Role of Severe Plastic Deformation in Suppressing Formation of R Phase and Ni4Ti3 Precipitate of NiTi Shape Memory Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
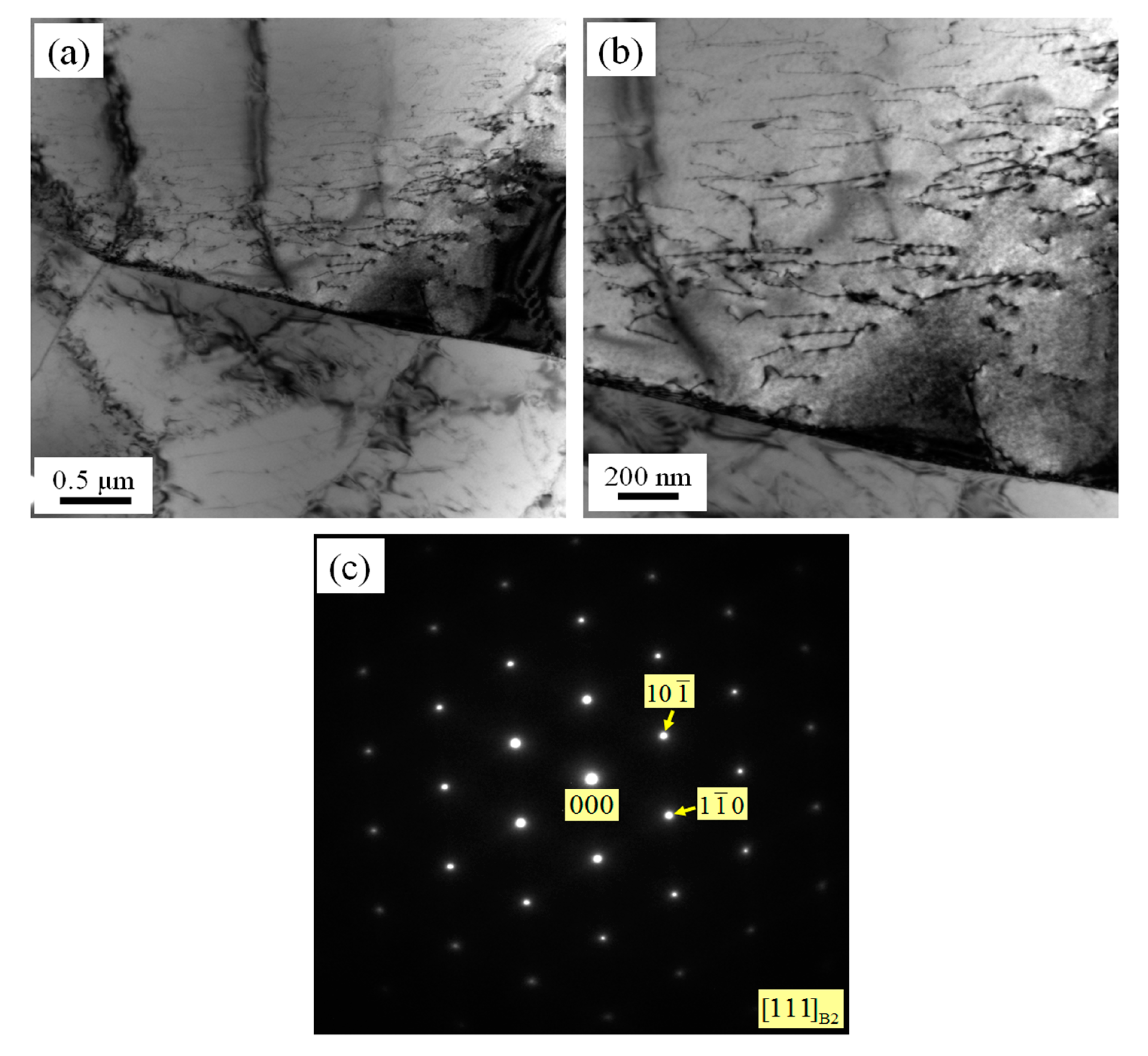
3.1. Microstructures of As-Rolled and Solution-Treated NiTi SMA Samples
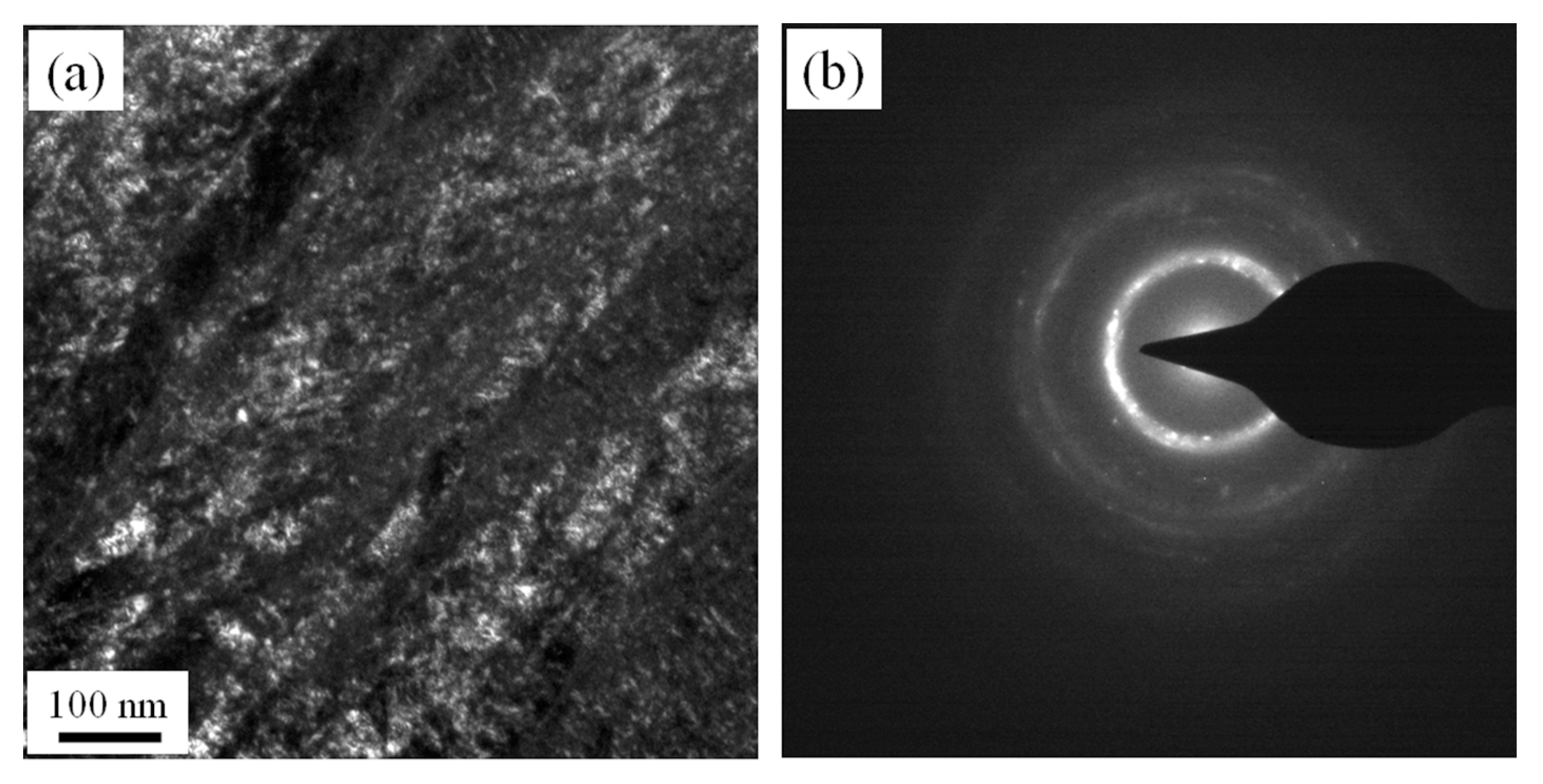
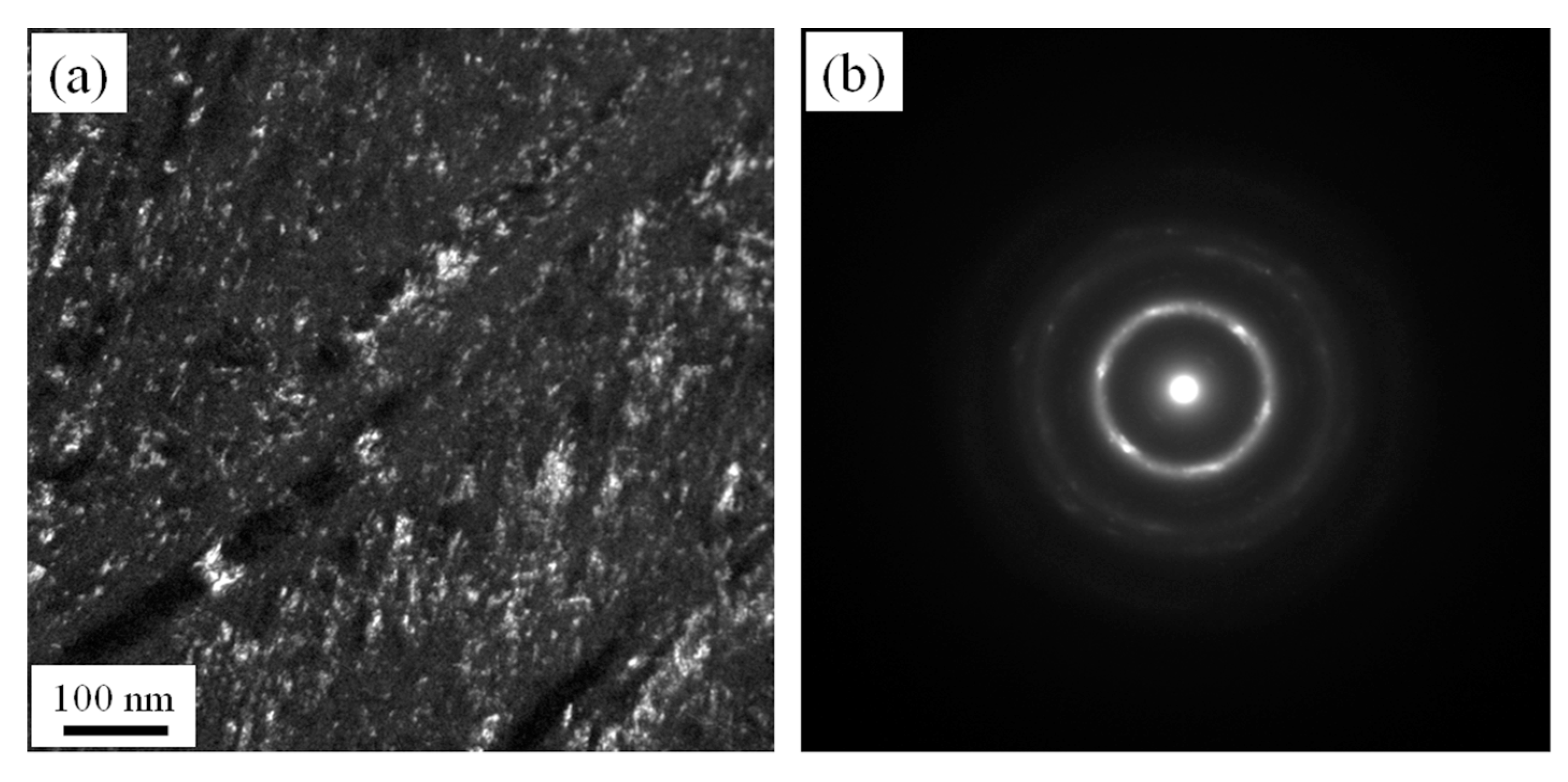
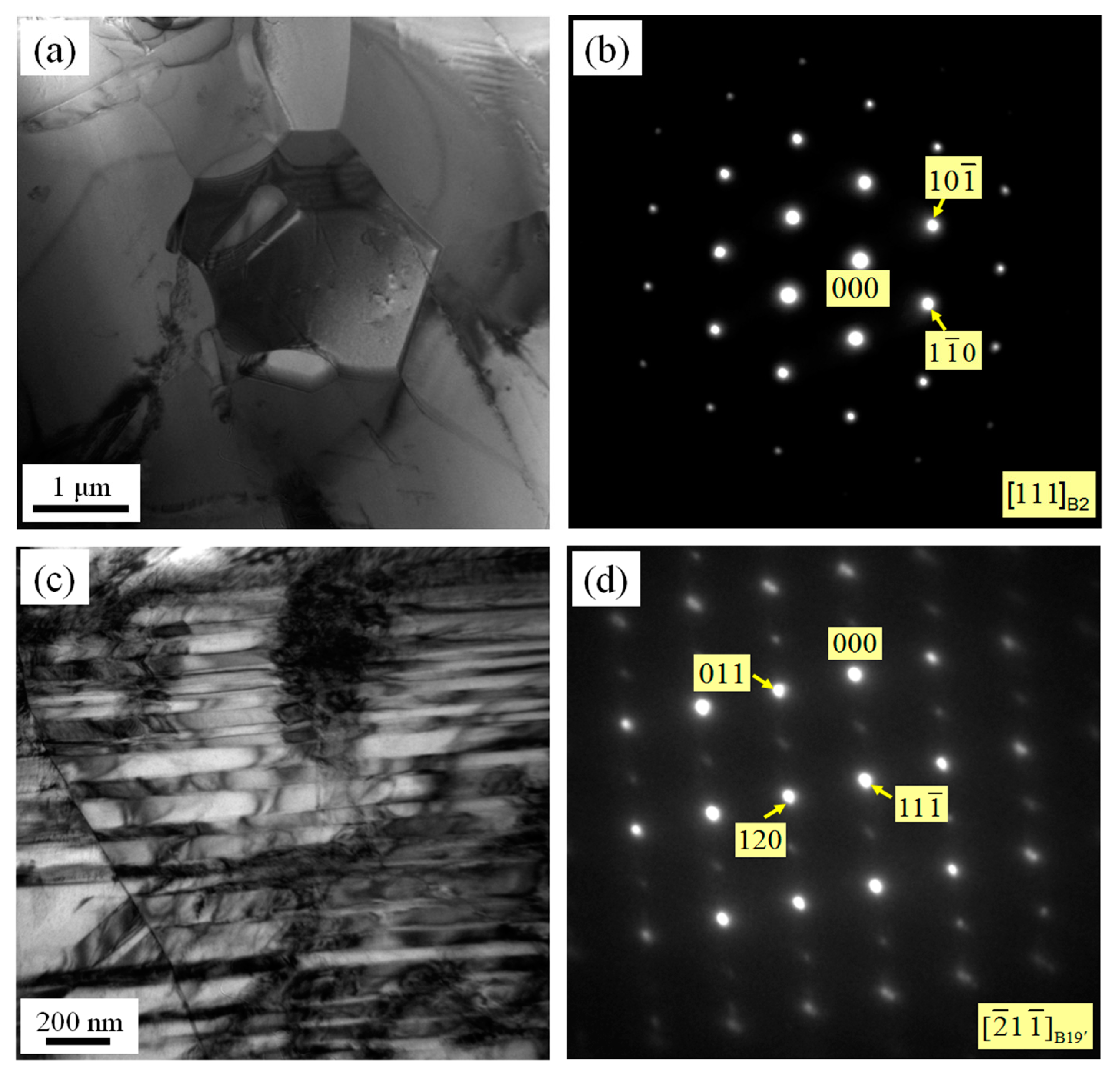
3.2. Microstructures of NiTi SMA Samples Subjected to SPD
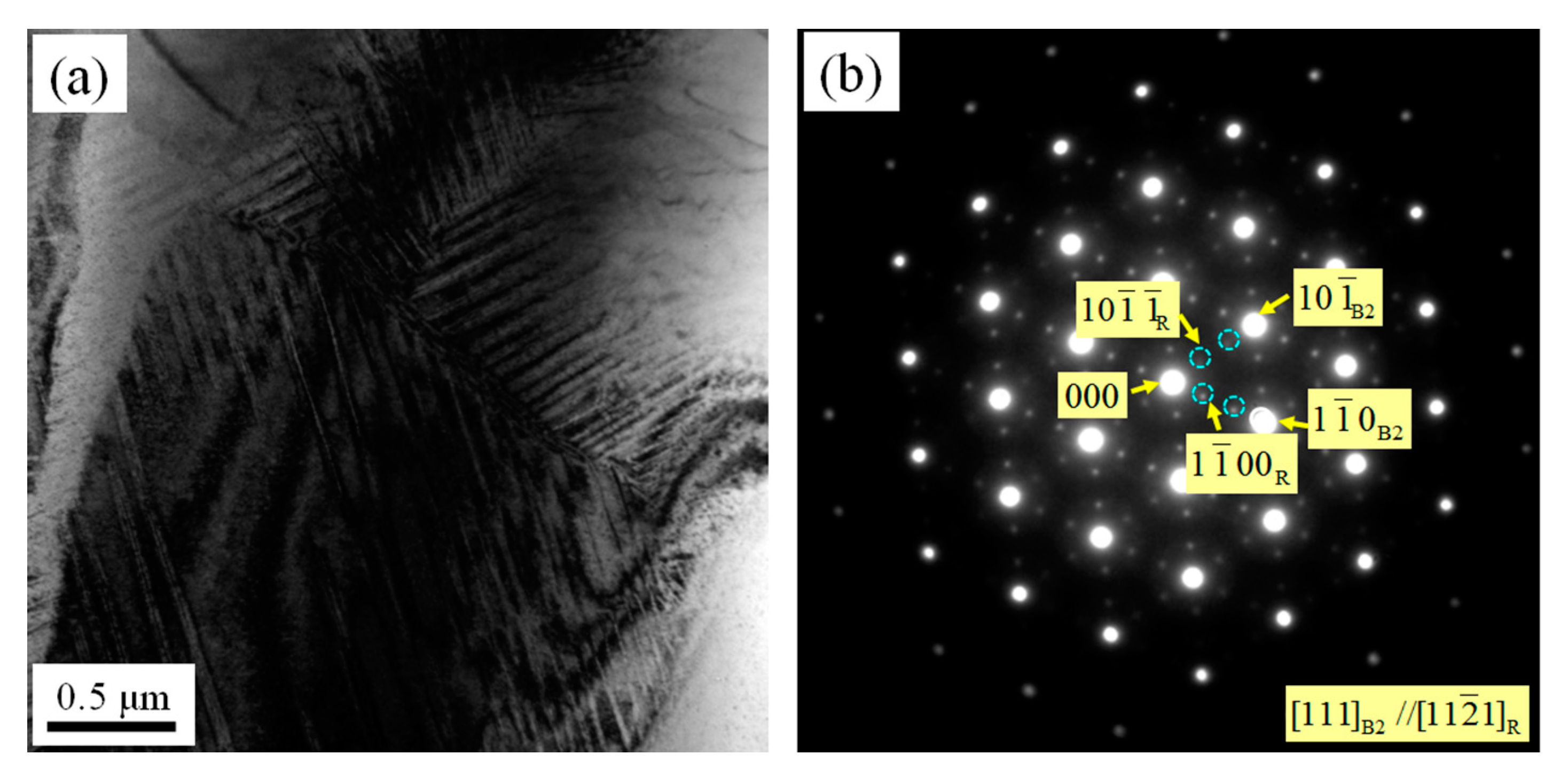
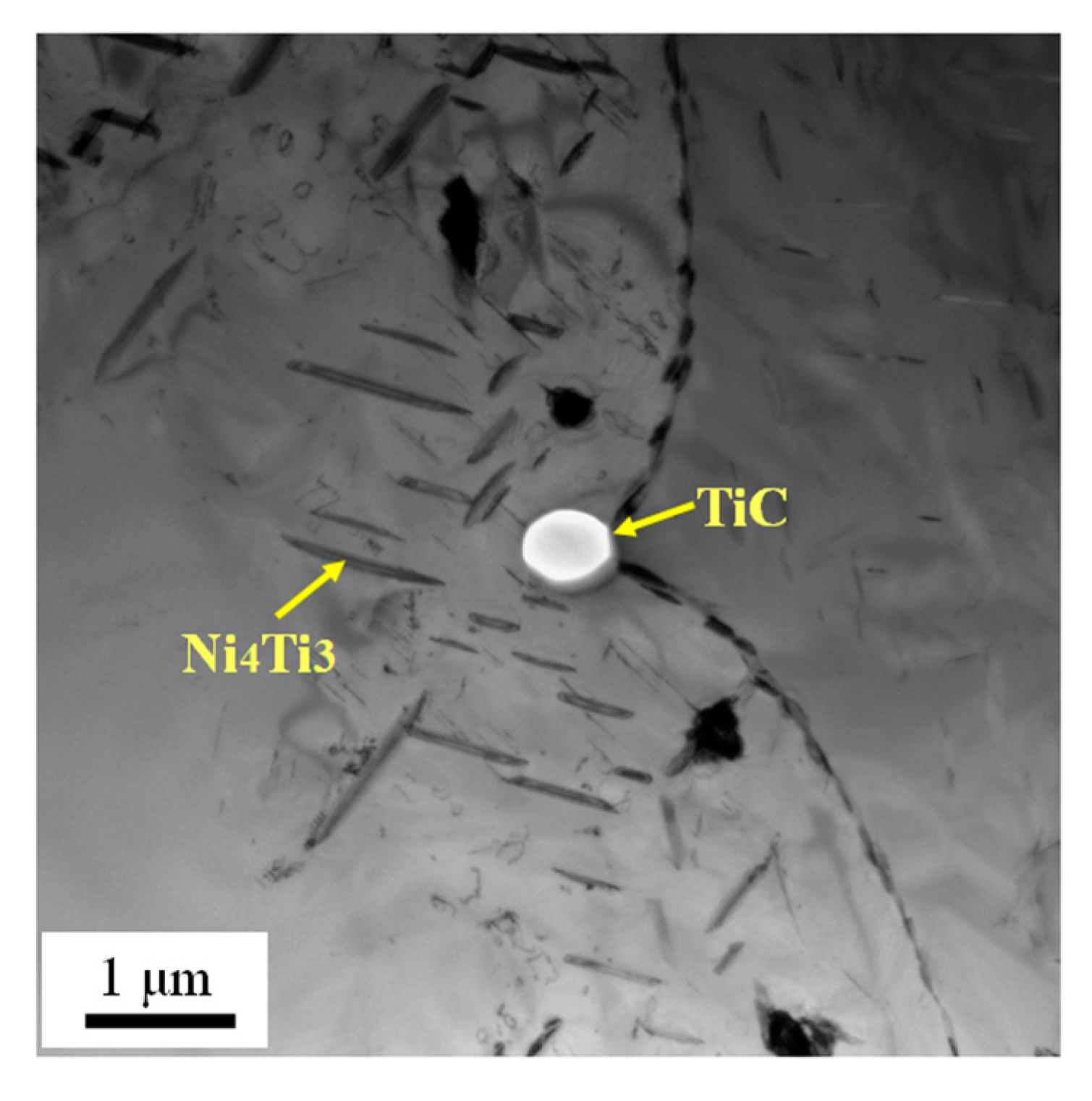
3.3. Microstructures of As-Rolled NiTi SMA Sample Subjected to Aging
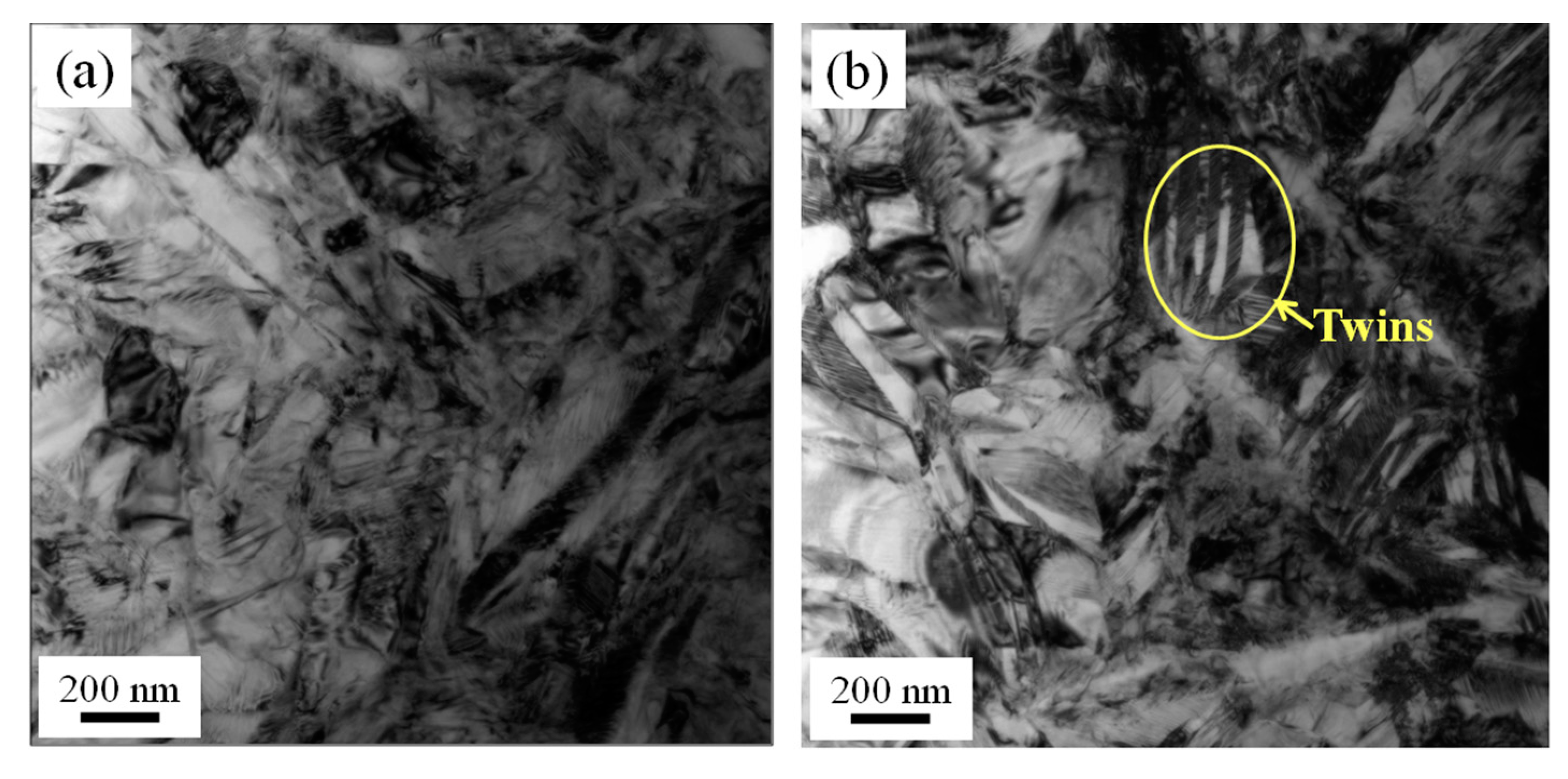
3.4. Microstructures of NiTi SMA Sample Subjected to Solution Treatment and Aging
3.5. Microstructures of As-Rolled NiTi SMA Sample Subjected to SPD and Aging
3.6. Microstructures of Solution-Treated NiTi SMA Sample Subjected to SPD and Aging
