The Biological Responses to Magnesium-Based Biodegradable Medical Devices
Abstract
:1. Introduction
2. Degradation of Mg-Based Alloys
3. Protein-Mediated Cell Adhesion
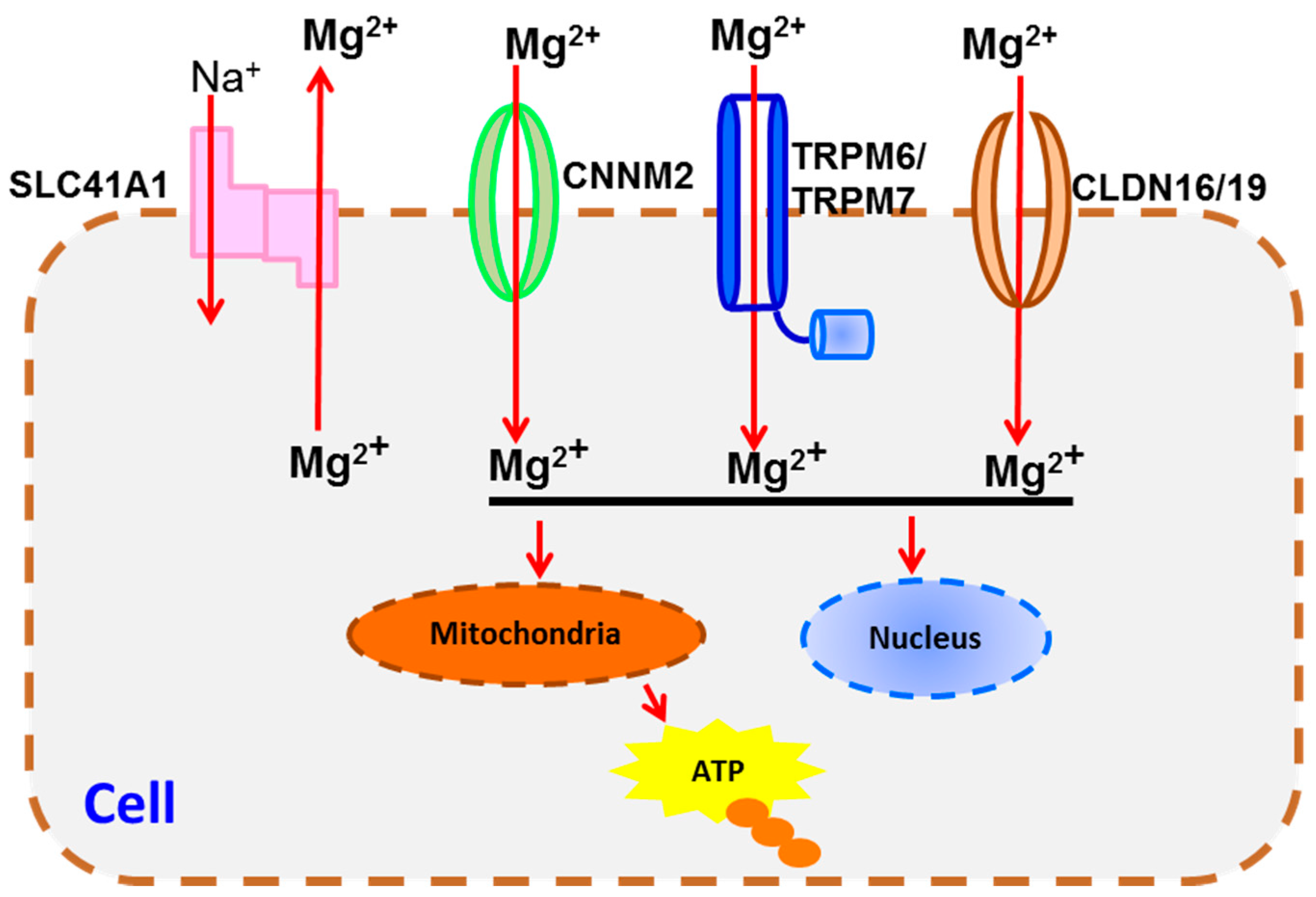
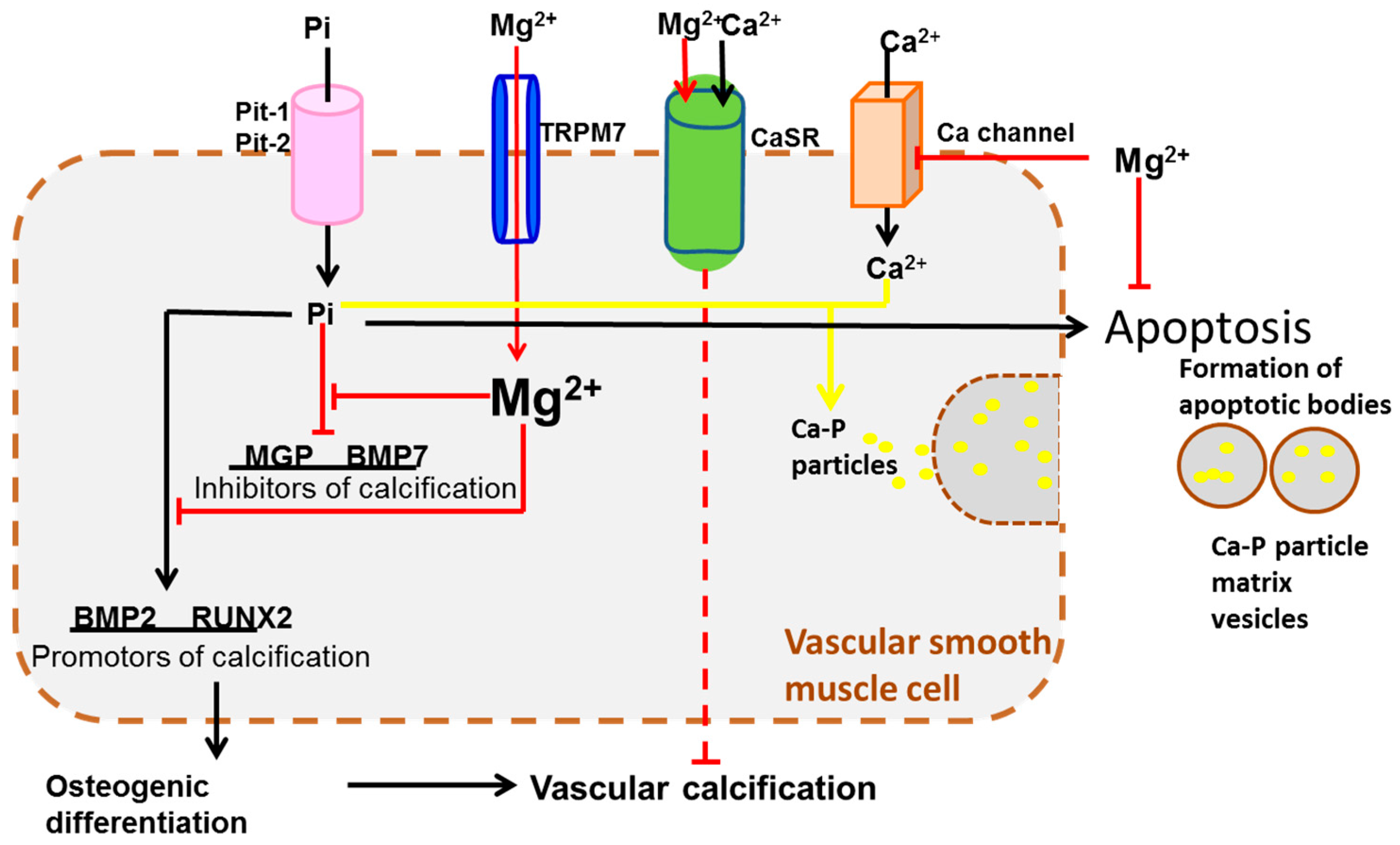
4. Transportation Signaling
5. Immune Responses
6. Tissue Growth
7. Systematic Integration
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuhlmann, J.; Bartsch, I.; Willbold, E.; Schuchardt, S.; Holz, O.; Hort, N.; Höche, D.; Heineman, W.R.; Witte, F. Fast escape of hydrogen from gas cavities around corroding magnesium implants. Acta Biomater. 2013, 9, 8714–8721. [Google Scholar] [CrossRef] [PubMed]
- McBride, E.D. Absorbable metal in bone surgery: A further report on the use of magnesium alloys. J. Am. Med. Assoc. 1938, 111, 2464–2467. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Song, Y.; Zhao, C.; Zhang, X.; Xie, C.; Zhang, Y.; Tao, H.; He, Y.; Jiang, Y. In vitro degradation, hemolysis and mc3t3-e1 cell adhesion of biodegradable Mg–Zn alloy. Mater. Sci. Eng. C 2009, 29, 1907–1912. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Wu, Y.; Maitz, M.F.; Wang, Z.; Koo, Y.; Zhao, A.; Sankar, J.; Kong, D.; Huang, N. Ex vivo blood vessel bioreactor for analysis of the biodegradation of magnesium stent models with and without vessel wall integration. Acta Biomater. 2017, 50, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Koo, Y.; Collins, B.; Xu, Z.; Sankar, J.; Yun, Y. Biodegradability and platelets adhesion assessment of magnesium-based alloys using a microfluidic system. PLoS ONE 2017, 12, e0182914. [Google Scholar] [CrossRef] [PubMed]
- McCord, C.P.; Prendergast, J.J.; Meek, S.F.; Harrold, G.C. Chemical gas gangrene from metallic magnesium. Ind. Med. 1942, 11, 71–75. [Google Scholar]
- Edwards, J.D. Application of the Interferometer to Gas Analysis; Government Publishing Office: Washington, DC, USA, 1919.
- Song, G.; Song, S.-Z. A possible biodegradable magnesium implant material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Salganik, R.I. The benefits and hazards of antioxidants: Controlling apoptosis and other protective mechanisms in cancer patients and the human population. J. Am. Coll. Nutr. 2001, 20, 464S–472S. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Colavitti, R.; Rovira, I.I.; Finkel, T. Redox-dependent transcriptional regulation. Circ. Res. 2005, 97, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Arnett, T.R.; Dempster, D.W. Effect of ph on bone resorption by rat osteoclasts in vitro. Endocrinology 1986, 119, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Bohr, C.; Hasselbalch, K.; Krogh, A. Concerning a biologically important relationship—The influence of the carbon dioxide content of blood on its oxygen binding. Skand. Arch. Physiol. 1904, 16, 402. [Google Scholar] [CrossRef]
