Structure and Martensitic Transformation in Rapidly Solidified CoNiAlFe Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
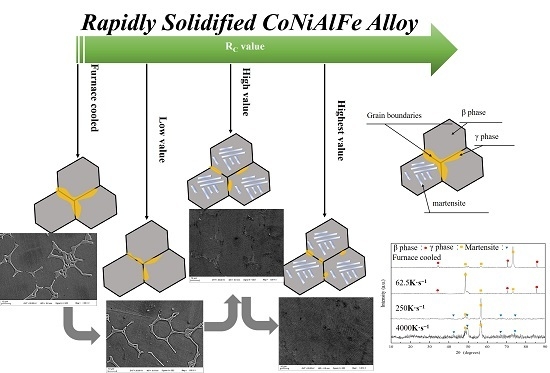
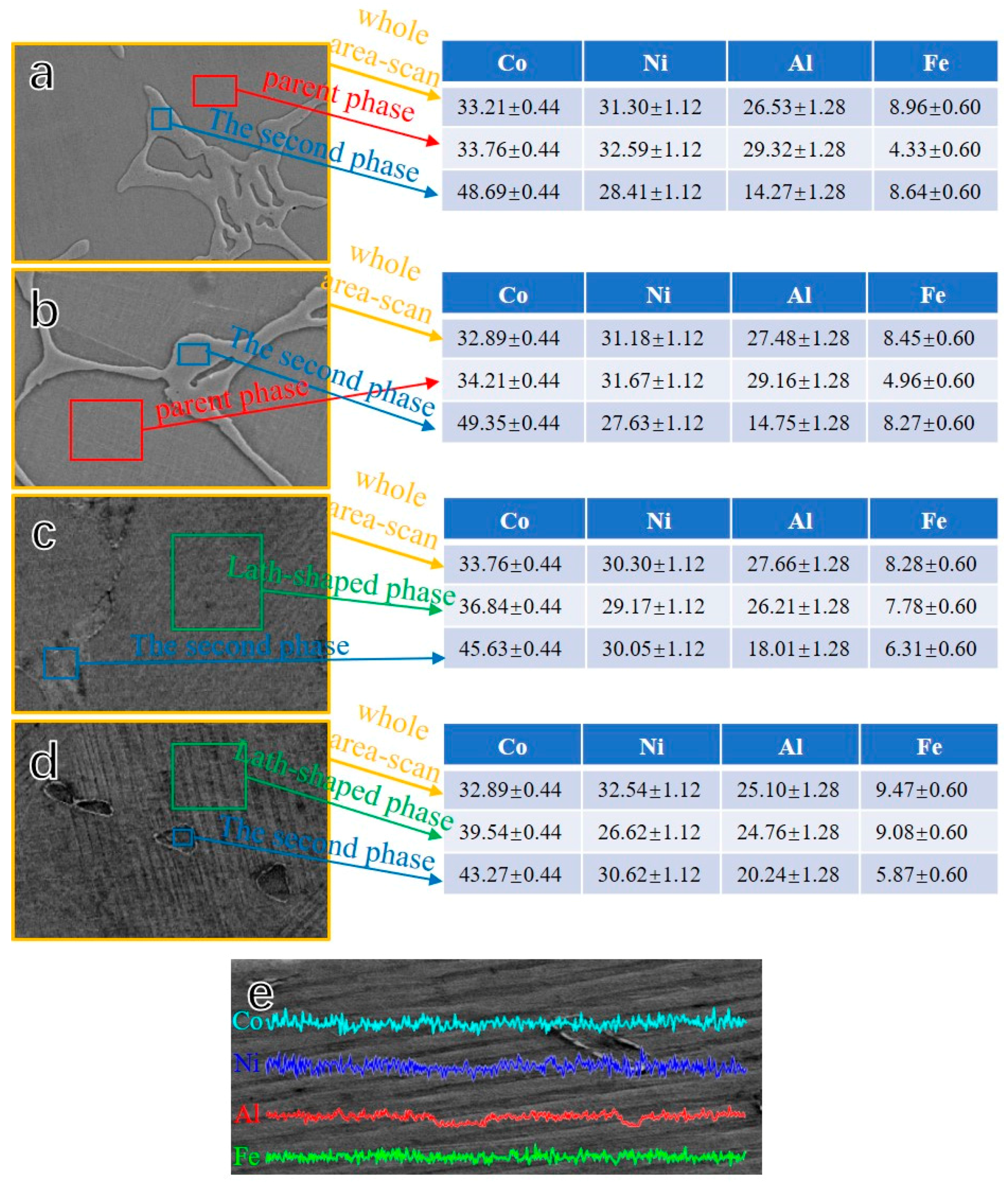
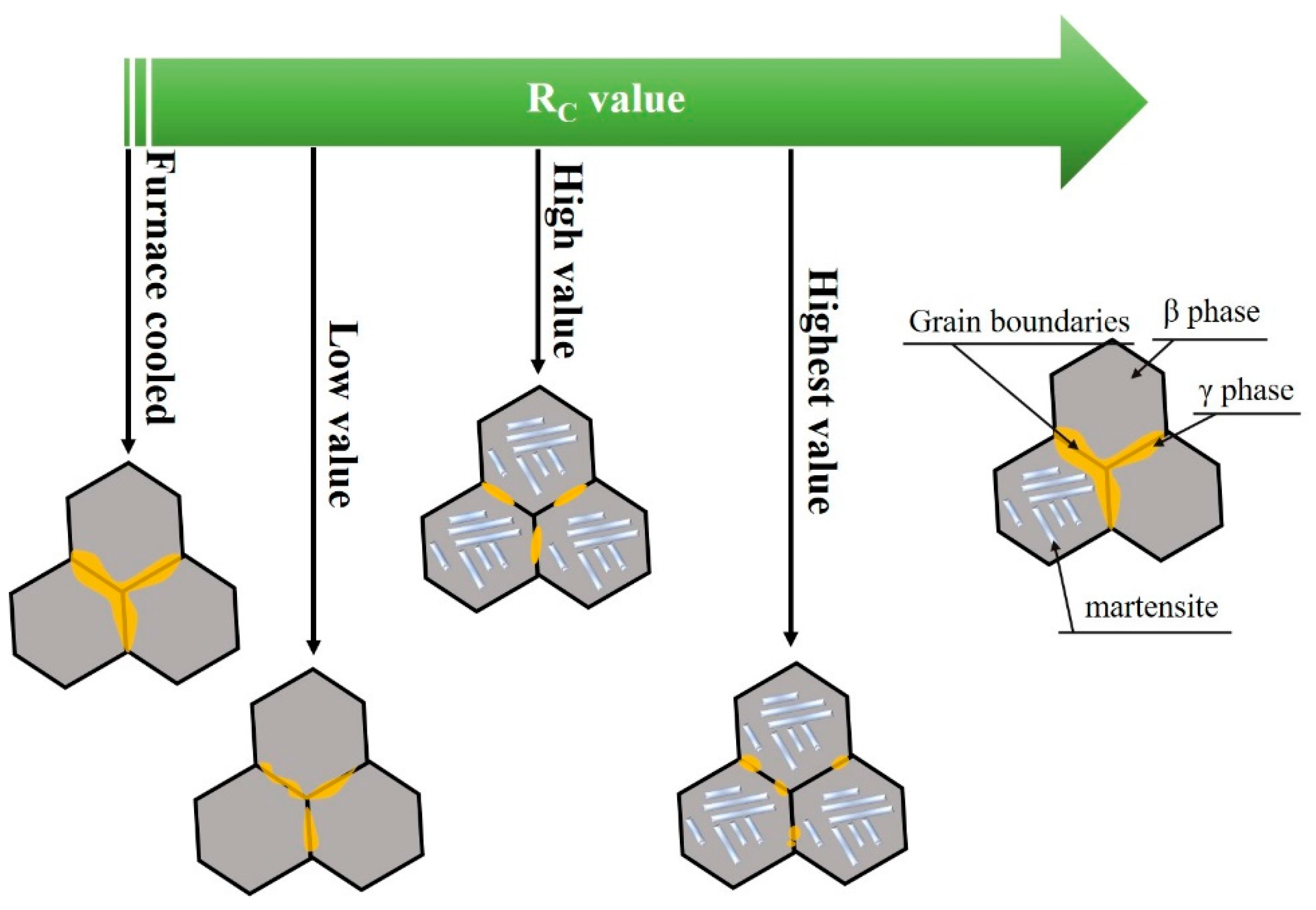
3.1. Microstructure
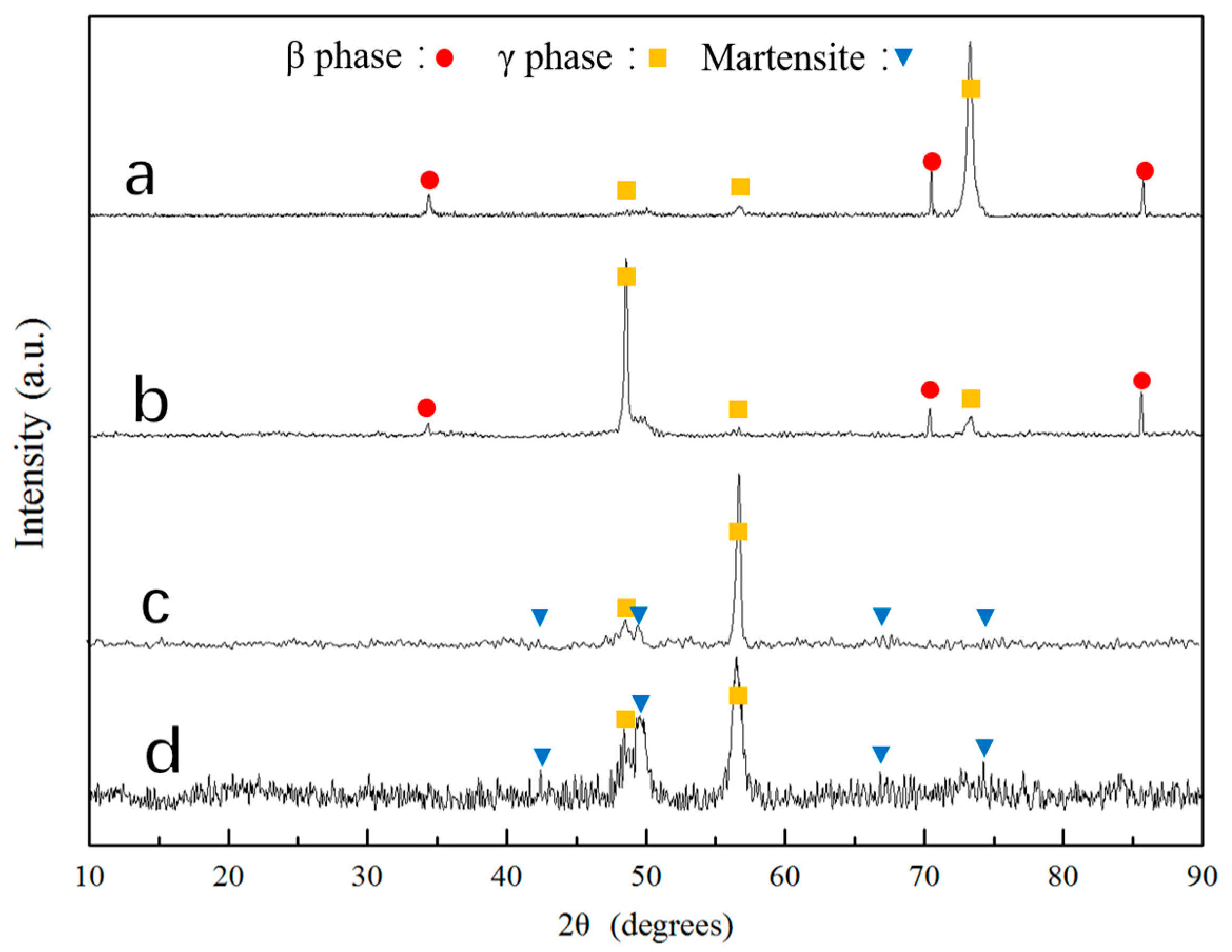
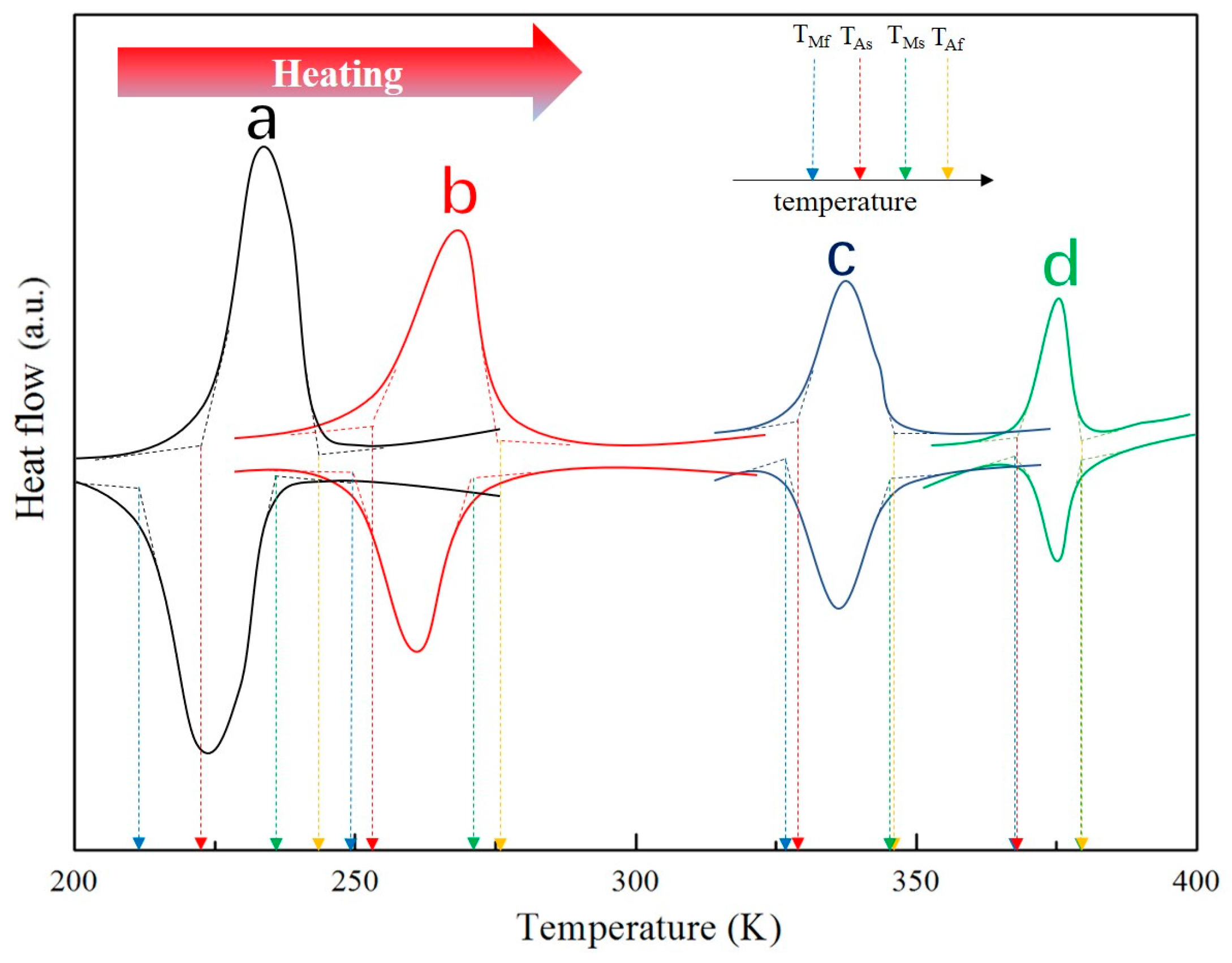
3.2. Martensitic Transformation
4. Conclusions
- (1)
- The microstructure of Co33Ni31Al27Fe9 alloy with furnace cooled and RS corresponded to a dual-phase structure, β phase + γ phase in furnace cooled sample, and D8; martensite + γ phase in D4 and D1. The γ phase at the grain boundaries tended to be limited and more fragile, when the RC value was raised.