4. Discussion
5. Conclusions
- (1)
- In the case of as-rolled NiTi SMA sample subjected to aging for 2 h at 600 °C, R phase appears in the B2 austenite matrix. The as-rolled NiTi SMA sample contains plenty of dislocation networks aroused by previous processing history. The dislocation networks are responsible for the formation of the R phase.
- (2)
- Solution treatment for 2 h at 850 °C can result in the annihilation of the dislocations in the as-rolled NiTi SMA sample. As a consequence, the Ni4Ti3 precipitates more easily arise in a solution-treated NiTi SMA sample when aged for 2 h at 600 °C.
- (3)
- SPD is capable of inducing amorphization of a NiTi SMA sample at room temperature. Amorphous NiTi SMA is able to be subjected to crystallization when aged for 2 h at 600 °C. Consequently, SPD and subsequent aging contribute to enhancing the transformation temperature of martensite. In addition, SPD plays an important role in suppressing the occurrence of R phase and Ni4Ti3 precipitate.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Otsuka, K.; Ren, X. Physical metallurgy of Ti-Ni-based shape memory alloys. Prog. Mater. Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef]
- Delobelle, V.; Chagnon, G.; Favier, D.; Alonso, T. Study of electropulse heat treatment of cold worked NiTi wire: From uniform to localised tensile behaviour. J. Mater. Process. Technol. 2016, 227, 244–250. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, Y.; Zhang, Y.; Li, H.; Liang, Y. Effect of solution treatment and aging on microstructural evolution and mechanical behavior of NiTi shape memory alloy. Trans. Nonferr. Met. Soc. 2013, 23, 3658–3667. [Google Scholar] [CrossRef]