- Schwartz, M.A.; Schaller, M.D.; Ginsberg, M.H. Integrins: Emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 1995, 11, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, W.J. Integrins and bone—Cell adhesion and beyond. Arch. Immunol. Ther. Exp. 1996, 45, 271–275. [Google Scholar]
- Damsky, C.H.; Ilić, D. Integrin signaling: It’s where the action is. Curr. Opin. Cell Biol. 2002, 14, 594–602. [Google Scholar] [CrossRef]
- Vroman, L.; Adams, A.; Fischer, G.; Munoz, P. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 1980, 55, 156–159. [Google Scholar] [PubMed]
- Coté, C.J.; Lerman, J.; Todres, I.D. A Practice of Anesthesia for Infants and Children E-Book: Expert Consult: Online and Print; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Ehtemam-Haghighi, S.; Liu, Y.; Cao, G.; Zhang, L.-C. Phase transition, microstructural evolution and mechanical properties of Ti-Nb-Fe alloys induced by fe addition. Mater. Des. 2016, 97, 279–286. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Prashanth, K.; Attar, H.; Chaubey, A.K.; Cao, G.; Zhang, L. Evaluation of mechanical and wear properties of Ti-xNb-7Fe alloys designed for biomedical applications. Mater. Des. 2016, 111, 592–599. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Versatility, modulation and signaling. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Zhu, X.; Ohtsubo, M.; Böhmer, R.M.; Roberts, J.M.; Assoian, R.K. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 1996, 133, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Van der Flier, A.; Sonnenberg, A. Structural and functional aspects of filamins. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2001, 1538, 99–117. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Howlett, C.R.; Zreiqat, H.; Wu, Y.; McFall, D.W.; McKenzie, D.R. Effect of ion modification of commonly used orthopedic materials on the attachment of human bone-derived cells. J. Biomed. Mater. Res. 1999, 45, 345–354. [Google Scholar] [CrossRef]
- Zreiqat, H.; Evans, P.; Howlett, C.R. Effect of surface chemical modification of bioceramic on phenotype of human bone-derived cells. J. Biomed. Mater. Res. 1999, 44, 389–396. [Google Scholar] [CrossRef]
- Bilek, M.; Evans, P.; Mckenzie, D.; McCulloch, D.; Zreiqat, H.; Howlett, C. Metal ion implantation using a filtered cathodic vacuum arc. J. Appl. Phys. 2000, 87, 4198–4204. [Google Scholar] [CrossRef]
- Kokubo, T. Bioceramics and Their Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Anast, C.S.; Mohs, J.M.; Kaplan, S.L.; Burns, T.W. Evidence for parathyroid failure in magnesium deficiency. Science 1972, 177, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Neil, W.; Forsyth, M.; Howlett, P.; Hutchinson, C.; Hinton, B. Corrosion of magnesium alloy ZE41—The role of microstructural features. Corros. Sci. 2009, 51, 387–394. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Smith, C.E.; Xu, Z.; Waterman, J.; Sankar, J. Cytocompatibility assessment of mgznca alloys. Emerg. Mater. Res. 2013, 2, 283–290. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, M.; Abidin, N.I.Z. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 86–100. [Google Scholar]
