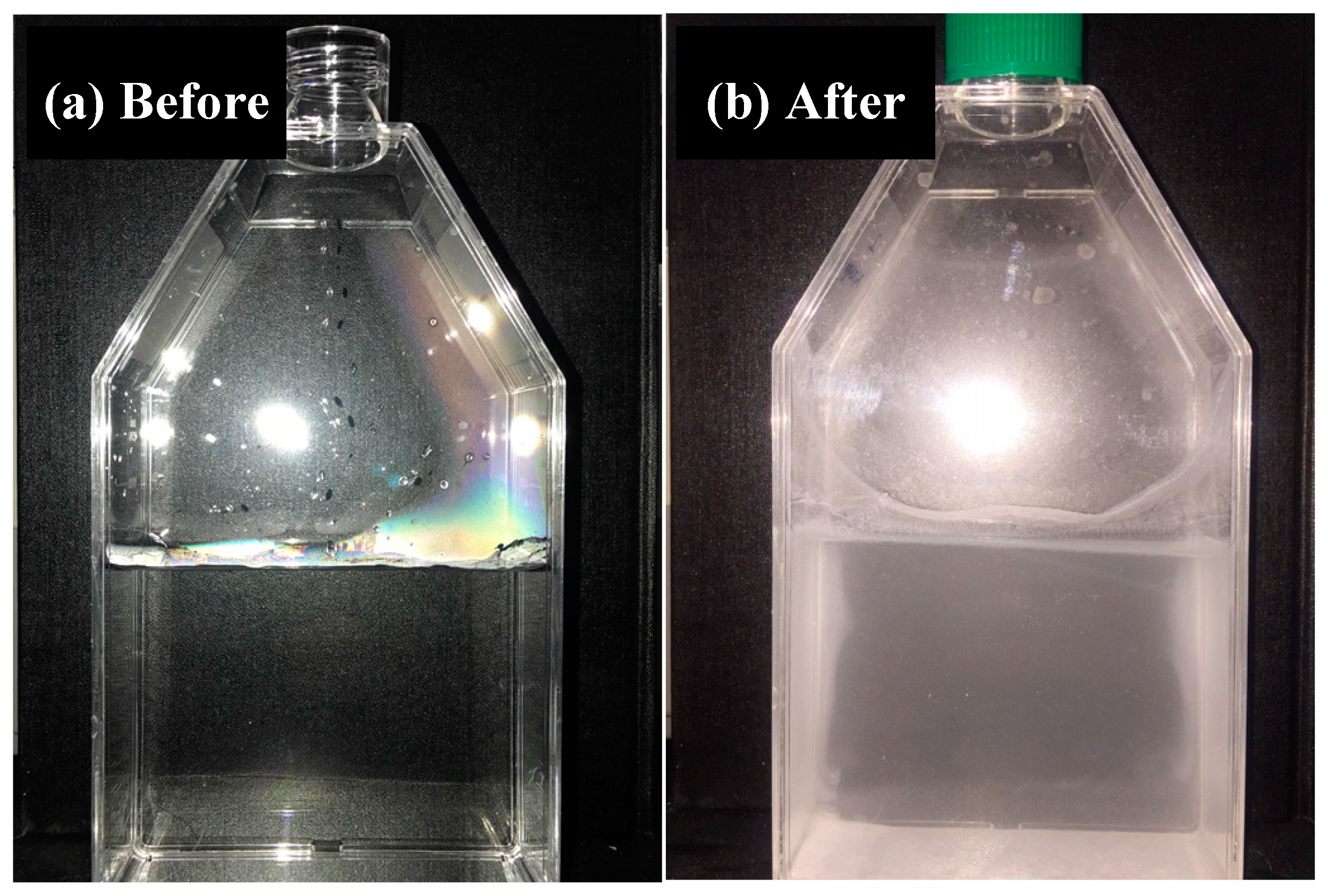
2.3. Leachability Tests
2.4. Toxicity Tests
2.5. Analytical Methods
3. Results
3.1. Physical Feasibility of the Lightweight Aggregate as Construction Materials for Bicycle Lanes
3.2. Environmental Compatibility of the Lightweight Aggregate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A

References
- Mine Reclamation Corporation. Yearbook of MIRECO Statistics; Mine Reclamation Corporation: Seoul, Korea, 2015. [Google Scholar]
- Mine Reclamation Corporation. Development of Characterizing Method for Mine Tailing; Mine Reclamation Corporation: Seoul, Korea, 2012. [Google Scholar]
- Ritcey, G.M. Tailings Management: Problems and Solutions in the Mining Industry; Elsevier Science: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Van Zyl, D. Mine waste disposal. In Geotechnical Practice for Waste Disposal; Daniel, D.E., Ed.; Springer: Boston, MA, USA, 1993; pp. 269–286. [Google Scholar]
- Fourie, A. Preventing catastrophic failures and mitigating environmental impacts of tailings storage facilities. Proc. Earth Planet. Sci. 2009, 1, 1067–1071. [Google Scholar] [CrossRef]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. Options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar]
- Benzaazoua, M.; Bussière, B.; Demers, I.; Aubertin, M.; Fried, É.; Blier, A. Integrated mine tailings management by combining environmental desulphurization and cemented paste backfill: Application to mine Doyon, Quebec, Canada. Miner. Eng. 2008, 21, 330–340. [Google Scholar] [CrossRef]
- Roy, S.; Adhikari, G.R.; Gupta, R.N. Use of gold mill tailings in making bricks: A feasibility study. Waste Manag. 2007, 25, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Ahmari, S.; Chen, R.; Zhang, L. Utilization of Mine Tailings As Road Base Material. In Proceedings of the GeoCongress, Oakland, CA, USA, 25–29 March 2012; pp. 3654–3661. [Google Scholar]
- Owens, P.; Newman, J. Structural lightweight aggregate concrete: The future? Concr. Lond. Concr. Soc. 1999, 33, 45–47. [Google Scholar]
- Tang, C.-W.; Chen, H.-J.; Wang, S.-Y.; Spaulding, J. Production of synthetic lightweight aggregate using reservoir sediments for concrete and masonry. Cem. Concr. Comp. 2011, 33, 292–300. [Google Scholar] [CrossRef]
