Effect of Sintering Time on the Densification, Microstructure, Weight Loss and Tensile Properties of a Powder Metallurgical Fe-Mn-Si Alloy
Abstract
:1. Introduction
2. Experiment
2.1. Powder Preparation
2.2. Press and Sinter
2.3. Characterisation and Analysis
2.4. Principle to Calculate the Vapor Pressure
3. Results
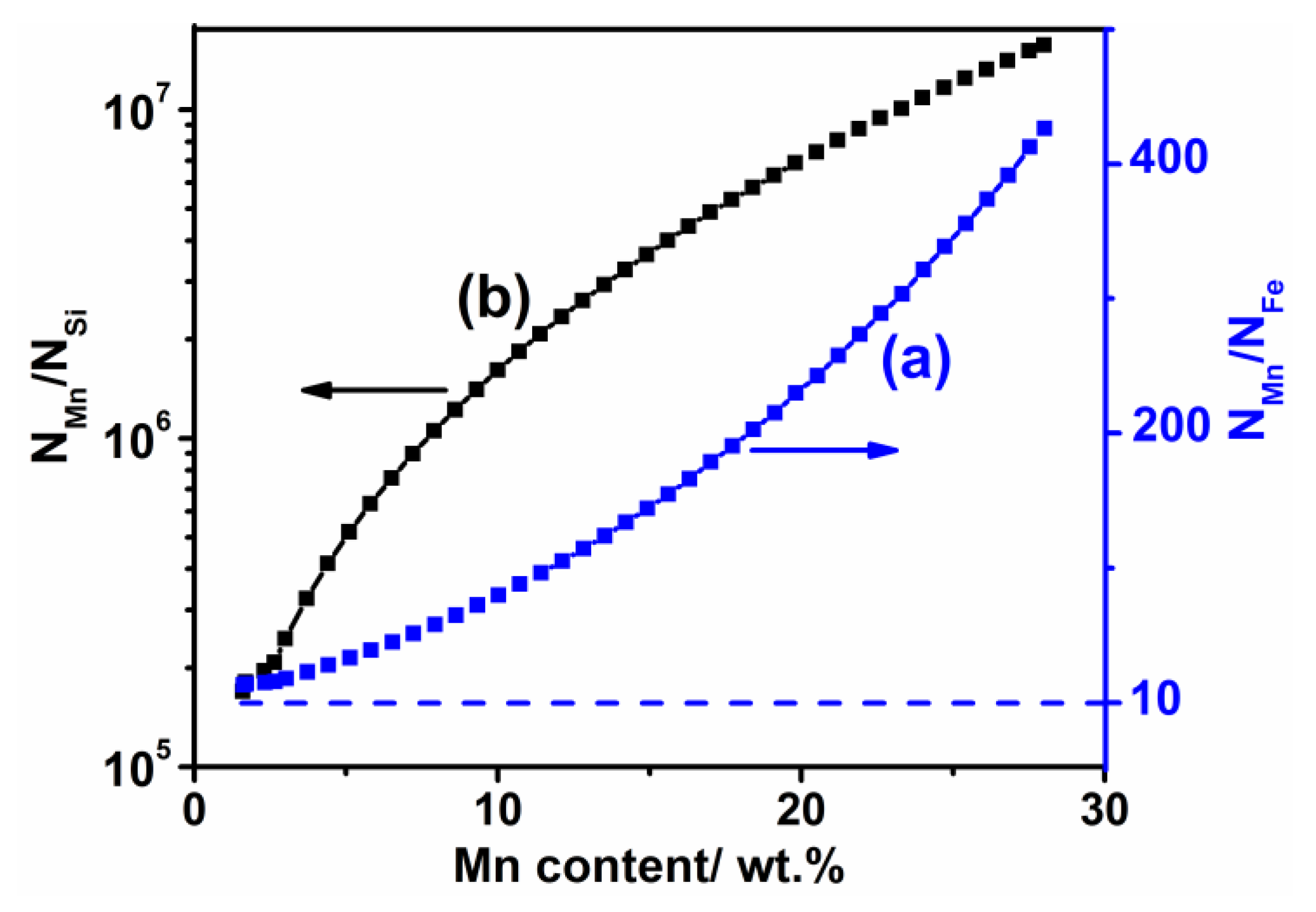