- Yun, Y.; Dong, Z.; Tan, Z.; Schulz, M.J. Development of an electrode cell impedance method to measure osteoblast cell activity in magnesium-conditioned media. Anal. Bioanal. Chem. 2010, 396, 3009–3015. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Dong, Z.; Lee, N.; Liu, Y.; Xue, D.; Guo, X.; Kuhlmann, J.; Doepke, A.; Halsall, H.B.; Heineman, W. Revolutionizing biodegradable metals. Mater. Today 2009, 12, 22–32. [Google Scholar] [CrossRef]
- Ingber, D.E. Tensegrity ii. How structural networks influence cellular information processing networks. J. Cell Sci. 2003, 116, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.G.; Dalton, B.A.; Johnson, G.; Underwood, P.A. Adsorption of fibronectin and vitronectin onto primaria™ and tissue culture polystyrene and relationship to the mechanism of initial attachment of human vein endothelial cells and BHK-21 fibroblasts. Biomaterials 1995, 16, 1057–1067. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Stewart, K.; Graves, S.E.; Hay, S.; Simmons, P.J. Integrin expression and function on human osteoblast-like cells. J. Bone Min. Res. 1997, 12, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Schulze-Tanzil, G.; de Souza, P.; John, T.; Rahmanzadeh, M.; Rahmanzadeh, R.; Merker, H.-J. Inhibition of mitogen-activated protein kinase kinase induces apoptosis of human chondrocytes. J. Biol. Chem. 2001, 276, 13289–13294. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, D.D.; Hanks, S.K.; Hunter, T.; van der Geer, P. Integrin-mediated signal transduction linked to ras pathway by GRB2 binding to focal adhesion kinase. Nature 1994, 372, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Shattil, S.J.; Newman, P.J. Integrins: Dynamic scaffolds for adhesion and signaling in platelets. Blood 2004, 104, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.G.; McFarland, C.; Dalton, B.A.; Johnson, G.; Evans, M.D.; Rolfe Howlett, C.; Underwood, P.A. Attachment of human bone cells to tissue culture polystyrene and to unmodified polystyrene: The effect of surface chemistry upon initial cell attachment. J. Biomater. Sci. Polym. Ed. 1994, 5, 245–257. [Google Scholar] [CrossRef]
- Howlett, C.R.; Evans, M.D.; Walsh, W.R.; Johnson, G.; Steele, J.G. Mechanism of initial attachment of cells derived from human bone to commonly used prosthetic materials during cell culture. Biomaterials 1994, 15, 213–222. [Google Scholar] [CrossRef]