- Tadayyon, G.; Mazinani, M.; Guo, Y.; Zebarjad, S.M.; Tofail, S.A.; Biggs, M.J. The effect of annealing on the mechanical properties and microstructural evolution of Ti-rich NiTi shape memory alloy. Mater. Sci. Eng. A 2016, 662, 564–577. [Google Scholar] [CrossRef]
- Tadayyon, G.; Mazinani, M.; Guo, Y.; Zebarjad, S.M.; Tofail, S.A.; Biggs, M.J. Study of the microstructure evolution of heat treated Ti-rich NiTi shape memory alloy. Mater. Charact. 2016, 112, 11–19. [Google Scholar] [CrossRef]
- Kuang, C.-H.; Chien, C.; Wu, S.-K. Multistage martensitic transformation in high temperature aged Ti 48 Ni 52 shape memory alloy. Intermetallics 2015, 67, 12–18. [Google Scholar] [CrossRef]
- Wang, X.; Verlinden, B.; Van Humbeeck, J. Effect of post-deformation annealing on the R-phase transformation temperatures in NiTi shape memory alloys. Intermetallics 2015, 62, 43–49. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, S.; Jin, Y.; Meng, X. Nanoscale interwoven structure of B2 and R-phase in Ni-Ti alloy film. Vacuum 2016, 129, 45–48. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Zhao, Y.; Liu, S.; Li, H.; Zhao, C.-Z. Influence of Ni4Ti3 precipitates on phase transformation of NiTi shape memory alloy. Trans. Nonferr. Met. Soc. 2015, 25, 4063–4071. [Google Scholar] [CrossRef]
- Resnina, N.; Belyaev, S.; Zeldovich, V.; Pilyugin, V.; Frolova, N.; Glazova, D. Variations in martensitic transformation parameters due to grains evolution during post-deformation heating of Ti-50.2 at.% Ni alloy amorphized by HPT. Thermochim. Acta 2016, 627, 20–30. [Google Scholar] [CrossRef]
- Shahmir, H.; Nili-Ahmadabadi, M.; Huang, Y.; Jung, J.M.; Kim, H.S.; Langdon, T.G. Shape memory effect in nanocrystalline NiTi alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2015, 626, 203–206. [Google Scholar] [CrossRef]
- Yu, C.; Aoun, B.; Cui, L.; Liu, Y.; Yang, H.; Jiang, X.; Cai, S.; Jiang, D.; Liu, Z.; Brown, D.E. Synchrotron high energy X-ray diffraction study of microstructure evolution of severely cold drawn NiTi wire during annealing. Acta Mater. 2016, 115, 35–44. [Google Scholar] [CrossRef]
- Shi, X.; Guo, F.; Zhang, J.; Ding, H.; Cui, L. Grain size effect on stress hysteresis of nanocrystalline NiTi alloys. J. Alloys Compd. 2016, 688, 62–68. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Liu, M.; Ren, Y.; Chen, F.; Yao, G.; Mei, Q. Evolution of microstructure and property of NiTi alloy induced by cold rolling. J. Alloys Compd. 2015, 653, 156–161. [Google Scholar] [CrossRef]
- Ke, C.; Cao, S.; Zhang, X. Phase field modeling of Ni-concentration distribution behavior around Ni4Ti3 precipitates in NiTi alloys. Comp. Mater. Sci. 2015, 105, 55–65. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, L.; Zhang, Y.; Liang, Y. Nanocrystallization and amorphization of NiTi shape memory alloy under severe plastic deformation based on local canning compression. J. Non-Cryst. Solids 2013, 367, 23–29. [Google Scholar] [CrossRef]
- Jiang, S.; Ming, T.; Zhao, Y.; Li, H.; Zhang, Y.; Liang, Y. Crystallization of amorphous NiTi shape memory alloy fabricated by severe plastic deformation. Trans. Nonferr. Met. Soc. 2014, 24, 1758–1765. [Google Scholar] [CrossRef]
- Bataillard, L.; Bidaux, J.-E.; Gotthardt, R. Interaction between microstructure and multiple-step transformation in binary NiTi alloys using in-situ transmission electron microscopy observations. Philos. Mag. A 1998, 78, 327–344. [Google Scholar] [CrossRef]
- Allafi, J.K.; Ren, X.; Eggeler, G. The mechanism of multistage martensitic transformations in aged Ni-rich NiTi shape memory alloys. Acta Mater. 2002, 50, 793–803. [Google Scholar] [CrossRef]
- Dlouhý, A.; Bojda, O.; Somsen, C.; Eggeler, G. Conventional and in-situ transmission electron microscopy investigations into multistage martensitic transformations in Ni-rich NiTi shape memory alloys. Mater. Sci. Eng. A 2008, 481, 409–413. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Jiang, S.; Zhang, Y. Role of Severe Plastic Deformation in Suppressing Formation of R Phase and Ni4Ti3 Precipitate of NiTi Shape Memory Alloy. Metals 2017, 7, 145. https://doi.org/10.3390/met7040145
Hu L, Jiang S, Zhang Y. Role of Severe Plastic Deformation in Suppressing Formation of R Phase and Ni4Ti3 Precipitate of NiTi Shape Memory Alloy. Metals. 2017; 7(4):145. https://doi.org/10.3390/met7040145
Chicago/Turabian StyleHu, Li, Shuyong Jiang, and Yanqiu Zhang. 2017. "Role of Severe Plastic Deformation in Suppressing Formation of R Phase and Ni4Ti3 Precipitate of NiTi Shape Memory Alloy" Metals 7, no. 4: 145. https://doi.org/10.3390/met7040145