- (2)
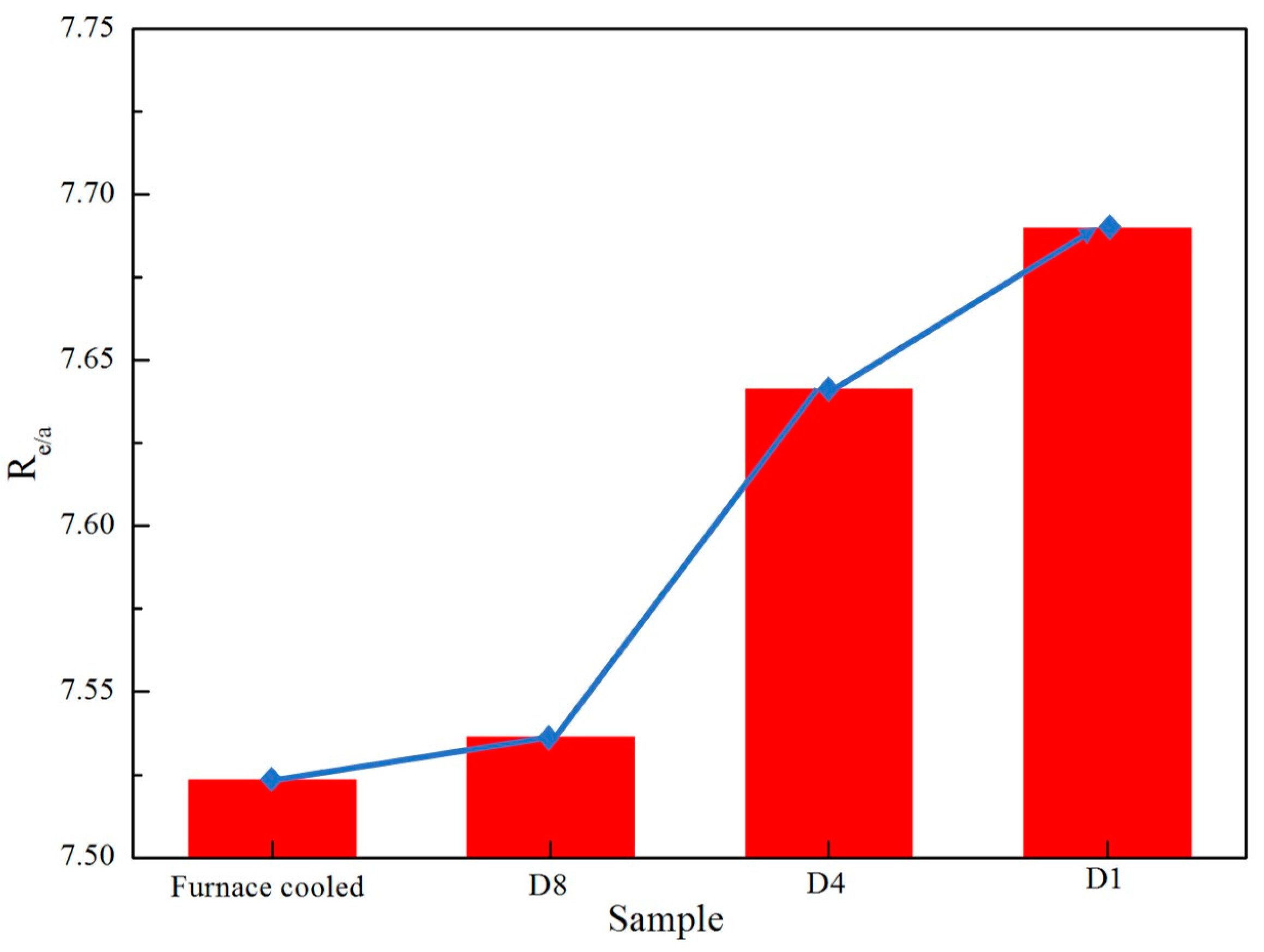
- By increasing the RC value, the elements Co and Fe in Co-rich γ phase presented apparent trend diffusion to the β phase and martensite.