- Kilpadi, K.L.; Chang, P.L.; Bellis, S.L. Hydroxylapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J. Biomed. Mater. Res. 2001, 57, 258–267. [Google Scholar] [CrossRef]
- Bale, M.D.; Wohlfahrt, L.A.; Mosher, D.F.; Tomasini, B.; Sutton, R.C. Identification of vitronectin as a major plasma protein adsorbed on polymer surfaces of different copolymer composition. Blood 1989, 74, 2698–2706. [Google Scholar] [PubMed]
- Fabrizius-Homan, D.J.; Cooper, S.L. A comparison of the adsorption of three adhesive proteins to biomaterial surfaces. J. Biomater. Sci. Polym. Ed. 1992, 3, 27–47. [Google Scholar] [CrossRef]
- Babensee, J.E.; Cornelius, R.M.; Brash, J.L.; Sefton, M.V. Immunoblot analysis of proteins associated with hema-mma microcapsules: Human serum proteins in vitro and rat proteins following implantation. Biomaterials 1998, 19, 839–849. [Google Scholar] [CrossRef]
- Rosengren, Å.; Pavlovic, E.; Oscarsson, S.; Krajewski, A.; Ravaglioli, A.; Piancastelli, A. Plasma protein adsorption pattern on characterized ceramic biomaterials. Biomaterials 2002, 23, 1237–1247. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, N.; Zhu, D. Biphasic responses of human vascular smooth muscle cells to magnesium ion. J. Biomed. Mater. Res. Part A 2016, 104, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A.; Windhugen, H.; Witte, F. Medical Implant for the Human or Animal Body. U.S. Patents US20,040,241,036 A1, 2 December 2004. [Google Scholar]
- Maxian, S.H.; Zawadsky, J.P.; Dunn, M.G. Effect of Ca/P coating resorption and surgical fit on the bone/implant interface. J. Biomed. Mater. Res. 1994, 28, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Norde, W. Driving forces for protein adsorption at solid surfaces. In Macromolecular Symposia; Wiley Online Library: New York, NY, USA, 1996; pp. 5–18. [Google Scholar]
- Ohno, Y.; Maehashi, K.; Yamashiro, Y.; Matsumoto, K. Electrolyte-gated graphene field-effect transistors for detecting pH and protein adsorption. Nano Lett. 2009, 9, 3318–3322. [Google Scholar] [CrossRef] [PubMed]
- Dee, K.C.; Puleo, D.A.; Bizios, R. An Introduction to Tissue-Biomaterial Interactions; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Maier, J.A.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2004, 1689, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Moomaw, A.S.; Maguire, M.E. The unique nature of Mg2+ channels. Physiology 2008, 23, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Günther, T. Concentration, compartmentation and metabolic function of intracellular free Mg2+. Magnes. Res. 2006, 19, 225–236. [Google Scholar] [PubMed]
