Hexagonal Boron Nitride Impregnated Silane Composite Coating for Corrosion Resistance of Magnesium Alloys for Temporary Bioimplant Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composite Coating Preparation
2.2. Sample Preparation and Coating Procedure
2.3. Electrochemical Measurements
2.4. Surface Morphology and Coating Characterisation
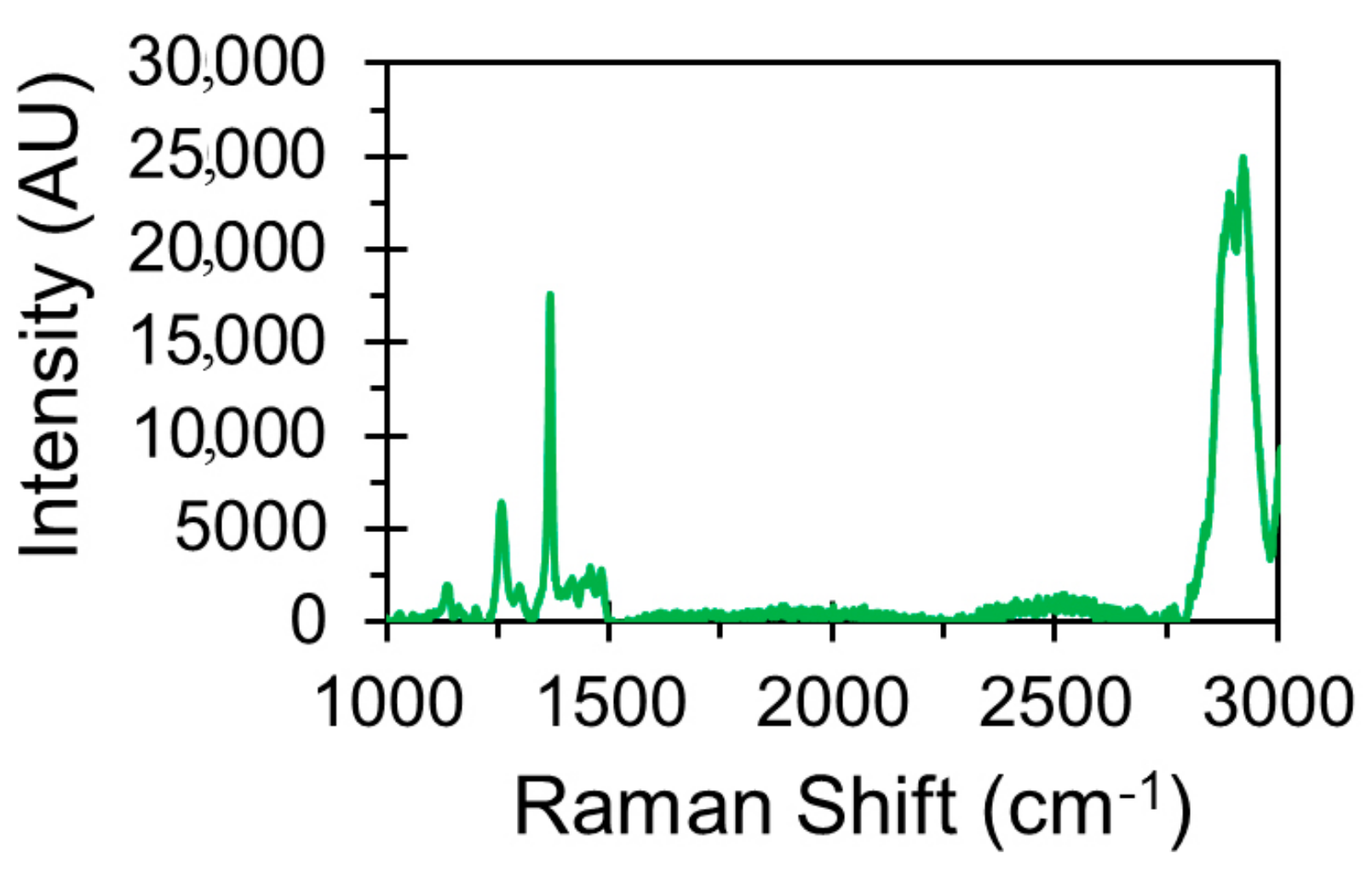
2.4.1. Raman Spectroscopy
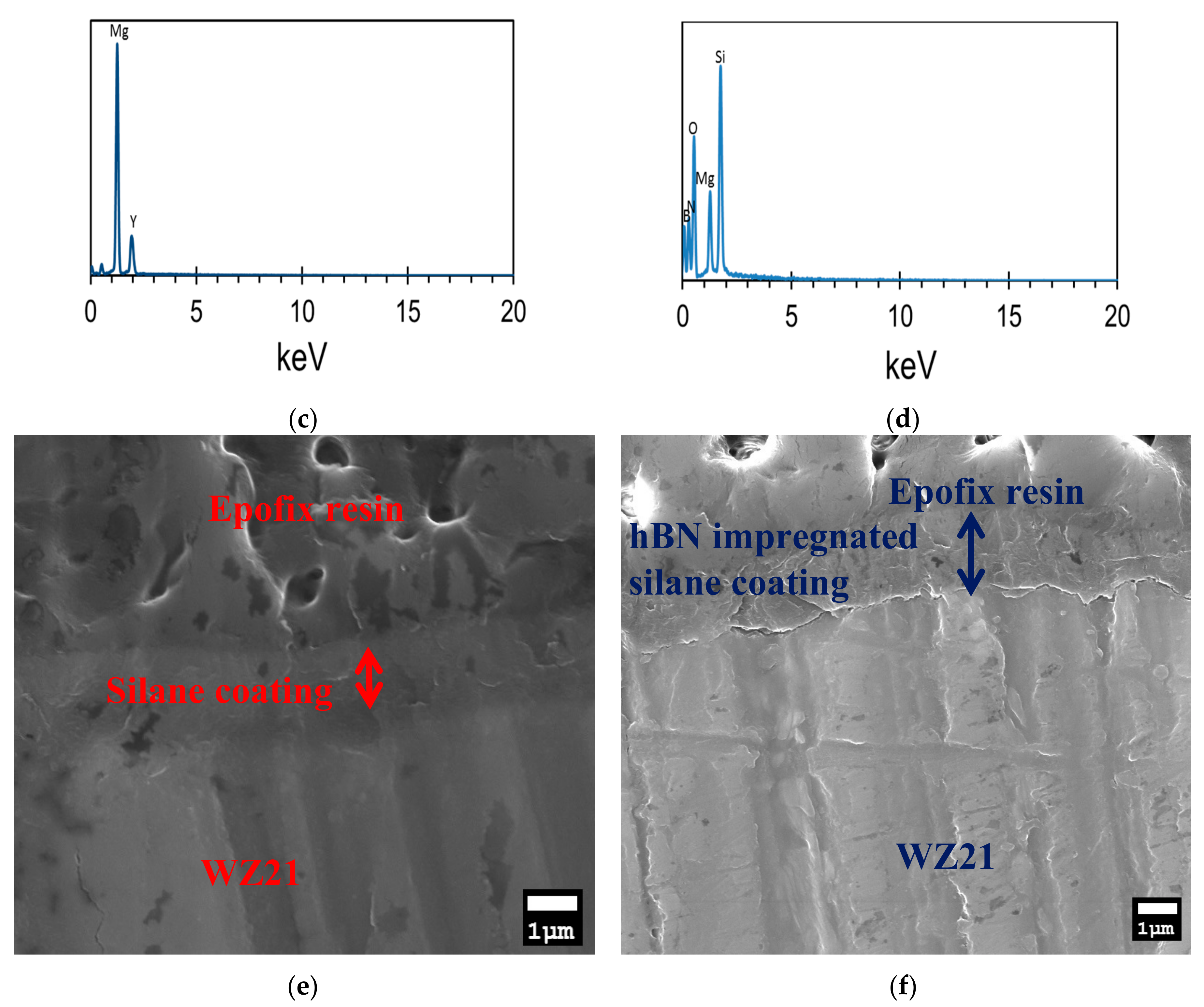
2.4.2. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
3. Results and Discussion
3.1. Chemical and Morphological Characterisation
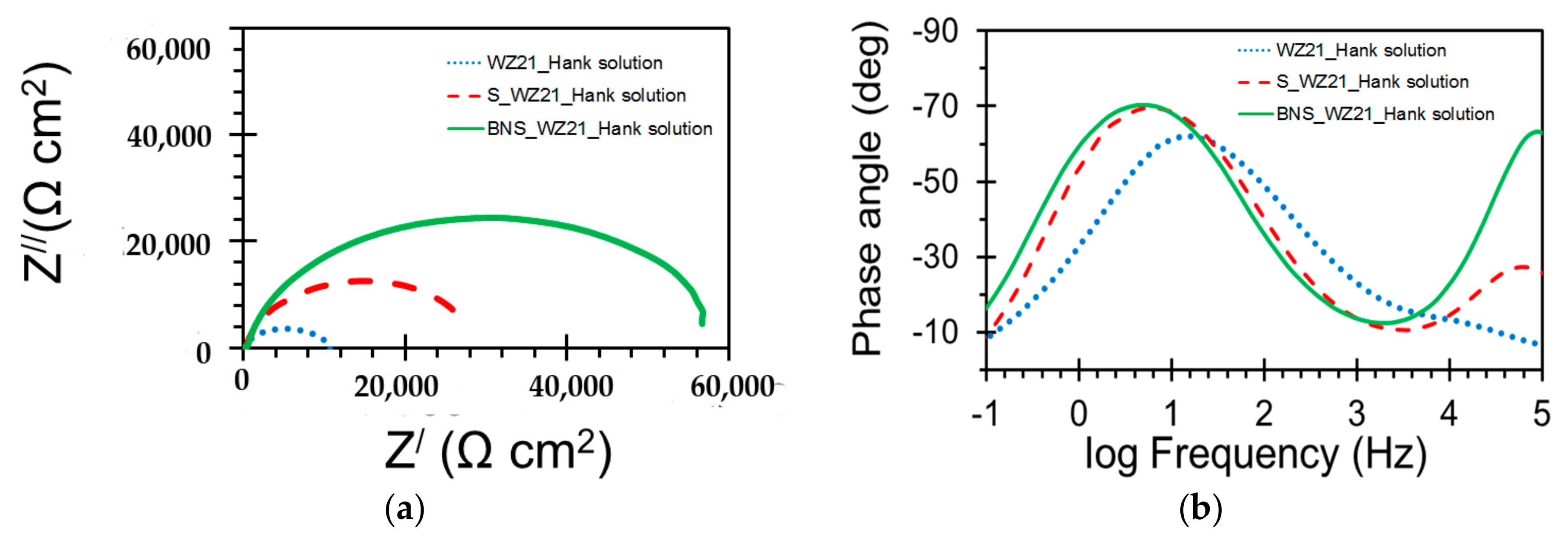
3.2. Electrochemical Characterisation
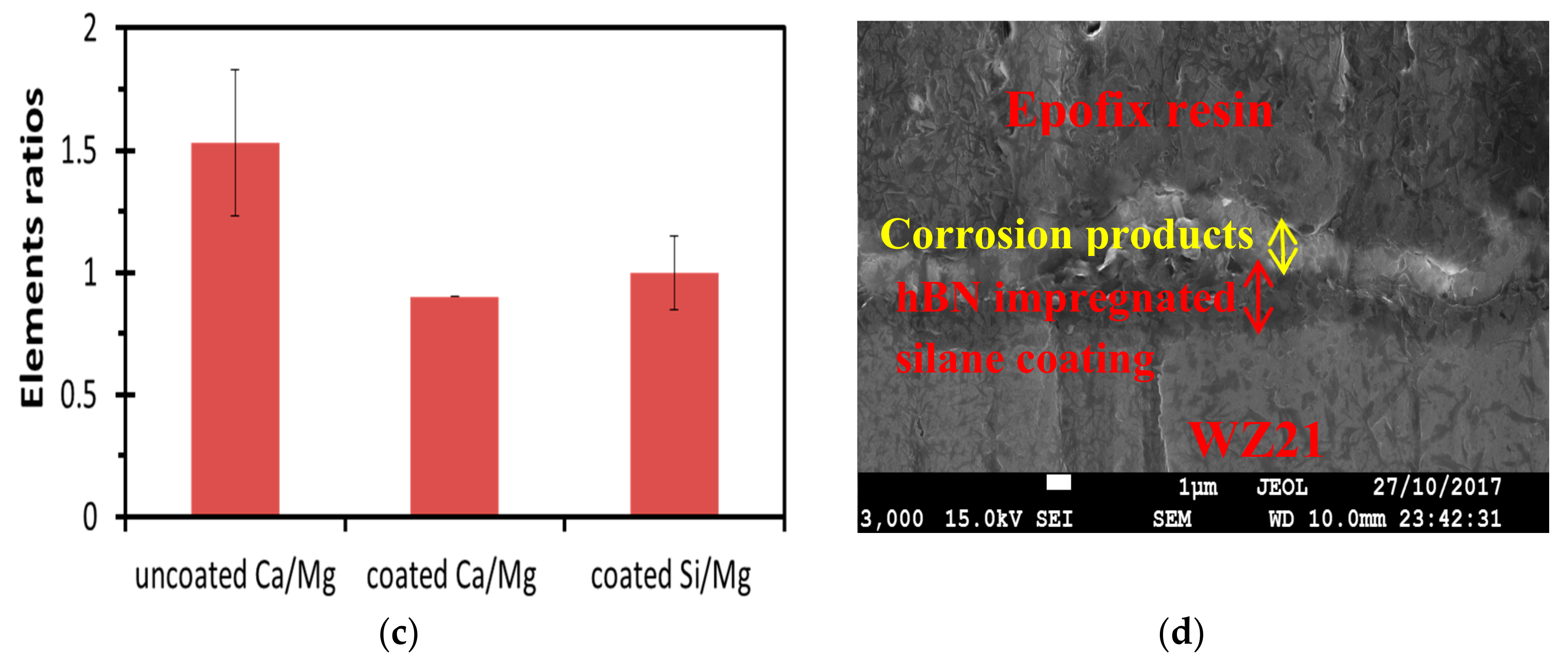
3.3. Post-Corrosion Morphology of Bare WZ21 and BNS_WZ21
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Xu, J. Biodegradable Alloys: The glass window of opportunities. Nat. Mater. 2009, 8, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.B.; Raman, R.K.S. In vitro degradation and mechanical integrity of calcium-containing magnesium alloys in modified-simulated body fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Horst-Artur, C.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, G.; Suwas, S. Biodegradable Mg and Mg based alloys for biomedical implants. Mater. Sci. Technol. 2014, 30, 515–520. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.K.; Vince, K.; Hodgson, M.A. Biodegradable surgical implants based on magnesium alloys—A review of current research. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Strasbourg, France, 8–12 June 2009; IOP Publishing: Bristol, UK, 2009. [Google Scholar]