- (3)
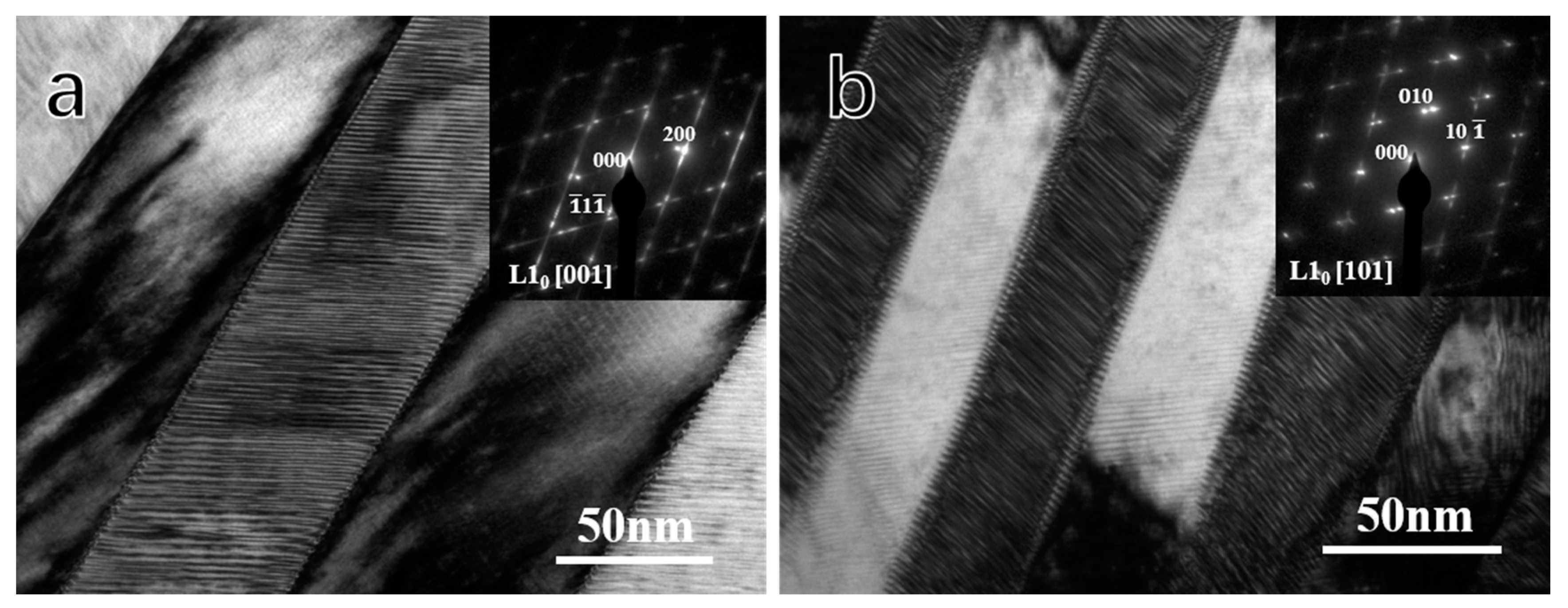
- The martensite structure of D4 corresponded to the L10 structure with a = b = 0.382 nm, c = 0.321 nm and in D1, the martensite equaled the L10 structure with a = b = 0.376 nm, c = 0.325 nm.
- (4)
- The one-step austenite-martensite phase transformation occurred during the process of heating and cooling. The phase transition temperature preserved a significant tendency of increasing with the rise of the RC value, even over the room temperature in D1. Additionally, the values of thermal hysteresis were about 11.8, 4.5, 2.3, and 0.7 K for furnace cooled sample, D8, D4, and D1, respectively.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, L.; Schneider, M.M.; Giri, A.; Cho, K.; Sohn, Y. Microstructural and crystallographic characteristics of modulated martensite, non-modulated martensite, and pre-martensitic tweed austenite in Ni-Mn-Ga alloys. Acta Mater. 2017, 134, 93–103. [Google Scholar] [CrossRef]
- Dai, Y.C.; Hou, L.; Fautrelle, Y.; Li, Z.B.; Esling, C.; Ren, Z.M.; Li, X. Martensitic transformation and detwinning in directionally solidified two-phase Ni-Mn-Ga alloys under uniaxial compression. J. Alloys Compd. 2017, 722, 721–728. [Google Scholar] [CrossRef]
- Mino, J.; Ipatov, M.; Gamcova, J.; Saksl, K.; Durisin, M.; Zhukova, V.; Vargova, Z.; Zhukov, A.; Varga, R. Magnetic Characterization of Melt-Spun Co-Ni-Ga Ferromagnetic Superelastic Alloy. Acta Phys. Pol. A 2017, 131, 1075–1077. [Google Scholar] [CrossRef]
- Vollmer, M.; Krooss, P.; Segel, C.; Weidner, A.; Paulsen, A.; Frenzel, J.; Schaperc, M.; Eggeler, G.; Maier, J.H.; Niendorfa, T. Damage evolution in pseudoelastic polycrystalline Co-Ni-Ga high-temperature shape memory alloys. J. Alloys Compd. 2015, 633, 288–295. [Google Scholar] [CrossRef]
- Timofeeva, E.E.; Panchenko, E.Y.; Chumlyakov, Y.I.; Maier, H.J.; Gerstein, G. Peculiarities of High-Temperature Superelasticity in Ni-Fe-Ga Single Crystals in Compression. Tech. Phys. Lett. 2017, 43, 320–323. [Google Scholar] [CrossRef]
- Chabungbam, S.; Borgohain, P.; Ghosh, S.; Singh, N.; Sahariah, M.B. Martensitic transformation and magnetism in Ni and Fe-rich compositions of Ni-Fe-Ga shape memory alloys. J. Alloys Compd. 2016, 689, 199–207. [Google Scholar] [CrossRef]
- Sharma, J.; Suresh, K.G. Investigation of multifunctional properties of Mn50Ni40−xCoxSn10 (x = 0–6) Heusler alloys. J. Alloys Compd. 2015, 620, 329–336. [Google Scholar] [CrossRef]
- Liu, J.; Fei, X.P.; Gong, Y.Y.; Xu, F. Phase Transition and Magnetocaloric Properties of Ni50Mn35-xIn15Cux Bulk Alloys and Ribbons. IEEE Trans. Magn. 2015, 51. 2503004. [Google Scholar] [CrossRef]