- Bo, S.; Pisu, E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr. Opin. Lipidol. 2008, 19, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7—Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2007, 1772, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.-L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.-P.; Penner, R.; Scharenberg, A.M. Ltrpc7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Wabakken, T.; Rian, E.; Kveine, M.; Aasheim, H.-C. The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2+ transporters. Biochem. Biophys. Res. Commun. 2003, 306, 718–724. [Google Scholar] [CrossRef]
- Meyer, T.E.; Verwoert, G.C.; Hwang, S.-J.; Glazer, N.L.; Smith, A.V.; van Rooij, F.J.; Ehret, G.B.; Boerwinkle, E.; Felix, J.F.; Leak, T.S.; et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet. 2010, 6, e1001045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-Y.; Shi, J.-D.; Yang, P.; Kumar, P.G.; Li, Q.-Z.; Run, Q.-G.; Su, Y.-C.; Scott, H.S.; Kao, K.-J.; She, J.-X. Molecular cloning and characterization of a novel gene family of four ancient conserved domain proteins (ACDP). Gene 2003, 306, 37–44. [Google Scholar] [CrossRef]
- Goytain, A.; Quamme, G.A. Functional characterization of ACDP2 (ancient conserved domain protein), a divalent metal transporter. Physiol. Genom. 2005, 22, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Will, C.; Breiderhoff, T.; Thumfart, J.; Stuiver, M.; Kopplin, K.; Sommer, K.; Günzel, D.; Querfeld, U.; Meij, I.C.; Shan, Q.; et al. Targeted deletion of murine cldn16 identifies extra-and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am. J. Physiol.-Ren. Physiol. 2010, 298, F1152–F1161. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Hussain, S.; Akhtar, N. Mutation in the tight-junction gene claudin 19 (CLDN19) and familial hypomagnesemia, hypercalciuria, nephrocalcinosis (FHHNC) and severe ocular disease. Am. J. Nephrol. 2011, 34, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Schaller, A.; Seelow, D.; Pandey, A.V.; Waldegger, S.; Lesslauer, A.; Vitzthum, H.; Suzuki, Y.; Luk, J.M.; Becker, C. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am. J. Hum. Genet. 2006, 79, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Renigunta, A.; Konrad, M.; Gomes, A.S.; Schneeberger, E.E.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J. Clin. Investig. 2008, 118, 619. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Renigunta, A.; Gomes, A.S.; Hou, M.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc. Natl. Acad. Sci. USA 2009, 106, 15350–15355. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Cittadini, A. Magnesium in cell proliferation and differentiation. Front. Biosci. 1999, 4, D607–D617. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Benz, E.J., Jr.; Silberstein, L.E.; Heslop, H.; Weitz, J.; Anastasi, J. Hematology: Basic Principles and Practice, Expert Consult Premium Edition-Enhanced Online Features; Elsevier Health Sciences: Amsterdam, The Netherland, 2012. [Google Scholar]