- Hänzi, A.C.; Gerber, I.; Schinhammer, M.; Löffler, J.F.; Uggowitzer, P.J. On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg-Y-Zn alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, N.T.; Williams, G.; Birbilis, N. Observations of the galvanostatic dissolution of pure magnesium. Corros. Sci. 2012, 65, 5–9. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, A.A.; Fekry, A.M.; Ameer, M.A. Electrochemical behavior of magnesium alloys as biodegradable materials in Hank’s solution. Electrochim. Acta 2010, 55, 6028–6035. [Google Scholar] [CrossRef]
- Zucchi, F.; Grassi, V.; Frignani, A.; Monticelli, C.; Trabanelli, G. Influence of a silane treatment on the corrosion resistance of a WE43 magnesium alloy. Surf. Coat. Technol. 2006, 200, 4136–4143. [Google Scholar] [CrossRef]
- Zomorodian, A.; Brusciotti, F.; Fernandes, A.; Carmezim, M.J.; Silva, T.M.; Fernandes, J.C.S.; Montemor, M.F. Anti-corrosion performance of a new silane coating for corrosion protection of AZ31 magnesium alloy in Hank’s solution. Surf. Coat. Technol. 2012, 206, 4368–4375. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Montemor, M.F.; Galio, A.F.; Zheludkevich, M.L.; Trindade, C.; Dick, L.F.; Ferreira, M.G.S. Novel hybrid sol-gel coatings for corrosion protection of AZ31B magnesium alloy. Electrochim. Acta 2008, 53, 4773–4783. [Google Scholar] [CrossRef]
- Hu, J.; Li, Q.; Zhong, X.K.; Li, L.Q.; Zhang, L. Organic coatings silane-based for AZ91D magnesium alloy. Thin Solid Films 2010, 519, 1361–1366. [Google Scholar] [CrossRef]