3.1. Weight Loss and Chemical Composition
3.2. Density and Porosity
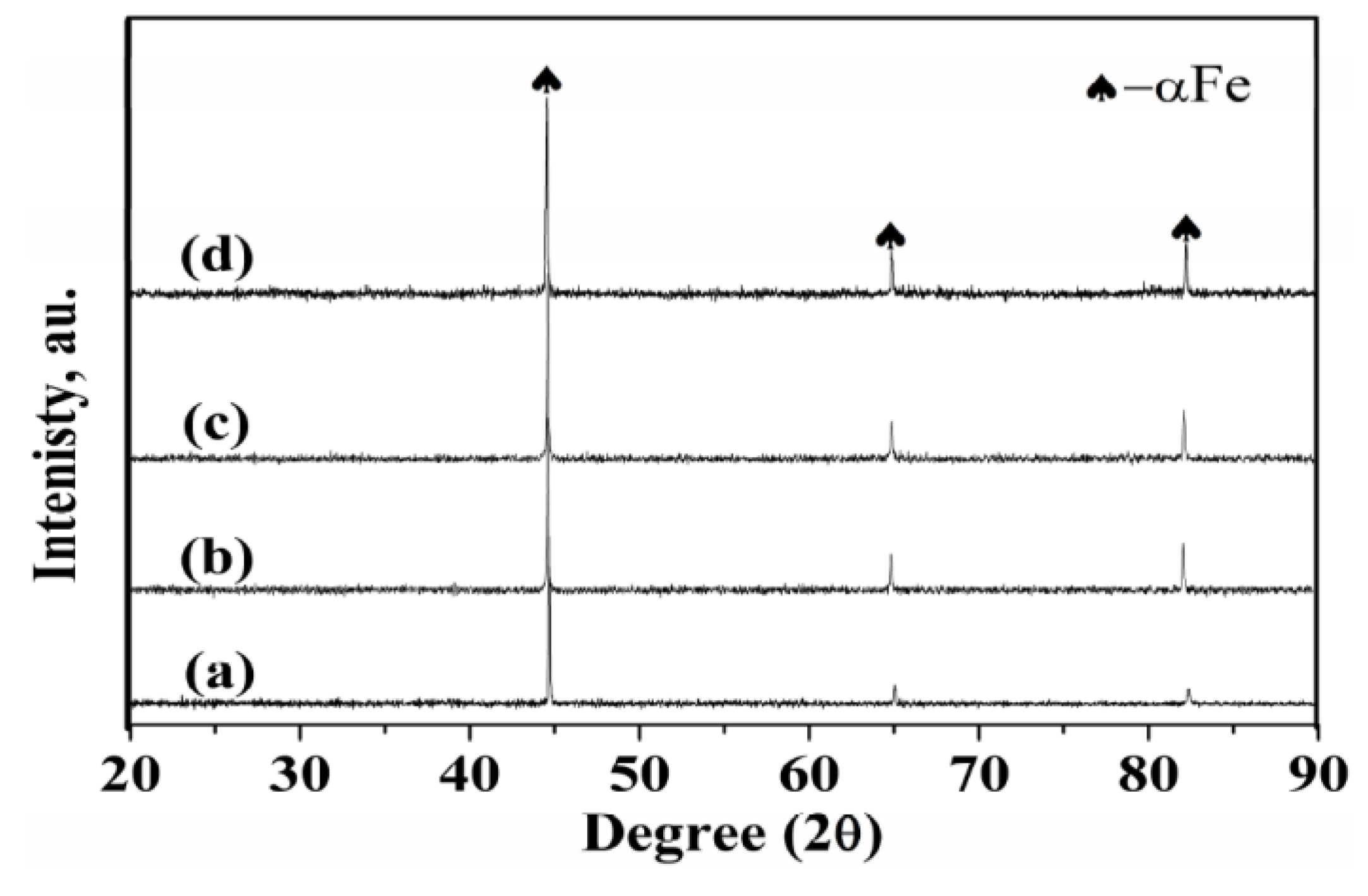
3.3. Phase Identification
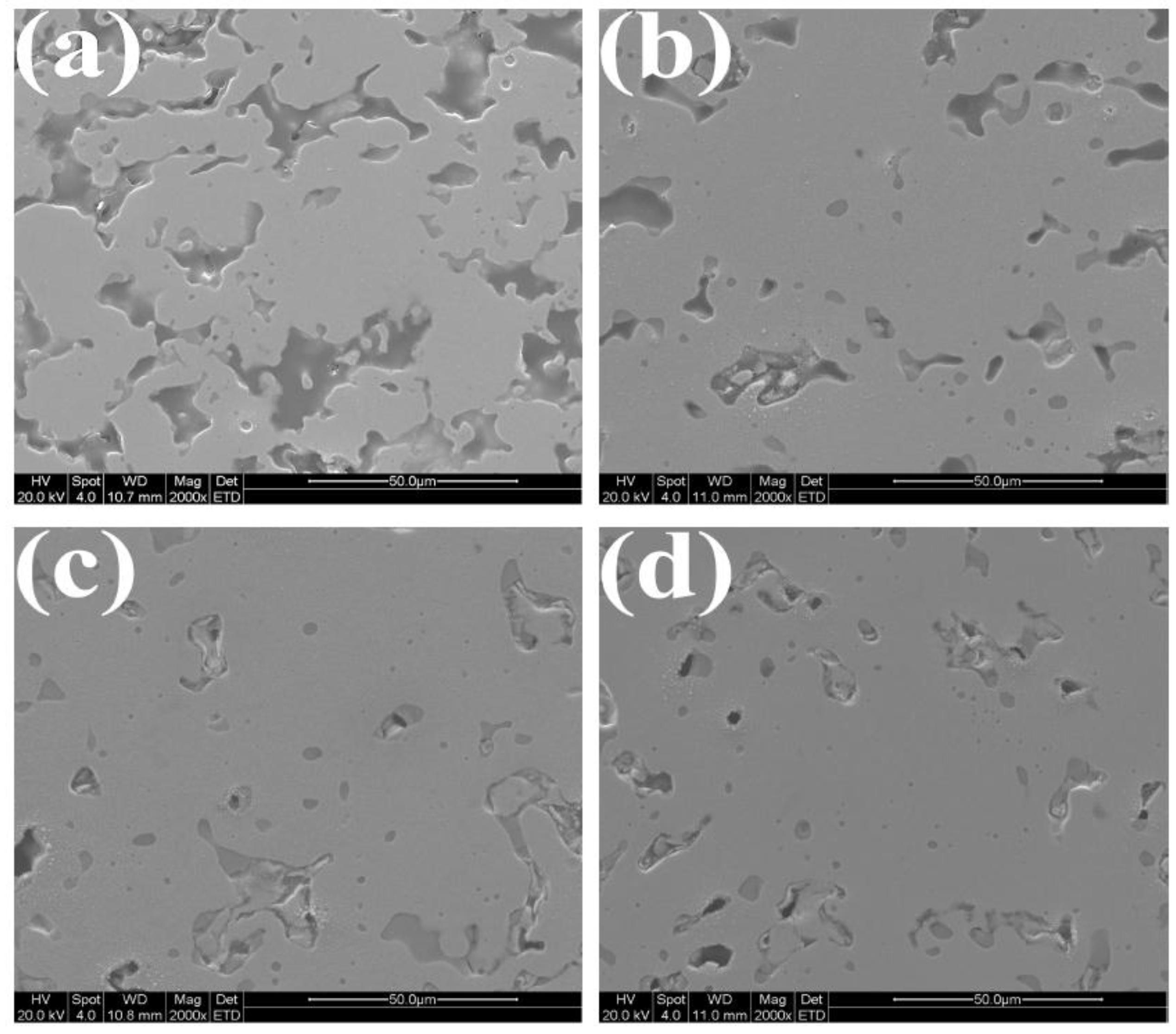
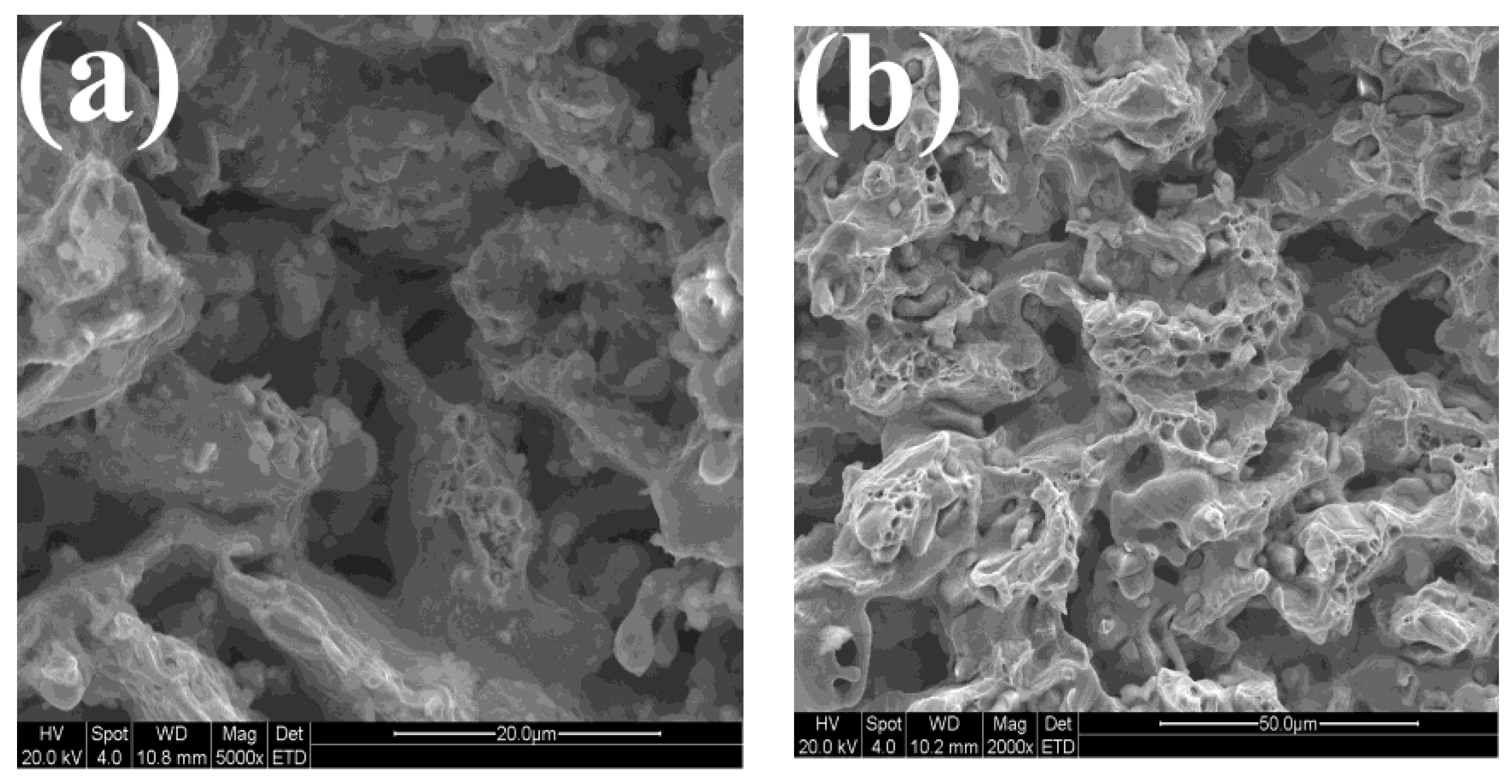
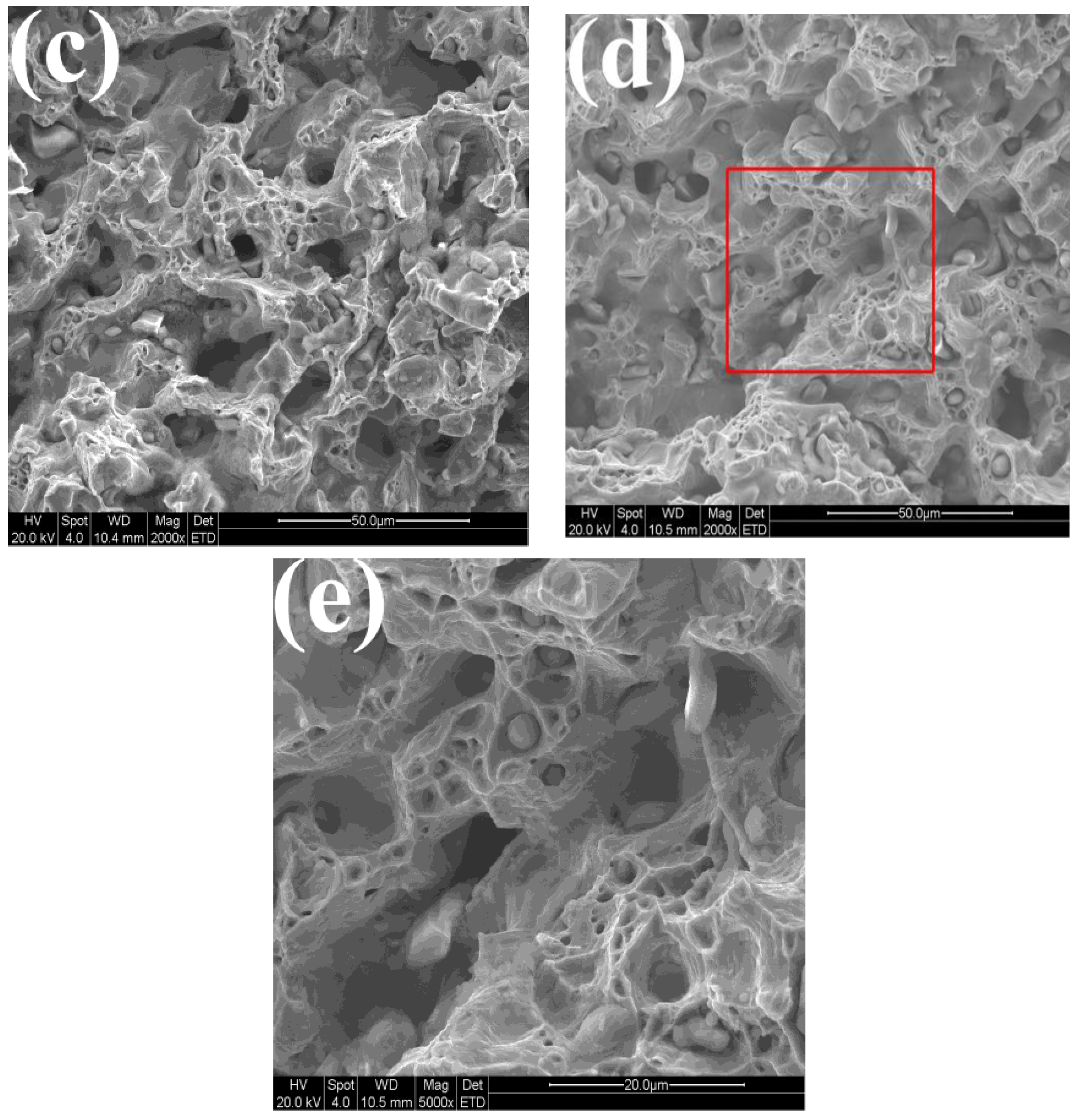
3.4. Microstructure
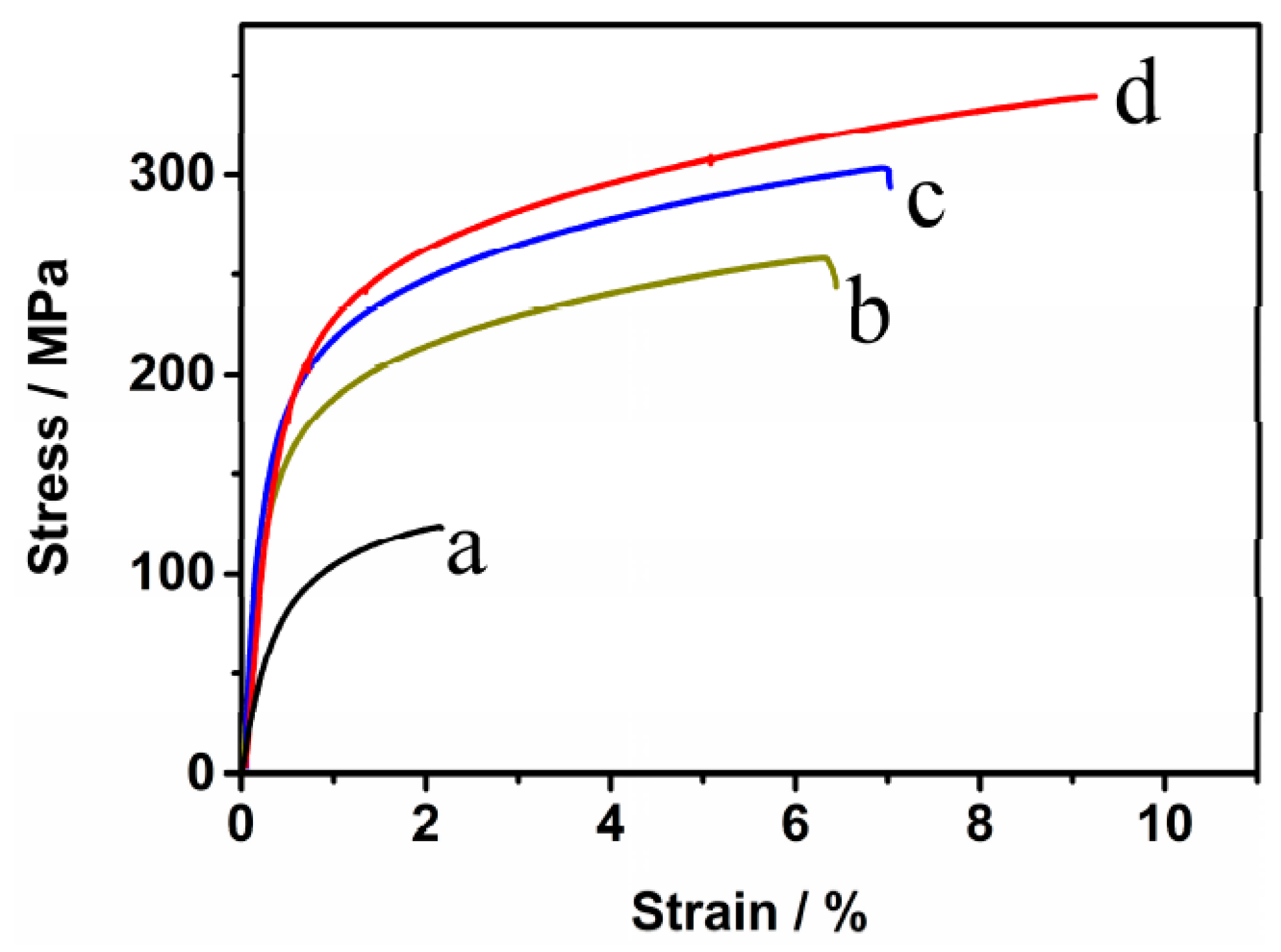
3.5. Tensile Properties
4. Discussion
4.1. Weight Loss Mechanism