- Wang, J.; He, Y.; Maitz, M.F.; Collins, B.; Xiong, K.; Guo, L.; Yun, Y.; Wan, G.; Huang, N. A surface-eroding poly (1,3-trimethylene carbonate) coating for fully biodegradable magnesium-based stent applications: Toward better biofunction, biodegradation and biocompatibility. Acta Biomater. 2013, 9, 8678–8689. [Google Scholar] [CrossRef] [PubMed]
- Runnels, L.W.; Yue, L.; Clapham, D.E. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat. Cell Biol. 2002, 4, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Paunier, L. Effect of magnesium on phosphorus and calcium metabolism. Monatsschr. Kinderheilkd. Organ Deutsch. Ges. Kinderheilkd. 1992, 140, S17–S20. [Google Scholar]
- Massy, Z.A.; Drüeke, T.B. Magnesium and cardiovascular complications of chronic kidney disease. Nat. Rev. Nephrol. 2015, 11, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Kircelli, F.; Peter, M.E.; Ok, E.S.; Celenk, F.G.; Yilmaz, M.; Steppan, S.; Asci, G.; Ok, E.; Passlick-Deetjen, J. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol. Dial. Transplant. 2011, 27, 514–521. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R. Formation and transformation of calcium phosphates: Relevance to vascular calcification. Z. Kardiol. 2001, 90, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-T.; Grabher, J.; LeGeros, R. Effects of magnesium on calcium phosphate formation. Magnesium 1987, 7, 123–132. [Google Scholar]
- Peters, F.; Epple, M. Simulating arterial wall calcification in vitro: Biomimetic crystallization of calcium phosphates under controlled conditions. Z. Kardiol. 2001, 90, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Altura, B.; Altura, B.; Carella, A.; Gebrewold, A.; Murakawa, T.; Nishio, A. Mg2+-Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can. J. Physiol. Pharmacol. 1987, 65, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Zimmerman, D.; Yusuf, H.; Burger, D.; Chignalia, A.Z.; Wadhera, V.; van Leeuwen, F.N.; Touyz, R.M. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 2010, 56, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Gamba, G.; Riccardi, D.; Lombardi, M.; Butters, R.; Kifor, O.; Sun, A.; Hediger, M.A.; Lytton, J.; Hebert, S.C. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 1993, 366, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, O.; Nikolov, I.G.; Joki, N.; Caudrillier, A.; Phan, O.; Mentaverri, R.; Maizel, J.; Hamada, Y.; Nguyen-Khoa, T.; Fukagawa, M. The calcimimetic R-568 retards uremia-enhanced vascular calcification and atherosclerosis in apolipoprotein E deficient (apoE−/−) mice. Atherosclerosis 2009, 205, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Biological responses to materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Anderson, J.M. Multinucleated giant cells. Curr. Opin. Hematol. 