- Ju, J.; Yang, L.; Hao, S.; Mao, Q.T.; Lou, S.T.; Liu, H. Microstructure Martensite Transition and Mechanical Properties Investigations of Polycrystalline Co-Ni-Al Alloys with Er Doping. J. Mater. Eng. Perform. 2017, 26, 1062–1068. [Google Scholar] [CrossRef]
- Yamakov, V.; Hochhalter, J.D.; Leser, W.P.; Warner, J.E.; Newman, J.A.; Pun, G.P.P.; Mishin, Y. Multiscale modeling of sensory properties of Co-Ni-Al shape memory particles embedded in an Al metal matrix. J. Mater. Sci. 2016, 51, 1204–1216. [Google Scholar] [CrossRef]
- Li, P.Z.; Karaca, H.E.; Chumlyakov, Y.I. Orientation dependent compression behavior of Co35Ni35Al30 single crystals. J. Alloys Compd. 2017, 718, 326–334. [Google Scholar] [CrossRef]
- Kokorin, V.V.; Konoplyuk, S.M.; Dalinger, A.; Maier, H.J. Influence of martensitic transformation on the magnetic transition in Ni-Mn-Ga. J. Magn. Magn. Mater. 2017, 432, 266–270. [Google Scholar] [CrossRef]
- Oikawa, K.; Wulff, L.; Iijima, T.; Gejima, F.; Ohmori, T.; Fujita, A.; Fukamichi, K.; Kainuma, R.; Ishida, K. Promising ferromagnetic Ni-Co-Al shape memory alloy system. Appl. Phys. Lett. 2001, 79, 3290–3292. [Google Scholar] [CrossRef]
- Ju, J.; Lou, S.T.; Yan, C.; Yang, L.; Li, T.; Hao, S.; Wang, X.Y.; Liu, H. Microstructure Magnetism and Magnetic Field Induced-Strain in Er-Doped Co-Ni-Al Polycrystalline Alloy. J. Electron. Mater. 2017, 46, 2540–2547. [Google Scholar] [CrossRef]
- Ju, J.; Xue, F.; Li, H. Microstructure and Magnetic Property Variation with Addition of Rare Earth Element Dy in Co-Ni-Al Ferromagnetic Shape Memory Alloy. J. Iron Steel Res. Int. 2015, 22, 858–863. [Google Scholar] [CrossRef]
- O’Handley, R.C. Model for strain and magnetization in magnetic shape-memory alloys. J. Appl. Phys. 1998, 83, 3263–3270. [Google Scholar] [CrossRef]
- Olszewski, J.; Zbroszczyk, J.; Hasiak, M.; Kaleta, J.; Nabialek, M.; Bragiel, P.; Sobczyka, K.; Ciurzyńska, W.; Świerczek, J.; Łukiewskaa, A. Microstructure and magnetic properties of Fe-Co-Nd-Y-B alloys obtained by suction casting method. J. Rare Earths 2009, 27, 680–683. [Google Scholar] [CrossRef]
- Ju, J.; Xue, F.; Sun, L.X. Effect of Rapid Solidification on the Microstructure and Magnetic-Field-Induced Strain of Co1.36Ni1.21AlFe0.12. Mater. Manuf. Process. 2015, 30, 637–643. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, M.B.; Zhan, J.; Peng, C.Y.; Hu, J.J.; Chen, K.; Zhou, Z.; Chen, Z.H. Investigation on the Microstructure Characterization and Aging Response of Rapidly Solidified Mg-6wt%Zn-5wt%Ca Alloy Produced by Atomization-Twin Roll Quenching Technology. Mater. Manuf. Process. 2012, 27, 125–129. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.G. Microstructure, shape memory effect and mechanical properties of rapidly solidified Co-Ni-Al magnetic shape memory alloys. Mater. Sci. Eng. A 2007, 454, 423–432. [Google Scholar] [CrossRef]