- Chen, H.-J.; Wang, S.-Y.; Tang, C.-W. Reuse of incineration fly ashes and reaction ashes for manufacturing lightweight aggregate. Constr. Build. Mater. 2010, 24, 46–55. [Google Scholar] [CrossRef]
- Molineux, C.J.; Newport, D.J.; Ayati, B.; Wang, C.; Connop, S.P.; Green, J.E. Bauxite residue (red mud) as a pulverised fuel ash substitute in the manufacture of lightweight aggregate. J. Clean. Prod. 2016, 112, 401–408. [Google Scholar] [CrossRef]
- Shafigh, P.; Nomeli, M.A.; Alengaram, U.J.; Mahmud, H.B.; Jumaat, M.Z. Engineering properties of lightweight aggregate concrete containing limestone powder and high volume fly ash. J. Clean. Prod. 2016, 135, 148–157. [Google Scholar] [CrossRef]
- Mun, K.J. Development and tests of lightweight aggregate using sewage sludge for nonstructural concrete. Constr. Build. Mater. 2007, 21, 1583–1588. [Google Scholar] [CrossRef]
- Turkdogan, E.T. Fundamentals of Steelmaking; Maney Publishing: Leeds, UK, 1996; Volume 614. [Google Scholar]
- Petkovic, G.; Engelsen, C.J.; Håøya, A.-O.; Breedveld, G. Environmental impact from the use of recycled materials in road construction: method for decision-making in Norway. Resour. Conserv. Recycl. 2004, 42, 249–264. [Google Scholar] [CrossRef]
- Chang, F.-C.; Lo, S.-L.; Lee, M.-Y.; Ko, C.-H.; Lin, J.-D.; Huang, S.-C.; Wang, C.-F. Leachability of metals from sludge-based artificial lightweight aggregate. J. Hazard. Mater. 2007, 146, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Maghool, F.; Arulrajah, A.; Du, Y.-J.; Horpibulsuk, S.; Chinkulkijniwat, A. Environmental impacts of utilizing waste steel slag aggregates as recycled road construction materials. Clean Technol. Environ. Policy 2017, 19, 949–958. [Google Scholar] [CrossRef]
- Park, H.; Ha, M.; Shin, D.; Kim, M.; Sohn, I. Potential utilization of hazardous mining wastes in the production of lightweight aggregates. Miner. Process. Extr. Metall. 2017, 1–10. [Google Scholar] [CrossRef]
- Korea Ministry of Environment. Korea Standard Testing Methods for Soil Contamination. Available online: http://www.law.go.kr/ (accessed on 1 October 2015).