2000, 7, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Gretzer, C.; Emanuelsson, L.; Liljensten, E.; Thomsen, P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J. Biomater. Sci. Polym. Ed. 2006, 17, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Luttikhuizen, D.T.; Harmsen, M.C.; Luyn, M.J.V. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006, 12, 1955–1970. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. Magnesium and immune function: An overview. Magnesium 1988, 7, 290–299. [Google Scholar] [PubMed]
- Mazur, A.; Maier, J.A.M.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Malpuech-Brugère, C.; Zimowska, W.; Rayssiguier, Y.; Mazur, A. Low magnesium promotes endothelial cell dysfunction: Implications for atherosclerosis, inflammation and thrombosis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2004, 1689, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.E.; March, C.J.; Cosman, D.; Widmer, M.B.; MacDonald, H.R.; McMahan, C.J.; Grubin, C.E.; Wignall, J.M.; Jackson, J.L.; Call, S.M.; et al. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science 1988, 241, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Yasukawa, K.; Harada, H.; Taga, T.; Watanabe, Y.; Matsuda, T.; Kashiwamura, S.-I.; Nakajima, K.; Koyama, K.; Iwamatsu, A.; et al. Complementary DNA for a novel human interleukin (BSF-2) that induces b lymphocytes to produce immunoglobulin. Nature 1986, 324, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Hedges, J.C.; Singer, C.A.; Gerthoffer, W.T. Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am. J. Respir. Cell Mol. Biol. 2000, 23, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.M.; Eifler, R.; Bach, F.; Maier, H.J. Magnesium degradation products: Effects on tissue and human metabolism. J. Biomed. Mater. Res. Part A 2014, 102, 3744–3753. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, B.M.; Kaczorowski, D.J.; Sugimoto, R.; Yang, R.; Wang, Y.; Billiar, T.R.; McCurry, K.R.; Bauer, A.J.; Nakao, A. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am. J. Transplant. 2008, 8, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Yu, Y.; Pei, Y.; Hou, L.; Chen, S.; Xiong, L.; Wang, G. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and hmgb1 release. Shock 2010, 34, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Guan, J.; Tamama, K. Hydrogen gas treatment prolongs replicative lifespan of bone marrow multipotential stromal cells in vitro while preserving differentiation and paracrine potentials. Biochem. Biophys. Res. Commun. 2010, 397, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, Y.P.; Li, Y.; Liu, W.W.; Xiang, H.G.; Fan, L.Y.; Sun, Q.; Xu, X.Y.; Cai, J.M.; Ruan, C.P.; et al. Hydrogen-rich saline ameliorates the severity of L-arginine-induced acute pancreatitis in rats. Biochem. Biophys. Res. Commun. 2010, 393, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Recent progress in corrosion and protection of magnesium alloys. Adv. Eng. Mater. 2005, 7, 563–586. [Google Scholar] [CrossRef]