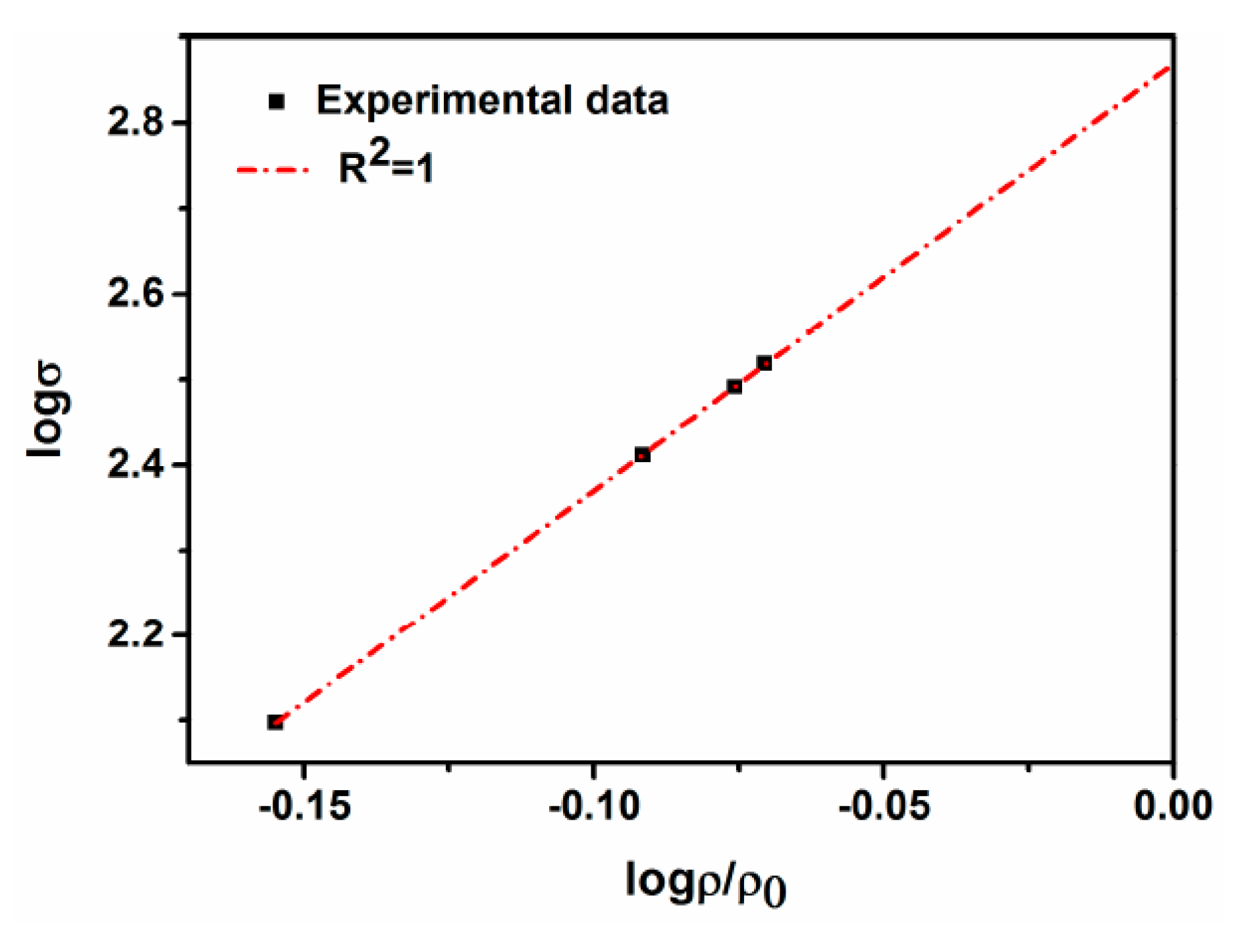
4.2. Densification
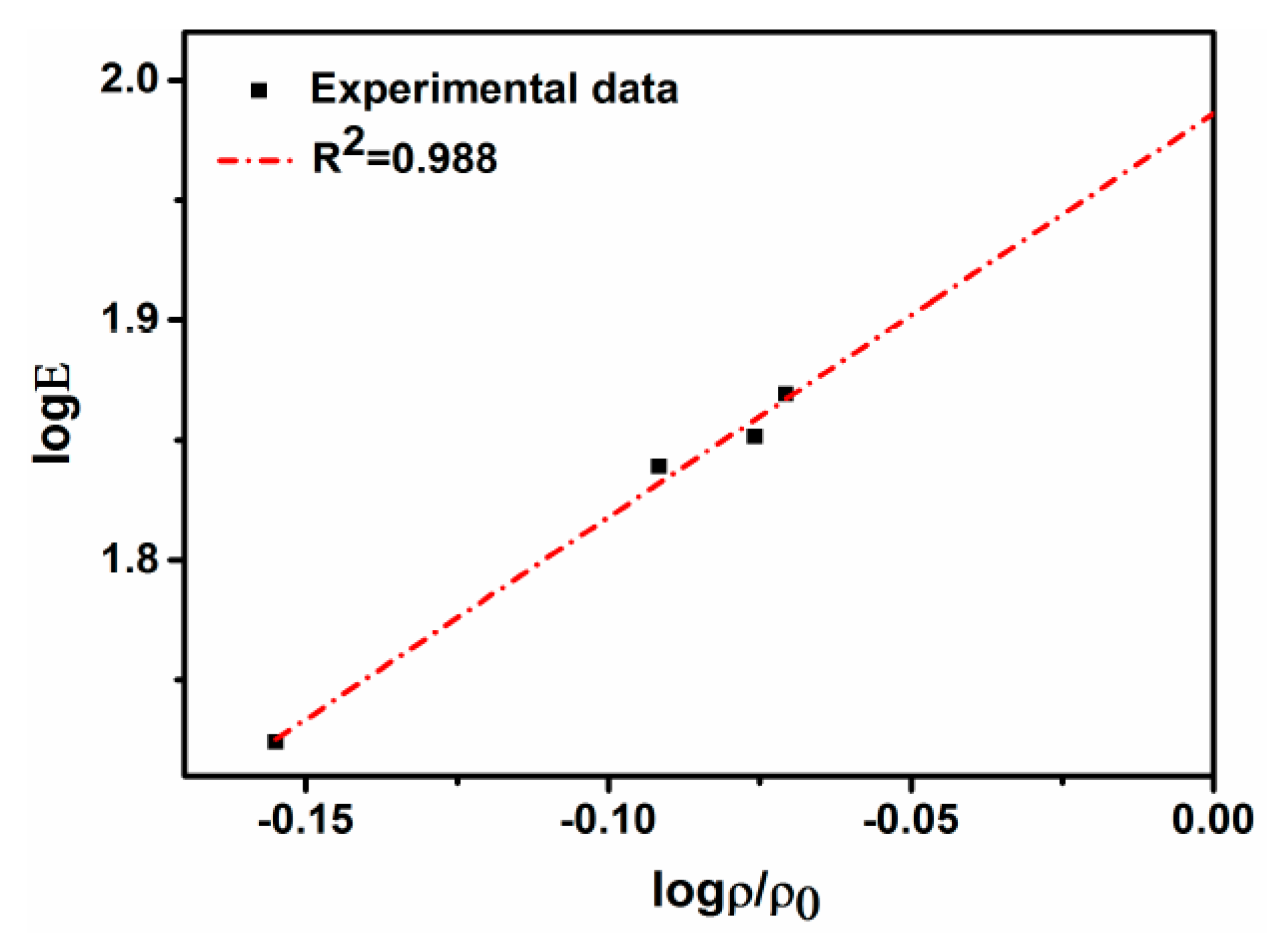
4.3. Tensile Properties and Fracture
5. Conclusions
- (1)
- The weight loss of the sintered Fe-Mn-Si compacts with no isothermal holding is only ~2%. The weight loss of the sintered Fe-Mn-Si compacts increases significantly to ~7.6% after the first hour of isothermal holding. A further increment in weight loss is very limited when the isothermal holding time is >1 h. An Mn depletion region exists on the surface layer of all of the sintered compacts. The length of the Mn depletion region (LD) increases with the increase of the isothermal holding time. The weight loss is mainly caused by the sublimation of Mn.