- Korea Ministry of Environment. Korea Standard Testing Methods for Solid Wastes. Available online: http://www.law.go.kr/ (accessed on 1 October 2015).
- United States of Environmental Protection Agency. T.C.L.P. EPA Method 1311; United States of Environmental Protection Agency: Washington, DC, USA, 1990.
- United States of Environmental Protection Agency. S.P.L.P. EPA Method 1312; United States of Environmental Protection Agency: Washington, DC, USA, 1994.
- British Standards Institution. British Standards. BS EN 12457-1. Characterisation of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludges. One Stage Batch Test at a Liquid To Solid Ratio of 2 L/kg for Materials with High Solid Content and with Particle Size below 4 mm (without or with Size Reduction); British Standards Institution: London, UK, 2002. [Google Scholar]
- Korea Ministry of Environment. Korea Standard Testing Methods for Water Quality. Available online: http://www.law.go.kr/ (accessed on 1 October 2015).
- Epstein, J. Estimation of microquantities of cyanide. Anal. Chem. 1947, 19, 272–274. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, Method 4500 FD, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Department of Planning, Transport and Infrastructure. Guide to Bikeway Pavement Design Construction & Maintenance for South Australia. Available online: https://www.dpti.sa.gov.au/__data/assets/pdf_file/0006/149964/DPTI_Bikeway_Pavement_Guidelines_2.pdf (accessed on 10 August 2016).
- Huang, S.-C.; Chang, F.-C.; Lo, S.-L.; Lee, M.-Y.; Wang, C.-F.; Lin, J.-D. Production of lightweight aggregates from mining residues, heavy metal sludge, and incinerator fly ash. J. Hazard. Mater. 2007, 144, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, C.R.; Makinde, A.; Bethanis, S. Properties of lightweight aggregate produced by rapid sintering of incinerator bottom ash. Resour. Conserv. Recycl. 2005, 43, 147–162. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, C.; Wu, Y. Characterization of red mud derived from a combined Bayer Process and bauxite calcination method. J. Hazard. Mater. 2007, 146, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Qu, J.K.; Qi, T.; Wei, G.Y.; Han, B.B. Activation pretreatment of limonitic laterite ores by alkali-roasting using NaOH. Int. J. Min. Met. Mater. 2012, 19, 100–105. [Google Scholar] [CrossRef]
- Mayes, W.M.; Younger, P.L.; Aumônier, J. Hydrogeochemistry of Alkaline steel slag leachates in the UK. Water Air Soil Pollut. 2008, 195, 35–50. [Google Scholar] [CrossRef]
- Mayes, W.M.; Younger, P.L. Buffering of alkaline steel slag leachate across a natural wetland. Environ. Sci. Technol. 2006, 40, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Proctor, D.M.; Shay, E.C.; Fehling, K.A.; Finley, B.L. Assessment of human health and ecological risks posed by the uses of steel-industry slags in the environment. Hum. Ecol. Risk Assess. 2002, 8, 681–711. [Google Scholar] [CrossRef]