- Zucchi, F.; Frignani, A.; Grassi, V.; Trabanelli, G. Organo-silane coatings for AZ31 magnesium alloy corrosion protection. Mater. Chem. Phys. 2008, 110, 263–268. [Google Scholar] [CrossRef]
- Ivanou, D.K.; Starykevich, M.; Lisenkov, A.D.; Zheludkevich, M.L.; Xue, H.B.; Lamaka, S.V.; Ferreira, M.G.S. Plasma anodized ZE41 magnesium alloy sealed with hybrid epoxy-silane coating. Corros. Sci. 2013, 73, 300–308. [Google Scholar] [CrossRef]
- Chakraborty Banerjee, P.; Singh Raman, R.K. Electrochemical impedance spectroscopic investigation of the role of alkaline pre-treatment in corrosion resistance of a silane coating on magnesium alloy, ZE41. Electrochim. Acta 2011, 56, 3790–3798. [Google Scholar] [CrossRef]
- Al-Saadi, S.; Banerjee, P.C.; Raman, R.K.S. Corrosion of bare and silane-coated mild steel in chloride medium with and without sulphate reducing bacteria. Prog. Org. Coat. 2017, 111, 231–239. [Google Scholar] [CrossRef]
- Ravenscroft-Chang, M.S.; Stohlman, J.M.; Molnar, P.; Natarajan, A.; Teliska, M.; Stancescu, M.; Krauthamer, V.; Hickman, J.J. Altered calcium dynamics in cardiac cells grown on silane-modified surfaces. Biomaterials 2010, 31, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Evans, M.E.; Jones, I.A.; Rudd, C.D.; Scotchford, C.A.; Walker, G.S. Preparation of poly(ε-caprolactone)/continuous bioglass fibre composite using monomer transfer moulding for bone implant. Biomaterials 2005, 26, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Abke, J.; Schnell, E.; Macionczyk, F.; Gbureck, U.; Mehrl, R.; Ruszczak, Z.; Kujat, R.; Englert, C.; Nerlich, M.; et al. Surface engineering of stainless steel materials by covalent collagen immobilization to improve implant biocompatibility. Biomaterials 2005, 26, 6962–6972. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Tsuru, K.; Hayakawa, S.; Osaka, A. Novel approach to fabricate porous gelatin–siloxane hybrids for bone tissue engineering. Biomaterials 2002, 23, 4765–4773. [Google Scholar] [CrossRef]
- Ren, L.; Tsuru, K.; Hayakawa, S.; Osaka, A. Synthesis and Characterization of Gelatin-Siloxane Hybrids Derived through Sol-Gel Procedure. J. Sol-Gel Sci. Technol. 2001, 21, 115–121. [Google Scholar] [CrossRef]
- Ren, L.; Tsuru, K.; Hayakawa, S.; Osaka, A. Sol–gel preparation and in vitro deposition of apatite on porous gelatin–siloxane hybrids. J. Non-Cryst. Solids 2001, 285, 116–122. [Google Scholar] [CrossRef]
- Dupraz, A.M.P.; Meer, S.A.T.; Wijn, J.R.D.; Goedemoed, J.H. Biocompatibility screening of silane-treated hydroxyapatite powders, for use as filler in resorbable composites. J. Mater. Sci. Mater. Med. 1996, 7, 731–738. [Google Scholar] [CrossRef]
- Nakkiew, W. Optimal Conditions of Electrostatic Spray Deposition (ESD) for Composite Biomaterials Coating for Biomedical Applications. Adv. Mater. Res. 2013, 748, 175–179. [Google Scholar] [CrossRef]
- Chetibi, L.; Hamana, D.; Achour, S. Growth and characterization of hydroxyapatite nanorice on TiO2 nanofibers. Mater. Chem. Phys. 2014, 144, 301–309. [Google Scholar] [CrossRef]
- Sutha, S.; Karunakaran, G.; Rajendran, V. Enhancement of antimicrobial and long-term biostability of the zinc-incorporated hydroxyapatite coated 316L stainless steel implant for biomedical application. Ceram. Int. 2013, 39, 5205–5212. [Google Scholar] [CrossRef]