- Lin, X.H.; Johnson, W.L. Formation of Ti-Zr-Cu-Ni bulk metallic glasses. J. Appl. Phys. 1995, 78, 6514–6519. [Google Scholar] [CrossRef]
- Rekik, H.; Krifa, M.; Bachaga, T.; Escoda, L.; Sunol, J.J.; Khitouni, M.; Chmingui, M. Structural and martensitic transformation of MnNiSn shape memory alloys. Int. J. Adv. Manuf. Technol. 2017, 90, 291–298. [Google Scholar] [CrossRef]
- Sanchez-Alarcos, V.; Recarte, V.; Perez-Landazabal, J.I.; Gomez-Polo, C.; Rodriguez-Velamazan, J.A. Role of magnetism on the martensitic transformation in Ni-Mn-based magnetic shape memory alloys. Acta Mater. 2012, 60, 459–468. [Google Scholar] [CrossRef]
- Ju, J.; Lou, S.T.; Yang, L.; Li, T.; Hao, S.; Yan, C. Effect of Electronic and Magnetic Valences on Phase Transition and Magnetic Properties in Co-Ni-Al-RE (RE = Gd, Dy and Er) Alloys. J. Electron. Mater. 2017, 46, 1390–1395. [Google Scholar] [CrossRef]

| Alloy | Diameter (mm) | RC (K·s−1) |
|---|---|---|
| D1 | 1 | 4000 |
| D4 | 4 | 250 |
| D8 | 8 | 62.5 |
| Alloy | Desired Composition (at %) | Actual Composition (at %) |
|---|---|---|
| Furnace cooled | Co33Ni31Al27Fe9 | Co33.1Ni31.5Al26.8Fe8.6 |
| D8 | Co32.8Ni31.4Al27.3Fe8.5 | |
| D4 | Co33.4Ni30.2Al28.1Fe8.3 | |
| D1 | Co32.9Ni32.4Al26.3Fe8.4 |
| Alloy | Experimental Conditions | Obtained Phases | |||
|---|---|---|---|---|---|
| Temperature (K) | Diameter (mm) | RC (K·s−1) | RC Degree | ||
| Furnace cooled | 1800 | - | <50 | Low RC value | β + γ |
| D8 | 1800 | 8 | 62.5 | Low RC value | β + γ |
| D4 | 1800 | 4 | 250 | Medium RC value | martensite + γ |
| D1 | 1800 | 1 | 4000 | High RC value | martensite + γ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Ju, J.; Shuai, L.; Liu, H.; Yan, C.; Liu, Z. Structure and Martensitic Transformation in Rapidly Solidified CoNiAlFe Alloy. Metals 2017, 7, 473. https://doi.org/10.3390/met7110473
Chen H, Ju J, Shuai L, Liu H, Yan C, Liu Z. Structure and Martensitic Transformation in Rapidly Solidified CoNiAlFe Alloy. Metals. 2017; 7(11):473. https://doi.org/10.3390/met7110473
Chicago/Turabian StyleChen, Huiling, Jia Ju, Liguo Shuai, Huan Liu, Chen Yan, and Zhuang Liu. 2017. "Structure and Martensitic Transformation in Rapidly Solidified CoNiAlFe Alloy" Metals 7, no. 11: 473. https://doi.org/10.3390/met7110473