- Rendal, C.; Kusk, K.O.; Trapp, S. The effect of pH on the uptake and toxicity of the bivalent weak base chloroquine tested on Salix viminalis and Daphnia magna. Environ. Toxicol. Chem. 2011, 30, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, K.E.; Christensen, G.M. Effects of various metals on survival, growth, reproduction, and metabolism of Daphnia magna. J. Fish. Res. Board Can. 1972, 29, 1691–1700. [Google Scholar] [CrossRef]
- Shi, C. Steel Slag—Its production, processing, characteristics, and cementitious properties. J. Mater. Civ. Eng. 2004, 16, 230–236. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Early strength development and hydration of alkali-activated blast furnace slag/fly ash blends. Adv. Chem. Res. 1999, 11, 189–196. [Google Scholar] [CrossRef]
- Cusidó, J.A.; Cremades, L.V. Environmental effects of using clay bricks produced with sewage sludge: Leachability and toxicity studies. Waste Manag. 2012, 32, 1202–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhang, M.H.; Ou, J.P. Abrasion resistance of concrete containing nano-particles for pavement. Wear 2006, 260, 1262–1266. [Google Scholar] [CrossRef]
| Element | Content (wt. %) | |||
|---|---|---|---|---|
| Mine Tailings | Red Mud | Waste Limestone | Lightweight Aggregate | |
| O | 50.00 | 37.50 | 31.80 | 36.00 |
| Si | 36.38 | 5.63 | 4.55 | 23.06 |
| Ca | 0.64 | 5.02 | 56.43 | 15.53 |
| Fe | 0.67 | 26.89 | 1.97 | 11.54 |
| Al | 7.29 | 13.19 | 1.84 | 7.02 |
| K | 3.94 | 0.05 | 0.85 | 2.21 |
| Ti | 0.11 | 4.59 | 0.16 | 1.76 |
| Na | 0.14 | 6.32 | - | 1.36 |
| S | 0.30 | 0.13 | 0.04 | 0.46 |
| Mg | 0.22 | 0.14 | 1.65 | 0.53 |
| Mn | 0.06 | 0.03 | 0.51 | 0.22 |
| Zr | 0.01 | 0.23 | - | 0.12 |
| Cr | 0.02 | 0.08 | - | 0.05 |
| Rb | 0.02 | - | 0.01 | 0.02 |
| Sr | 0.01 | - | 0.1 | 0.03 |
| Pb | 0.01 | - | - | - |
| Element | Mine Tailings | Red Mud | Waste Limestone |
|---|---|---|---|
| As | 12.64 ± 0.24 | 15.23 ± 0.40 | 6.91 ± 0.21 |
| Cd | ND | 1.32 ± 0.05 | ND |
| cyanide | ND | ND | ND |
| Cr | 87.53 ± 0.55 | 290.58 ± 2.32 | 4.18 ± 0.05 |
| Cr(VI) | ND | ND | ND |
| Cu | 13.83 ± 0.32 | 10.90 ± 0.10 | 27.83 ± 1.70 |
| F | 308.67 ± 28.57 | 472.00 ± 50.48 | 424.33 ± 3.51 |
| Hg | ND | ND | ND |
| Ni | 2.27 ± 0.06 | 19.90 ± 0.10 | 21.83 ± 0.84 |
| Pb | 107.93 ± 2.36 | 40.27 ± 0.25 | 5.83 ± 0.40 |
| Zn | 28.77 ± 1.34 | 20.43 ± 0.40 | 15.50 ± 1.50 |
| Element | Mine Tailings | Red Mud | Waste Limestone | Lightweight Aggregate | Regulatory Standards | ||
|---|---|---|---|---|---|---|---|
| (A) KSTM-SW (pH 5.8–6.3) (unit: mg·L−1) | Korea Waste Control Act | ||||||
| As | ND | ND | ND | ND | 1.5 | ||
| Cd | ND | ND | ND | ND | 0.3 | ||
| CN | ND | ND | ND | ND | 1 | ||
| Cr | ND | 0.653 ± 0.025 | ND | 0.045 ± 0.006 | - | ||
| Cr(VI) | ND | ND | ND | ND | 1.5 | ||
| Cu | ND | ND | ND | ND | 3.0 | ||
| Hg | ND | ND | ND | ND | 0.005 | ||
| Ni | ND | ND | ND | ND | - | ||
| Pb | ND | ND | ND | ND | 3.0 | ||
| Zn | ND | ND | ND | ND | |||
| Ca | 51.81 ± 0.39 | 1.67 ± 0.49 | 6.92 ± 0.14 | 1116.85 ± 45.50 | |||
| Mg | 4.35 ± 0.06 | 0.01 ± 0.00 | 1.49 ± 0.02 | 0.03 ± 0.00 | |||
| Leachate pH | 8.05 ± 0.14 | 11.85 ± 0.01 | 9.58 ± 0.13 | 12.65 ± 0.03 | |||
| (B) TCLP (pH 2.88) (unit: mg·L−1) | USEPA Land Disposal Restrictions Regulations | ||||||