- Lee, G.-W.; Park, M.; Kim, J.; Lee, J.I.; Yoon, H.G. Enhanced thermal conductivity of polymer composites filled with hybrid filler. Compos. Part A Appl. Sci. Manuf. 2006, 37, 727–734. [Google Scholar]
- Xie, B.H.; Huang, X.; Zhang, G.J. High thermal conductive polyvinyl alcohol composites with hexagonal boron nitride microplatelets as fillers. Compos. Sci. Technol. 2013, 85, 98–103. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.C.; Kuwahara, H.; Golberg, D. Large-Scale Fabrication of Boron Nitride Nanosheets and Their Utilization in Polymeric Composites with Improved Thermal and Mechanical Properties. Adv. Mater. 2009, 21, 2889–2893. [Google Scholar] [CrossRef]
- Harada, M.; Hamaura, N.; Ochi, M.; Agari, Y. Thermal conductivity of liquid crystalline epoxy/BN filler composites having ordered network structure. Compos. Part B Eng. 2013, 55, 306–313. [Google Scholar] [CrossRef]
- TabkhPaz, M.; Shajari, S.; Mahmoodi, M.; Park, D.Y.; Suresh, H.; Park, S.S. Thermal conductivity of carbon nanotube and hexagonal boron nitride polymer composites. Compos. Part B Eng. 2016, 100, 19–30. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.; Jia, Q.; Wu, G.L.; Ji, T.Z. Improved thermal conductivity and dielectric properties of hBN/PTFE composites via surface treatment by silane coupling agent. Compos. Part B Eng. 2017, 111, 83–90. [Google Scholar] [CrossRef]
- Jeong, S.H.; Song, J.B.; Kim, K.L.; Choi, Y.H.; Lee, H. Enhanced thermal properties of epoxy composite containing cubic and hexagonal boron nitride fillers for superconducting magnet applications. Compos. Part B Eng. 2016, 107, 22–28. [Google Scholar] [CrossRef]