- (2)
- The density of the sintered ternary Fe-Mn-Si alloys increases drastically during the first hour of isothermal holding, while densification slows down when the isothermal holding time is >1 h. The drop in open porosities mainly occurs during the first hour of isothermal holding.
- (3)
- The surface of the sintered Fe-Mn-Si is comprised of a single α-Fe phase. The sintered compacts in locations ≥LD consist of a major γ-austenite and minor ε-martensite.
- (4)
- The tensile properties of the sintered compacts increase with the increase of the sintering time. The tensile strength, elongation, and elasticity drastically increase from 125 MPa, 2.1%, and 52 GPa for the samples with no isothermal holding to 258 MPa, 6.4%, and 69 GPa for the samples with isothermal holding for 1 h.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ölander, A. An electrochemical investigation of solid cadmium-gold alloys. J. Am. Chem. Soc. 1932, 54, 3819–3833. [Google Scholar] [CrossRef]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Rao, A.; Srinivasa, A.; Reddy, J. Design of Shape Memory Alloy (SMA) Actuators, 1st ed.; Springer: New York, NY, USA, 2015; pp. 1–30. [Google Scholar]
- Lee, W.; Weber, B.; Leinenbach, C. Recovery stress formation in a restrained Fe-Mn-Si-based shape memory alloy used for prestressing or mechanical joining. Constr. Build. Mater. 2015, 95, 600–610. [Google Scholar] [CrossRef]
- Dasgupta, R. A look into Cu-based shape memory alloys: Present scenario and future prospects. J. Mater. Res. 2014, 29, 1681–1698. [Google Scholar] [CrossRef]
- Dong, Z.; Kajiwara, S.; Kikuchi, T.; Sawaguchi, T. Effect of pre-deformation at room temperature on shape memory properties of stainless type Fe-15Mn-5Si-9Cr-5Ni-(0.5–1.5) NbC alloys. Acta Mater. 2005, 53, 4009–4018. [Google Scholar] [CrossRef]
- Tanaka, Y.; Himuro, Y.; Kainuma, R.; Sutou, Y.; Omori, T.; Ishida, K. Ferrous polycrystalline shape-memory alloy showing huge superelasticity. Science 2010, 327, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Kubo, H.; Maruyama, T. Mechanical properties of Fe-Mn-Si based SMA and the application. Mater. Trans. 2006, 47, 571–579. [Google Scholar] [CrossRef]
- Cladera, A.; Weber, B.; Leinenbach, C.; Czaderski, C.; Shahverdi, M.; Motavalli, M. Iron-based shape memory alloys for civil engineering structures: An overview. Constr. Build. Mater. 2014, 63, 281–293. [Google Scholar] [CrossRef]
- Sawaguchi, T.; Nikulin, I.; Ogawa, K.; Sekido, K.; Takamori, S.; Maruyama, T.; Chiba, Y.; Kushibe, A.; Inoue, Y.; Tsuzaki, K. Designing Fe-Mn-Si alloys with improved low-cycle fatigue lives. Scr. Mater. 2015, 99, 49–52. [Google Scholar] [CrossRef]