- Zhang, E.; Xu, L.; Yu, G.; Pan, F.; Yang, K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. Part A 2009, 90, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, J.; Wang, Y. Evaluation of cyto-toxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluid. Surf. Coat. Technol. 2004, 185, 92–98. [Google Scholar] [CrossRef]
- Hulshoff, J.; Van Dijk, K.; De Ruijter, J.; Rietveld, F.; Ginsel, L.; Jansen, J. Interfacial phenomena: An in vitro study of the effect of calcium phosphate (Ca-P) ceramic on bone formation. J. Biomed. Mater. Res. 1998, 40, 464–474. [Google Scholar] [CrossRef]
- Geis-Gerstorfer, J.; Schille, C.; Schweizer, E.; Rupp, F.; Scheideler, L.; Reichel, H.P.; Hort, N.; Nolte, A.; Wendel, H.P. Blood triggered corrosion of magnesium alloys. Mater. Sci. Eng. B 2011, 176, 1761–1766. [Google Scholar] [CrossRef]
- Damsky, C.H. Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone 1999, 25, 95–96. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Z.; Tang, E.; Fan, Z.; McCauley, L.; Franceschi, R.; Guan, K.; Krebsbach, P.H.; Wang, C.-Y. Inhibition of osteoblastic bone formation by nuclear factor-κB. Nat. Med. 2009, 15, 682–689. [Google Scholar] [CrossRef] [PubMed]
| Biological Responses | Mg2+ | Ca-P | H2 |
|---|---|---|---|
| Cell Adhesion | α5β1-, α3β1-, β1-integrins [44], Shc [46], FAK [44], vitronectin and fibronectin [47,49], SERPINE 1 [56] | ||
| Transportation Signaling | TRPM6/7 [67,81,82], SLC41A1 [68], CLDN16/19 [76], CNNM2 [69] | CaSR [90,91], BGP [84] | |
| Immune Response | IL-8, PDGF, TGF-β1, Angio1, βFGF, VEGF, ET-1, CXCR-1, HIF-1α [39]; HMOX1 [56], IL-1, TNFα, IL-6 [97]; IL-1 α and IL-1 β [100]; BSF-2 [100] | TNF-α, IL-6, IL-1β, CCL2 and IL-10, TNF-γ, IL-12, CAM-1 [103]; HMGB-1 [104]; PGE2 [105], NF-κB [106] | |
| Tissue Growth | collagen I extracellular matrix protein [44]; EGF, FGF, GM-CSF, TGF-β, VEGF, PDGF [110]; IKK-NF-κB [110,115]; α5α1 and α3α1 [114] | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wang, J.; Russell, T.; Sankar, J.; Yun, Y. The Biological Responses to Magnesium-Based Biodegradable Medical Devices. Metals 2017, 7, 514. https://doi.org/10.3390/met7110514
Liu L, Wang J, Russell T, Sankar J, Yun Y. The Biological Responses to Magnesium-Based Biodegradable Medical Devices. Metals. 2017; 7(11):514. https://doi.org/10.3390/met7110514
Chicago/Turabian StyleLiu, Lumei, Juan Wang, Teal Russell, Jagannathan Sankar, and Yeoheung Yun. 2017. "The Biological Responses to Magnesium-Based Biodegradable Medical Devices" Metals 7, no. 11: 514. https://doi.org/10.3390/met7110514