- Husain, E.; Narayanan, T.N.; Taha-Tijerina, J.J.; Vinod, S.; Vajtai, R.; Ajayan, P.M. Marine Corrosion Protective Coatings of Hexagonal Boron Nitride Thin Films on Stainless Steel. ACS Appl. Mater. Interfaces 2013, 5, 4129–4135. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, L.; Singh, R.R.K.; Hofstetter, J.; Uggowitzer, P.J. In-vitro characterization of stress corrosion cracking of aluminium-free magnesium alloys for temporary bio-implant applications. Mater. Sci. Eng. C 2014, 42, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Geick, R.; Perry, C.H.; Rupprecht, G. Normal Modes in Hexagonal Boron Nitride. Phys. Rev. 1966, 146, 543–547. [Google Scholar] [CrossRef]
- Šapić, I.M.; Bistričić, L.; Volovšek, V.; Dananić, V. Vibrational Analysis of 3-Glycidoxypropyltrimethoxysilane Polymer. Macromol. Symp. 2014, 339, 122–129. [Google Scholar] [CrossRef]
- Ma, P.C.; Kim, J.K.; Tang, B.Z. Functionalization of carbon nanotubes using a silane coupling agent. Carbon 2006, 44, 3232–3238. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy Theory, Experiment, and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Banerjee, P.; Woo, R.P.; Grayson, S.M.; Majumder, A.; Raman, R.K.S. Influence of Zeolite Coating on the Corrosion Resistance of AZ91D Magnesium Alloy. Materials 2014, 7, 6092–7004. [Google Scholar] [CrossRef] [PubMed]
- Singh Raman, R.K.; Banerjee, P.C.; Lobo, D.E.; Gullapalli, H.; Sumandasa, M.; Choudhary, L.; Tkacz, R.; Ajayan, P.M.; Majumder, M. Protecting copper from electrochemical degradation by graphene coating. Carbon 2012, 50, 4040–4045. [Google Scholar] [CrossRef]