- Xu, Z.; Hodgson, M.A.; Cao, P. A comparative study of powder metallurgical (PM) and wrought Fe-Mn-Si alloys. Mater. Sci. Eng. A 2015, 630, 116–124. [Google Scholar] [CrossRef]
- Xu, Z.; Hodgson, M.A.; Cao, P. Microstructure and degradation behavior of forged Fe-Mn-Si alloys. Int. J. Mod. Phys. B 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.; Ruan, L. In vitro investigation of Fe30Mn6Si shape memory alloy as potential biodegradable metallic material. Mater. Lett. 2011, 65, 540–543. [Google Scholar] [CrossRef]
- Xu, Z.; Hodgson, M.A.; Cao, P. Effect of Immersion in Simulated Body Fluid on the Mechanical Properties and Biocompatibility of Sintered Fe-Mn-Based Alloys. Metals 2016, 6, 309. [Google Scholar] [CrossRef]
- Xu, Z.; Hodgson, M.A.; Cao, P. Effects of Mechanical Milling and Sintering Temperature on the Densification, Microstructure and Tensile Properties of the Fe-Mn-Si Powder Compacts. J. Mater. Sci. Technol. 2016, 32, 1161–1170. [Google Scholar] [CrossRef]
- Cintas, J.; Cuevas, F.; Montes, J.; Herrera, E. High-strength PM aluminium by milling in ammonia gas and sintering. Scr. Mater. 2005, 53, 1165–1170. [Google Scholar] [CrossRef]
- McNeese, M.D.; Lagoudas, D.C.; Pollock, T.C. Processing of TiNi from elemental powders by hot isostatic pressing. Mater. Sci. Eng. A 2000, 280, 334–348. [Google Scholar] [CrossRef]
- Angelo, P.; Subramanian, R. Powder Metallurgy: Science, Technology and Applications; PHI Learning Pvt. Ltd.: New Delhi, India, 2008. [Google Scholar]
- Rahimian, M.; Parvin, N.; Ehsani, N. The effect of production parameters on microstructure and wear resistance of powder metallurgy Al-Al2O3 composite. Mater. Des. 2011, 32, 1031–1038. [Google Scholar] [CrossRef]
- German, R.M. Powder Metallurgy and Particulate Materals Processing: The Processes, Materials, Products, Properties and Applications, 1st ed.; Metal Powder Industries Federation: Princeton, NJ, USA, 2005; pp. 221–260. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Zhu, M.; Dai, L.; Gu, N.; Cao, B.; Ouyang, L. Synergism of mechanical milling and dielectric barrier discharge plasma on the fabrication of nano-powders of pure metals and tungsten carbide. J. Alloys Compd. 2009, 478, 624–629. [Google Scholar] [CrossRef]
- Gheisari, K.; Javadpour, S.; Oh, J.; Ghaffari, M. The effect of milling speed on the structural properties of mechanically alloyed Fe-45%Ni powders. J. Alloys Compd. 2009, 472, 416–420. [Google Scholar] [CrossRef]
- Garroni, S.; Enzo, S.; Delogu, F. Mesostructural refinement in the early stages of mechanical alloying. Scr. Mater. 2014, 83, 49–52. [Google Scholar] [CrossRef]