| As | ND | ND | ND | ND | 5.0 | ||
| Cd | ND | ND | ND | ND | 1.0 | ||
| CN | ND | ND | ND | ND | - | ||
| Cr | 0.263 ± 0.057 | 0.244 ± 0.015 | ND | 0.055 ± 0.011 | 5.0 | ||
| Cr(VI) | ND | ND | ND | ND | - | ||
| Cu | 0.967 ± 0.695 | ND | 0.474 ± 0.099 | 0.133 ± 0.192 | - | ||
| Hg | ND | ND | ND | ND | - | ||
| Ni | ND | ND | 0.134 ± 0.189 | ND | - | ||
| Pb | ND | ND | ND | ND | 5.0 | ||
| Zn | 0.591 ± 0.046 | 0.005 ± 0.000 | 0.105 ± 0.035 | ND | - | ||
| Leachate pH | 3.37 ± 0.01 | 5.27 ± 0.02 | 5.73 ± 0.01 | 12.62 ± 0.02 | |||
| (C) SPLC (pH 5) (unit: mg·L−1) | USEPA Land Disposal Restrictions Regulations | ||||||
| As | ND | ND | ND | ND | 5.0 | ||
| Cd | ND | ND | ND | ND | 1.0 | ||
| CN | ND | ND | ND | ND | - | ||
| Cr | ND | 0.417 ± 0.021 | 0.010 ± 0.014 | 0.036 ± 0.001 | 5.0 | ||
| Cr(VI) | ND | ND | ND | ND | - | ||
| Cu | 0.129 ± 0.183 | 1.399 ± 2.384 | 0.199 ± 0.305 | ND | - | ||
| Hg | ND | ND | ND | ND | - | ||
| Ni | ND | ND | ND | ND | - | ||
| Pb | ND | ND | ND | 0.106 ± 0.139 | 5.0 | ||
| Zn | ND | 0.097 ± 0.158 | ND | ND | - | ||
| Leachate pH | 8.61 ± 0.11 | 11.67 ± 0.01 | 9.60 ± 0.01 | 12.84 ± 0.01 | |||
| (D) EU Method (pH 5–7.5) (unit: mg·kg−1) | European Legislation for Solid Wastes | ||||||
| Inert Waste | Non-Hazardous Waste | Hazardous Waste | |||||
| As | ND | 0.071 ± 0.000 | ND | ND | 0.5 | 2 | 25 |
| Cd | ND | ND | ND | ND | 0.04 | 1 | 5 |
| CN | ND | ND | ND | ND | - | - | - |
| Cr | ND | 0.034 ± 0.006 | ND | 0.032 ± 0.009 | 0.5 | 10 | 70 |
| Cr(VI) | ND | ND | ND | ND | - | - | - |
| Cu | ND | 0.260 ± 0.175 | 0.023 ± 0.000 | ND | 2 | 50 | 100 |
| Hg | ND | ND | ND | ND | - | - | - |
| Ni | ND | ND | ND | ND | 0.40 | 10 | 40 |
| Pb | ND | ND | ND | ND | 0.5 | 10. | 50 |
| Zn | ND | 0.060 ± 0.009 | 0.005 ± 0.000 | ND | 4 | 50 | 200 |
| Leachate pH | 7.78 ± 0.09 | 12.32 ± 0.00 | 9.39 ± 0.03 | 12.84 ± 0.01 | |||
| Sample | Mine Tailings | Red Mud | Waste Limestone | Lightweight Aggregate |
|---|---|---|---|---|
| 24 h TU | 1.36 | 12.8 | 0.17 | 10.67 |
| 48 h TU | 1.46 | 30.4 | 1.50 | 10.67 |
| Sample | As-Is Leachate | pH-Adjusted Leachate | Ca-Removed Leachate | Ca-Removed and pH-Adjusted Leachate |
|---|---|---|---|---|
| Ca Concentration (mg·L−1) | 1116.85 ± 45.50 | 1116.85 ± 45.50 | 96.32 ± 1.49 | 96.32 ± 1.49 |
| pH | 12.65 ± 0.03 | 5.97 ± 0.02 | 11.72 ± 0.01 | 6.00 ± 0.01 |
| 24 h TU | 10.67 | 1.31 | 3.47 | 0.60 |
| 48 h TU | 10.67 | 1.33 | 6.67 | 1.50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, W.J.; Shin, D.; Park, H.; Nam, K. Environmental Compatibility of Lightweight Aggregates from Mine Tailings and Industrial Byproducts. Metals 2017, 7, 390. https://doi.org/10.3390/met7100390
Ju WJ, Shin D, Park H, Nam K. Environmental Compatibility of Lightweight Aggregates from Mine Tailings and Industrial Byproducts. Metals. 2017; 7(10):390. https://doi.org/10.3390/met7100390
Chicago/Turabian StyleJu, Won Jung, Doyun Shin, Hyunsik Park, and Kyoungphile Nam. 2017. "Environmental Compatibility of Lightweight Aggregates from Mine Tailings and Industrial Byproducts" Metals 7, no. 10: 390. https://doi.org/10.3390/met7100390