- Zhu, D.; Ooij, W.J. Corrosion protection of AA 2024-T3 by bis-[3-(triethoxysilyl)propyl]tetrasulfide in sodium chloride solution. Corros. Sci. 2003, 45, 2177–2197. [Google Scholar] [CrossRef]
- Cabral, A.M.; Duarte, R.G.; Montemor, M.F.; Ferreira, M.G.S. A comparative study on the corrosion resistance of AA2024-T3 substrates pre-treated with different silane solutions. Prog. Org. Coat. 2005, 54, 322–331. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S. Electrochemical study of modified bis-[triethoxysilylpropyl] tetrasulfide silane films applied on the AZ31 Mg alloy. Electrochim. Acta 2007, 52, 7486–7495. [Google Scholar] [CrossRef]
- Wang, H.; Akid, R. A room temperature cured sol–gel anticorrosion pre-treatment for Al 2024-T3 alloys. Corros. Sci. 2007, 49, 4491–4503. [Google Scholar] [CrossRef]
- Banerjee, P.C.; Singh, R.; Durandet, Y.; McAdam, G. Electrochemical investigation of the influence of laser surface melting on the microstructure and corrosion behaviour of ZE41 magnesium alloy–An EIS based study. Corros. Sci. 2011, 53, 1505–1514. [Google Scholar] [CrossRef]
- Banerjee, P.C.; Singh, R.R.K.; Durandet, Y.; McAdam, G. Influence of Laser Surface Melting on the Microstructure and Corrosion Behaviour of ZE41 Magnesium Alloy. Mater. Sci. Forum 2009, 618–619, 263–268. [Google Scholar] [CrossRef]
- Harandi, S.E.; Banerjee, P.C.; Easton, C.D.; Raman, R.K.S. Influence of bovine serum albumin in Hanks’ solution on the corrosion and stress corrosion cracking of a magnesium alloy. Mater Sci. Eng. C 2017, 80, 335–345. [Google Scholar] [CrossRef] [PubMed]