- Zhang, Z.; Sandström, R.; Frisk, K.; Salwén, A. Characterization of intermetallic Fe-Mn-Si powders produced by casting and mechanical ball milling. Powder Technol. 2003, 137, 139–147. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.; Zhao, Z.; Ma, R.; Hu, T.; Xie, Y. Mechanical alloying of Fe-Mn and Fe-Mn-Si. Mater. Sci. Eng. A 1999, 271, 8–13. [Google Scholar] [CrossRef]
- Saito, T.; Kapusta, C.; Takasaki, A. Synthesis and characterization of Fe–Mn–Si shape memory alloy by mechanical alloying and subsequent sintering. Mater. Sci. Eng. A 2014, 592, 88–94. [Google Scholar] [CrossRef]
- Šalak, A.; Selecka, M.; Bureš, R. Manganese in ferrous powder metallurgy. Powder Metall. Prog. 2001, 1, 41–58. [Google Scholar]
- Hryha, E.; Dudrova, E.; Nyborg, L. Critical aspects of alloying of sintered steels with manganese. Metall. Mater. Trans. A 2010, 41, 2880–2897. [Google Scholar] [CrossRef]
- Hryha, E.; Gierl, C.; Nyborg, L.; Danninger, H.; Dudrova, E. Surface composition of the steel powders pre-alloyed with manganese. Appl. Surf. Sci. 2010, 256, 3946–3961. [Google Scholar] [CrossRef]
- Xu, Z.; Hodgson, M.A.; Cao, P. Weight loss behavior of a vacuum sintered powder metallurgical Fe-Mn-Si alloy. J. Mater. Res. 2017, 32, 644–655. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM) International. ASTM B962–14, Standard Test Methods for Density of Compacted or Sintered Powder Metallurgy (PM) Products Using Archimedes’ Principle; ASTM International: West Conshohocken, PA, USA, 2014; p. 7. [Google Scholar]
- Lide, D.R. CRC Handbook of Physics and Chemistry, 85th ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Redlich, O.; Kister, A. Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 1948, 40, 345–348. [Google Scholar] [CrossRef]
- Forsberg, A.; Ågren, J. Thermodynamic evaluation of the Fe-Mn-Si system and the γ/ε martensitic transformation. J. Phase Equilib. 1993, 14, 354–363. [Google Scholar] [CrossRef]
- Saunders, N.; Miodownik, A.P. CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide; Pergamon: Oxford, UK, 1998; Volume 1, pp. 1–100. [Google Scholar]
- Raghavan, V.; Raynor, G.V.; Rivlin, V.G. Phase Diagrams of Ternary Iron Alloys; Indian Institute of Metals: New Delhi, India, 1987; pp. 363–377. [Google Scholar]
- Jones, H.A.; Mackay, G. The rates of evaporation and the vapor pressures of tungsten, molybdenum, platinum, nickel, iron, copper and silver. Phys. Rev. 1927, 30, 201. [Google Scholar] [CrossRef]
- Langmuir, I. Vapor pressures, evaporation, condensation and adsorption. J. Am. Chem. Soc. 1932, 54, 2798–2832. [Google Scholar] [CrossRef]