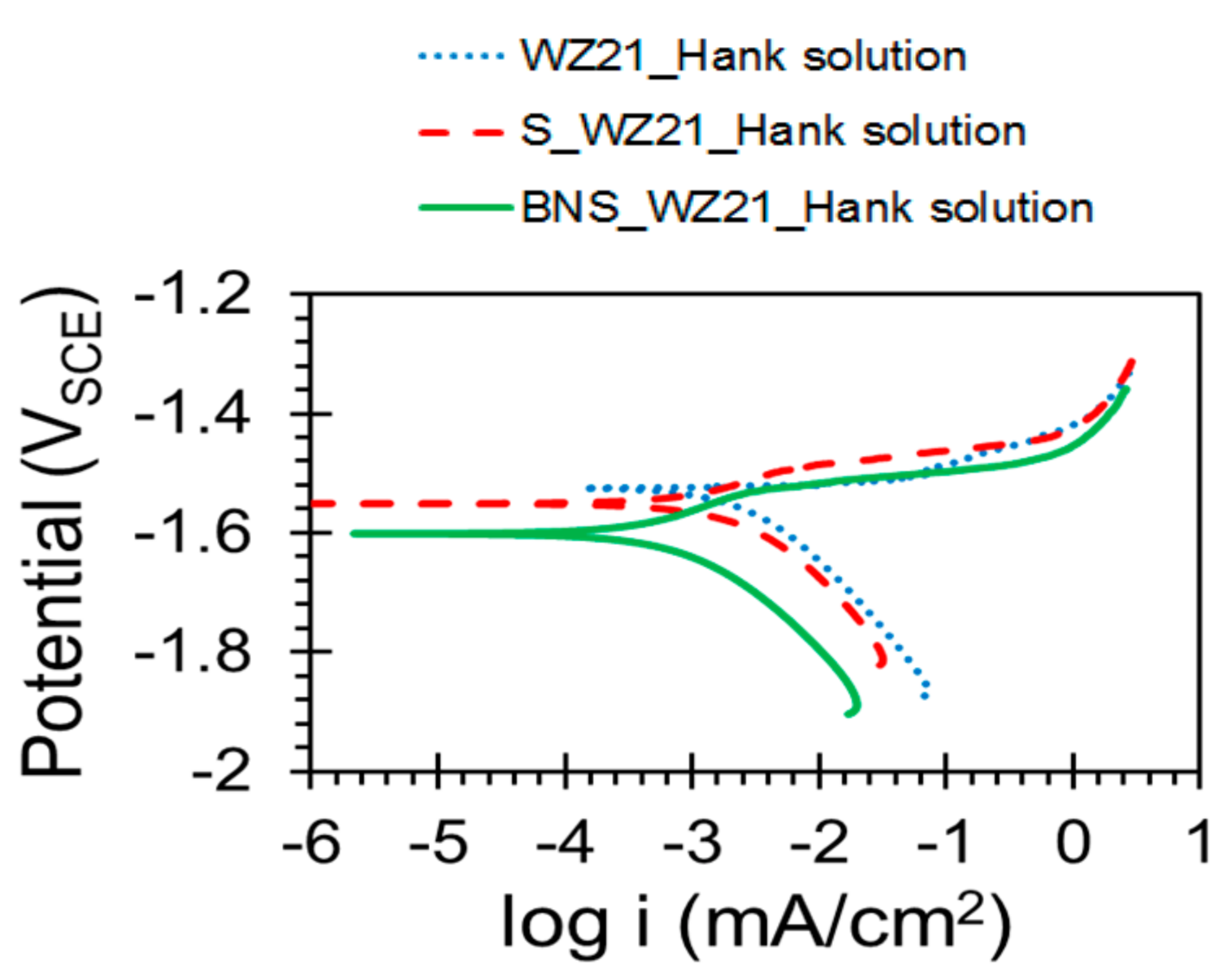
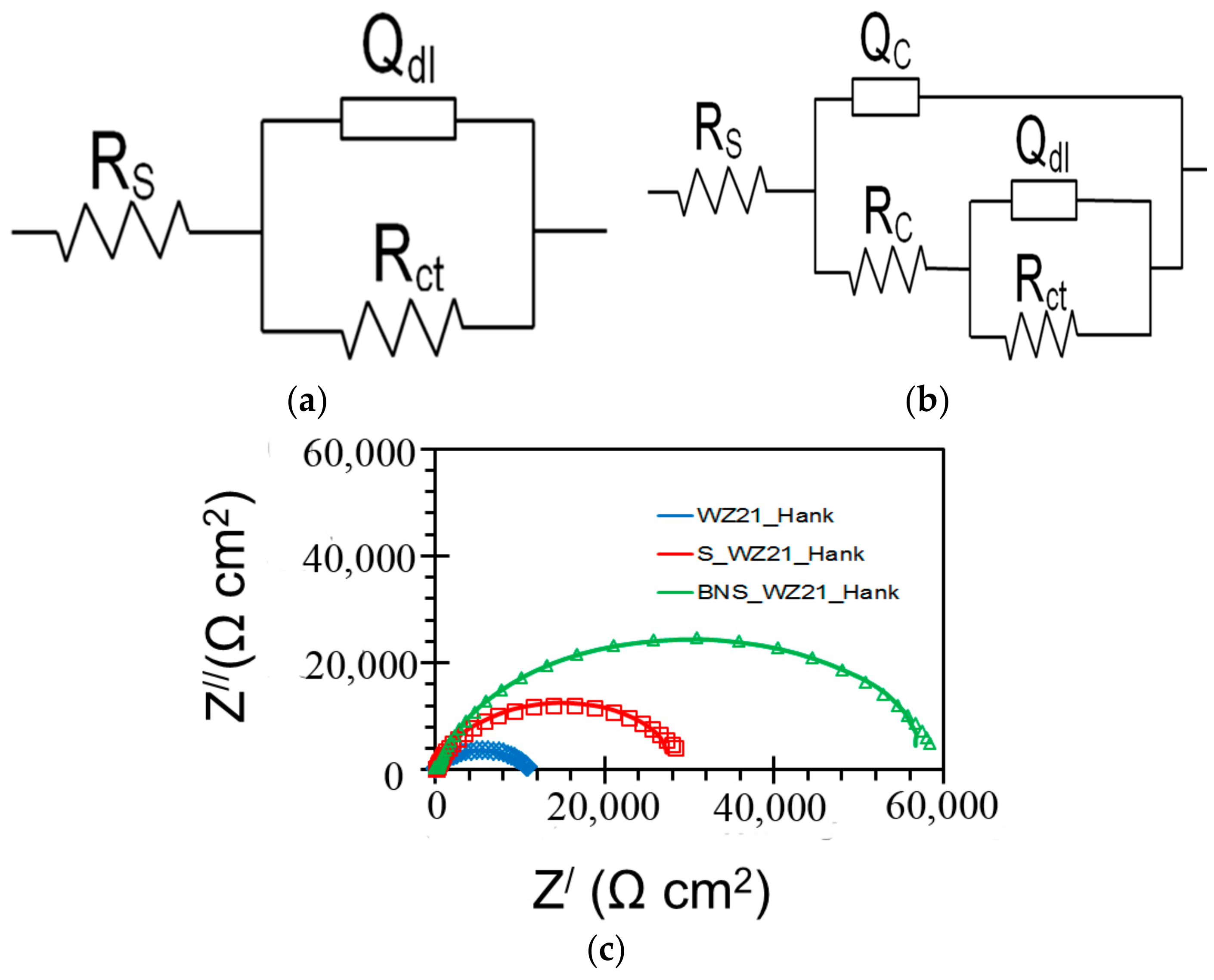
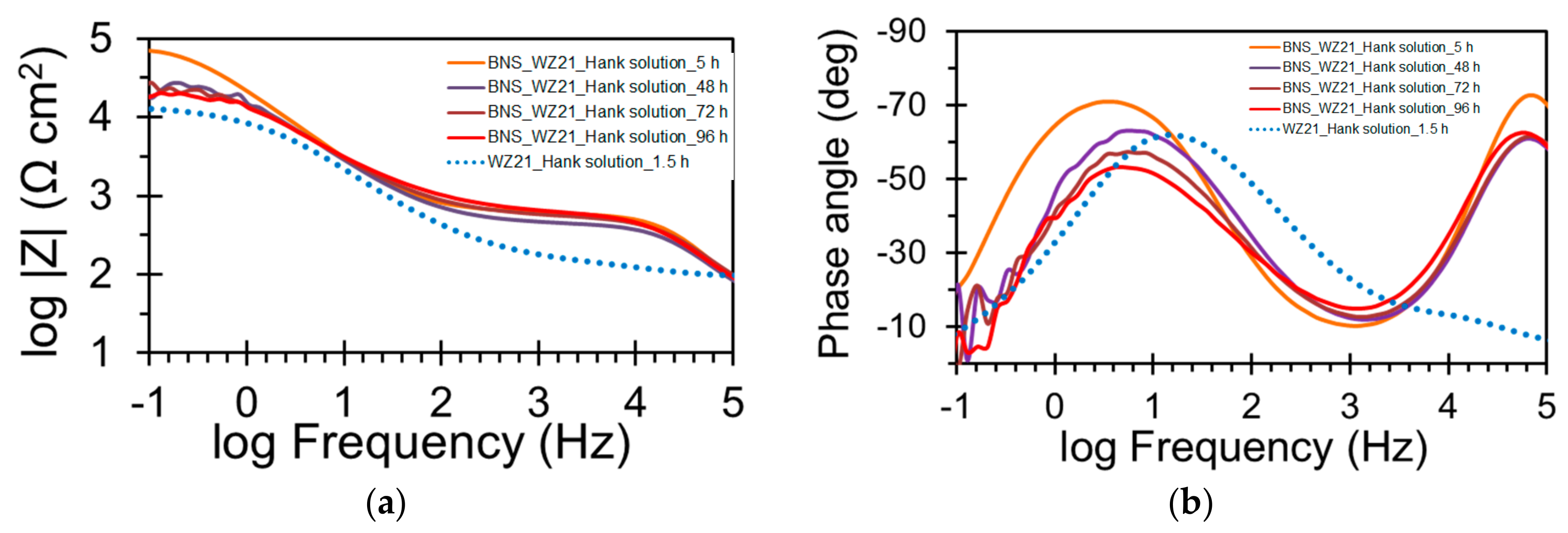
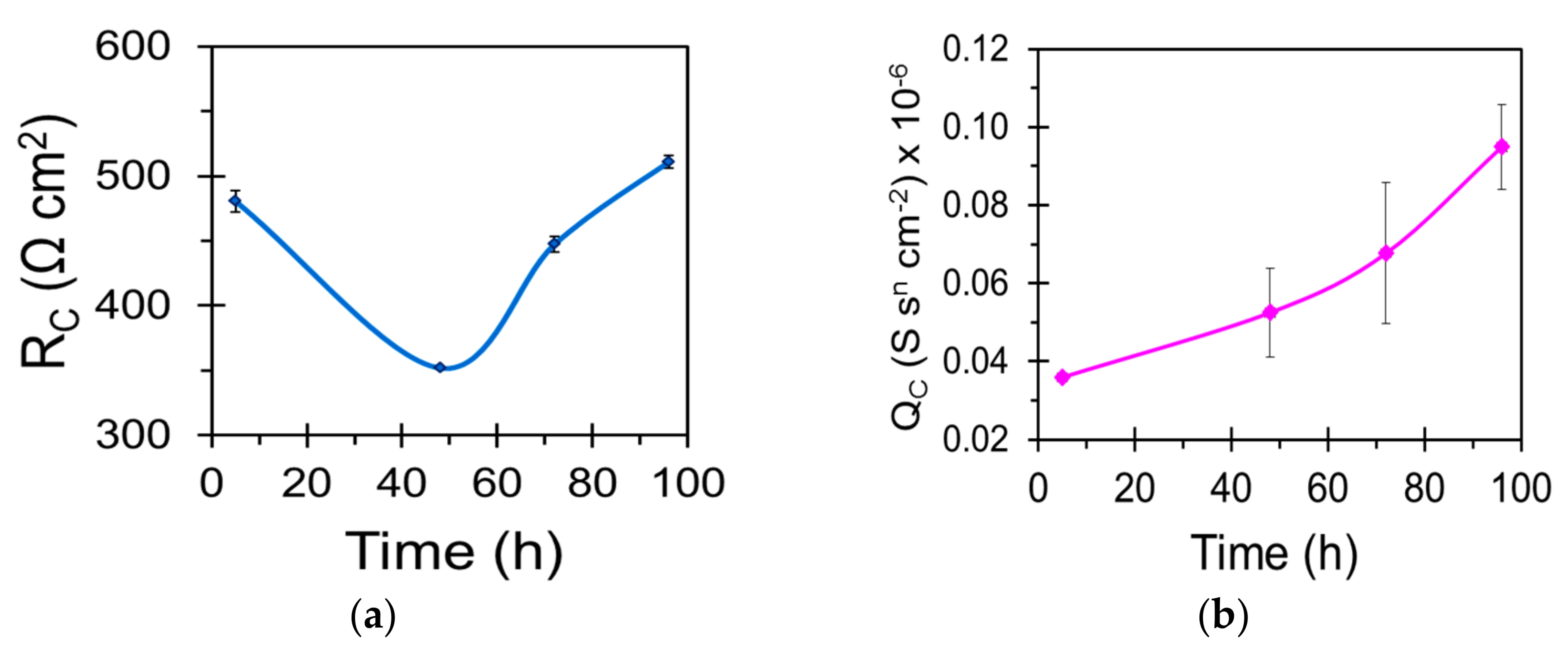
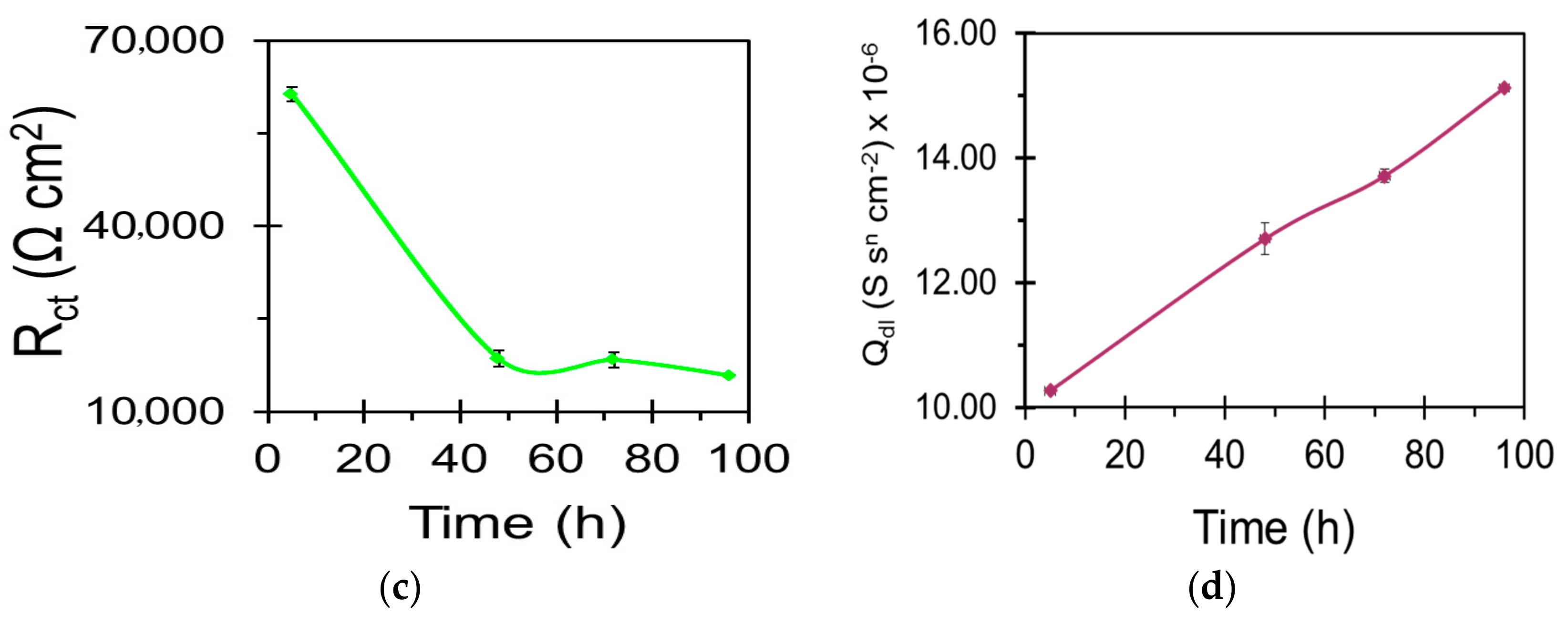

| Sample | Ecorr (VSCE) | icorr (µA/cm2) |
|---|---|---|
| WZ21 | −1.525 (±0.0083) | 1.217 (±0.269) |
| S_WZ21 | −1.546 (±0.009) | 0.538 (±0.059) |
| BNS_WZ21 | −1.594 (±0.007) | 0.292 (±0.006) |
| Sample | Qc (S·sn·cm−2) | nc | Rc (Ω·cm2) | Qdl (S sn cm−2) | ndl | Rct (Ω·cm2) |
|---|---|---|---|---|---|---|
| WZ21 | - | - | - | 2.639 × 10−5 | 0.7 | 11,048 |
| S_WZ21 | 2.164 × 10−6 | 0.6 | 213 | 1.106 × 10−5 | 0.9 | 29,736 |
| BNS_WZ21 | 5.169 × 10−8 | 0.9 | 403 | 8.735 × 10−6 | 0.9 | 59,364 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saadi, S.; Banerjee, P.C.; Anisur, M.R.; Raman, R.K.S. Hexagonal Boron Nitride Impregnated Silane Composite Coating for Corrosion Resistance of Magnesium Alloys for Temporary Bioimplant Applications. Metals 2017, 7, 518. https://doi.org/10.3390/met7120518
Al-Saadi S, Banerjee PC, Anisur MR, Raman RKS. Hexagonal Boron Nitride Impregnated Silane Composite Coating for Corrosion Resistance of Magnesium Alloys for Temporary Bioimplant Applications. Metals. 2017; 7(12):518. https://doi.org/10.3390/met7120518
Chicago/Turabian StyleAl-Saadi, Saad, Parama Chakraborty Banerjee, M.R. Anisur, and R.K. Singh Raman. 2017. "Hexagonal Boron Nitride Impregnated Silane Composite Coating for Corrosion Resistance of Magnesium Alloys for Temporary Bioimplant Applications" Metals 7, no. 12: 518. https://doi.org/10.3390/met7120518