- Chen, G.; Cao, P.; He, Y.; Shen, P.; Gao, H. Effect of aluminium evaporation loss on pore characteristics of porous FeAl alloys produced by vacuum sintering. J. Mater. Sci. 2012, 47, 1244–1250. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999; pp. 100–200. [Google Scholar]
- Kim, J.; Lee, S.-J.; de Cooman, B.C. Effect of Al on the stacking fault energy of F-18Mn-0.6C twinning-induced plasticity. Scr. Mater. 2011, 65, 363–366. [Google Scholar] [CrossRef]
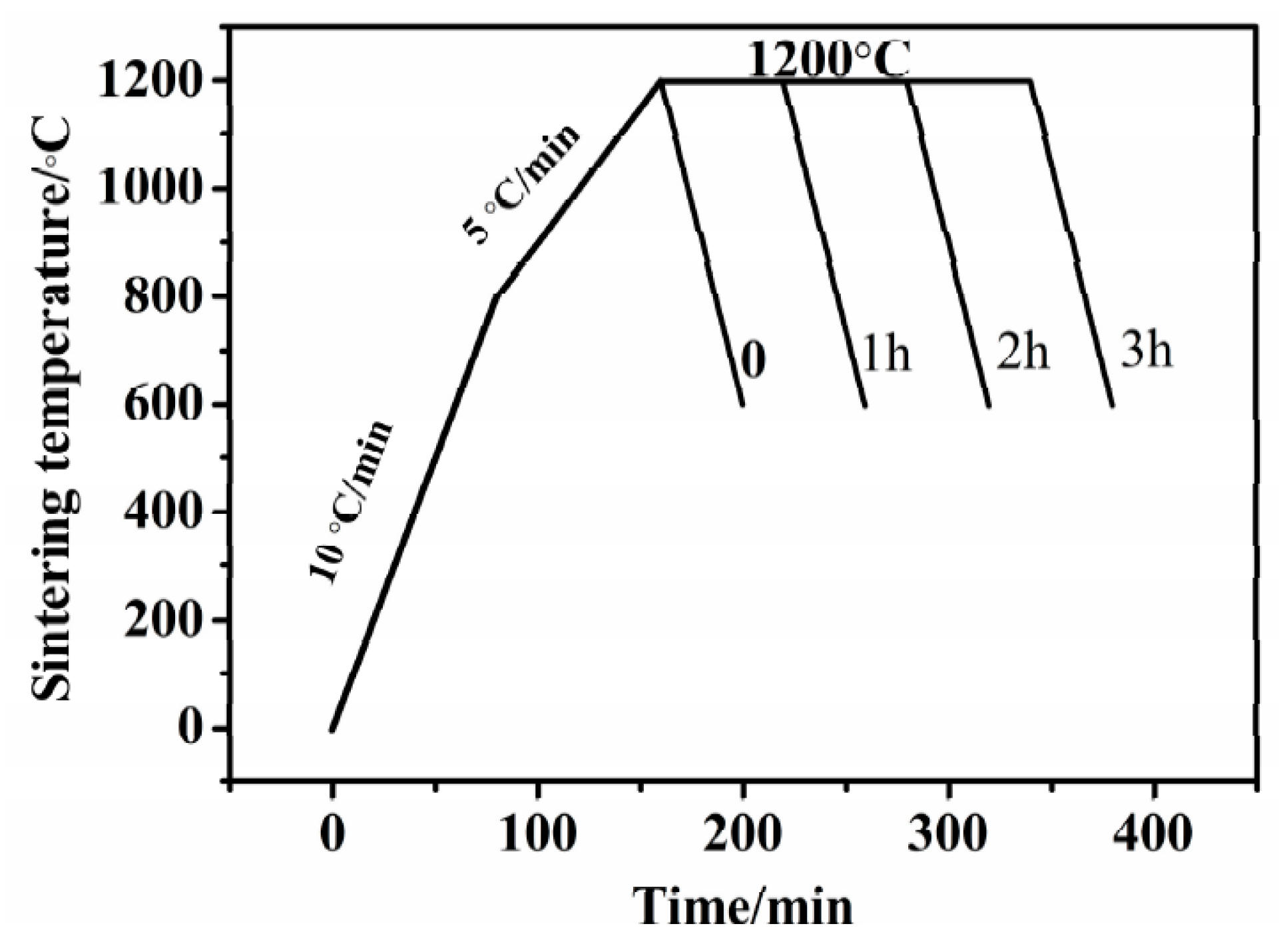

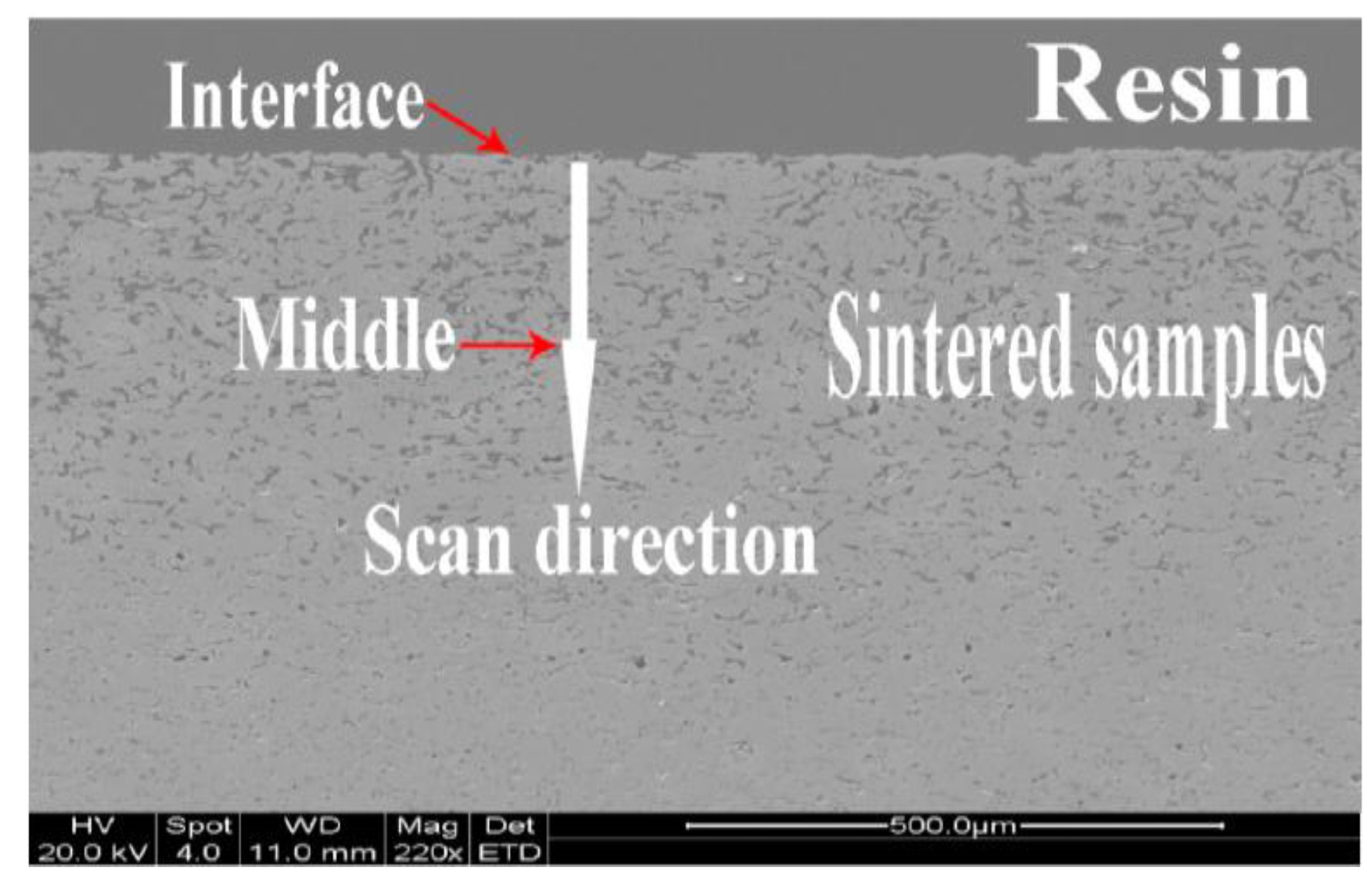
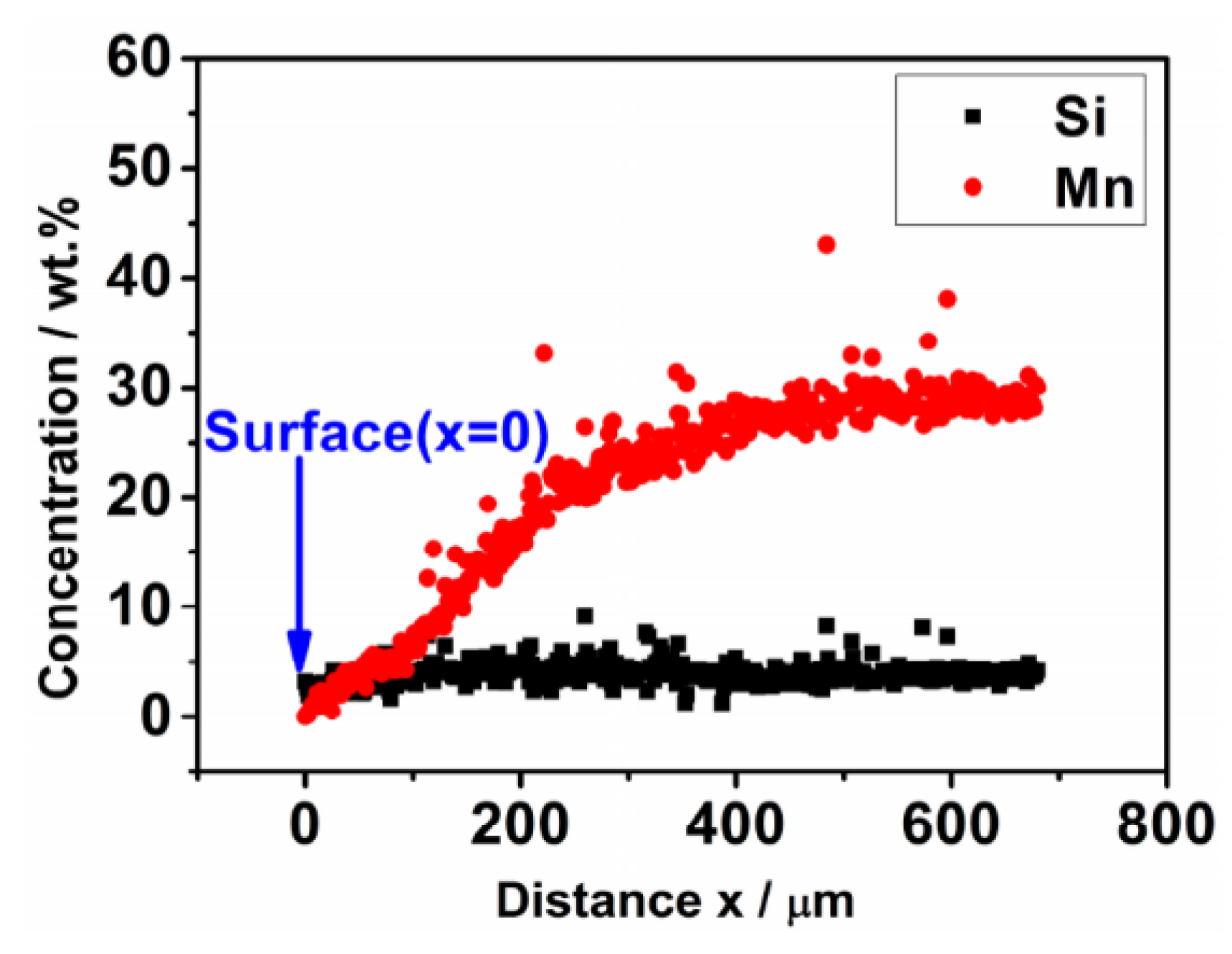
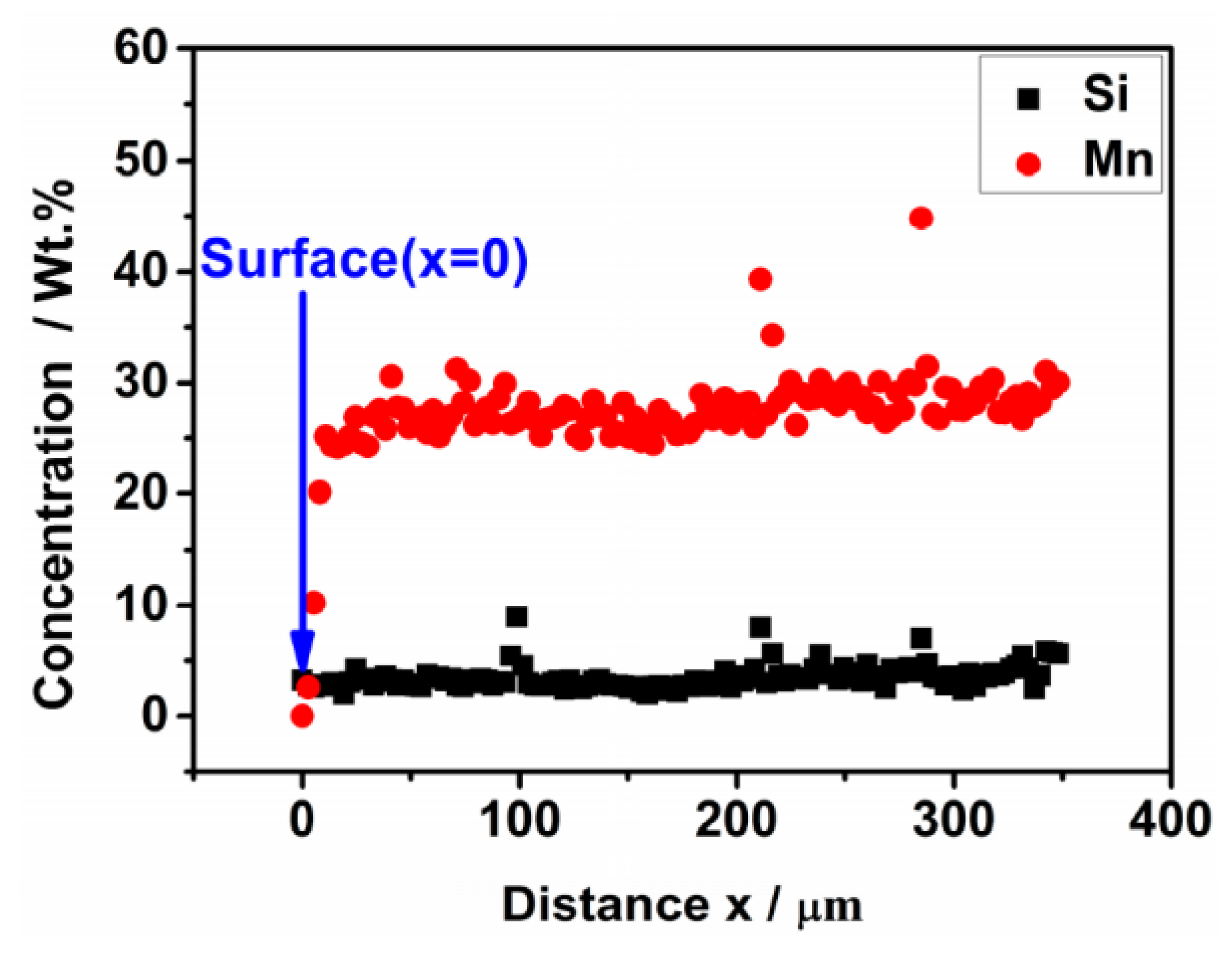



| Holding Time/h | Chemical Composition on the Surface x = 0 (wt. %) | LD (μm) | Chemical Composition (wt. %) at Positions ≥LD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn | Si | O | Fe | Mn | Si | O | Fe | ||
| 0 | 1.12 ± 0.12 | 3.21 ± 0.03 | 0.45 ± 0.04 | Bal. | 12 ± 5 | 27.65 ± 0.38 | 3.07 ± 0.04 | 0.45 ± 0.03 | Bal. |
| 1 | 1.14 ± 0.08 | 3.18 ± 0.06 | 0.43 ± 0.07 | Bal. | 405 ± 29 | 27.58 ± 0.41 | 3.09 ± 0.06 | 0.39 ± 0.06 | Bal. |
| 2 | 1.19 ± 0.09 | 3.09 ± 0.05 | 0.46 ± 0.07 | Bal. | 445 ± 31 | 27.67 ± 0.38 | 3.11 ± 0.04 | 0.47 ± 0.08 | Bal. |
| 3 | 1.13 ± 0.07 | 3.11 ± 0.06 | 0.47 ± 0.05 | Bal. | 500 ± 36 | 27.49 ± 0.45 | 3.05 ± 0.09 | 0.42 ± 0.09 | Bal. |
| Sintering Temperature/°C | Holding Time/h | Ultimate Tensile Strength/MPa | Fracture Strain/% | Young’s Modulus/GPa |
|---|---|---|---|---|
| 1200 | 0 | 125 ± 9 | 2 ± 1 | 53 ± 4 |
| 1 | 258 ± 9 | 6 ± 1 | 69 ± 3 | |
| 2 | 310 ± 15 | 8 ± 1 | 71 ± 3 | |
| 3 | 330 ± 23 | 9 ± 1 | 74 ± 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Hodgson, M.A.; Chang, K.; Chen, G.; Yuan, X.; Cao, P. Effect of Sintering Time on the Densification, Microstructure, Weight Loss and Tensile Properties of a Powder Metallurgical Fe-Mn-Si Alloy. Metals 2017, 7, 81. https://doi.org/10.3390/met7030081
Xu Z, Hodgson MA, Chang K, Chen G, Yuan X, Cao P. Effect of Sintering Time on the Densification, Microstructure, Weight Loss and Tensile Properties of a Powder Metallurgical Fe-Mn-Si Alloy. Metals. 2017; 7(3):81. https://doi.org/10.3390/met7030081
Chicago/Turabian StyleXu, Zhigang, Michael A. Hodgson, Keke Chang, Gang Chen, Xiaowen Yuan, and Peng Cao. 2017. "Effect of Sintering Time on the Densification, Microstructure, Weight Loss and Tensile Properties of a Powder Metallurgical Fe-Mn-Si Alloy" Metals 7, no. 3: 81. https://doi.org/10.3390/met7030081
APA StyleXu, Z., Hodgson, M. A., Chang, K., Chen, G., Yuan, X., & Cao, P. (2017). Effect of Sintering Time on the Densification, Microstructure, Weight Loss and Tensile Properties of a Powder Metallurgical Fe-Mn-Si Alloy. Metals, 7(3), 81. https://doi.org/10.3390/met7030081







