New Low-Sn Zr Cladding Alloys with Excellent Autoclave Corrosion Resistance and High Strength
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
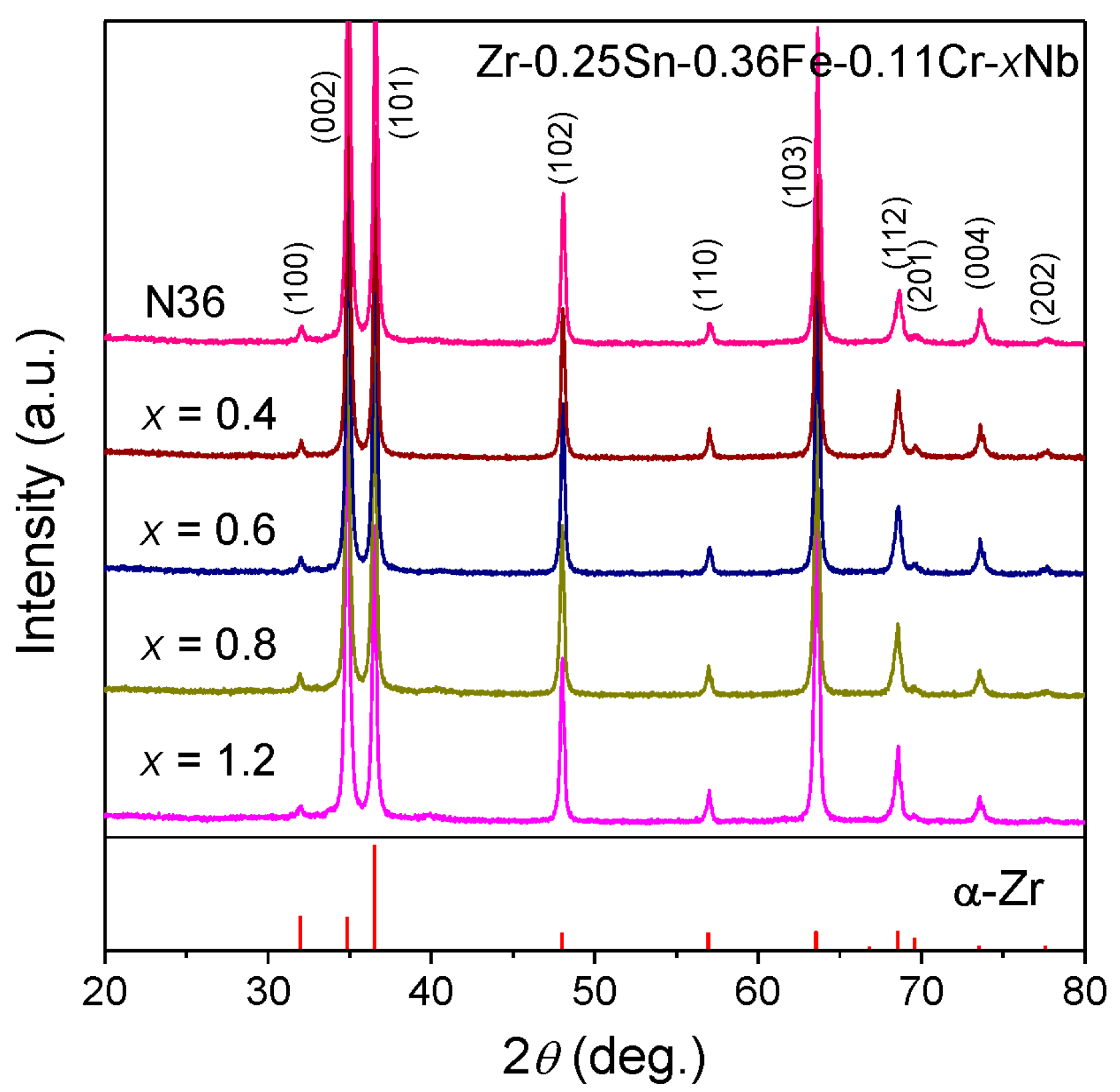
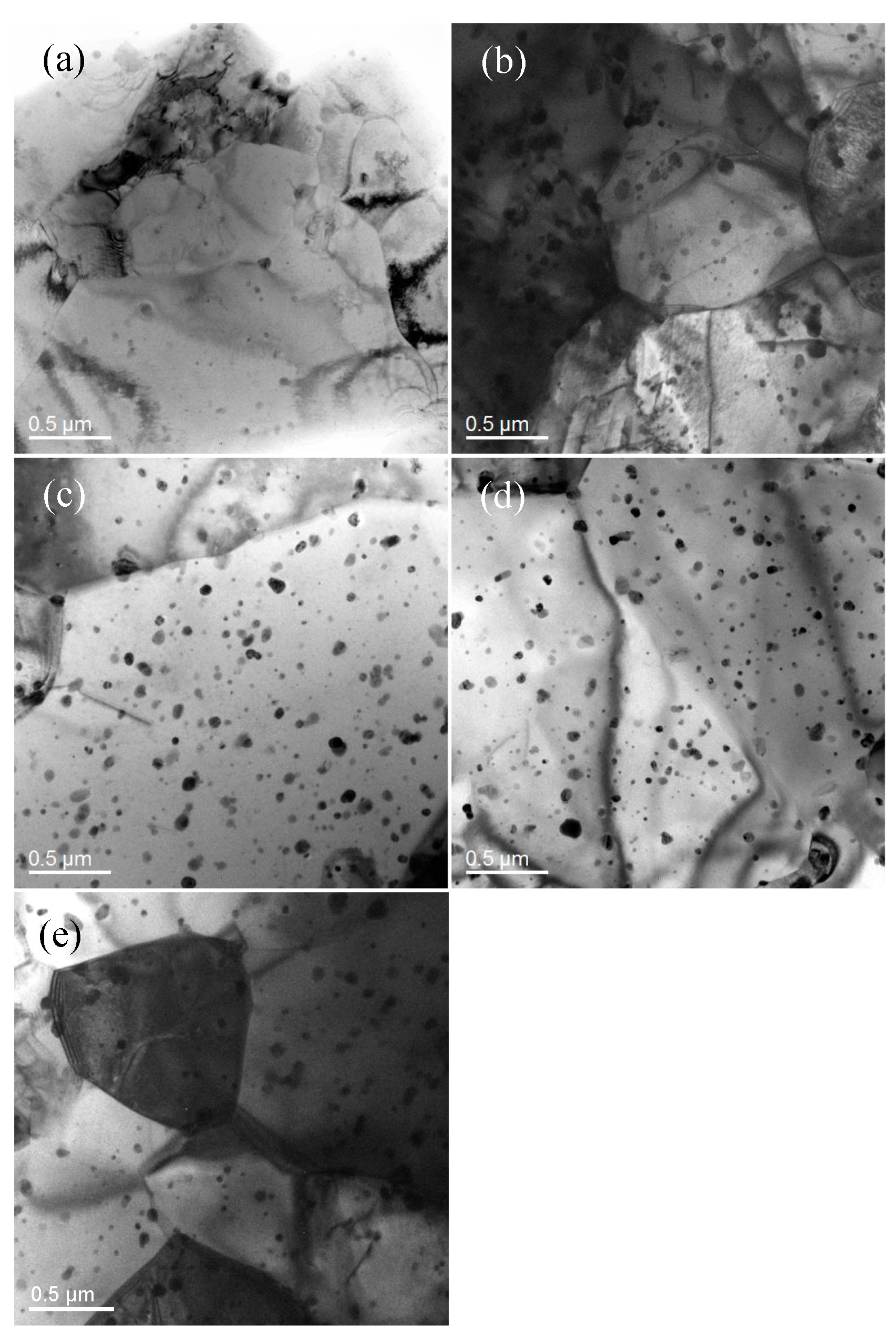
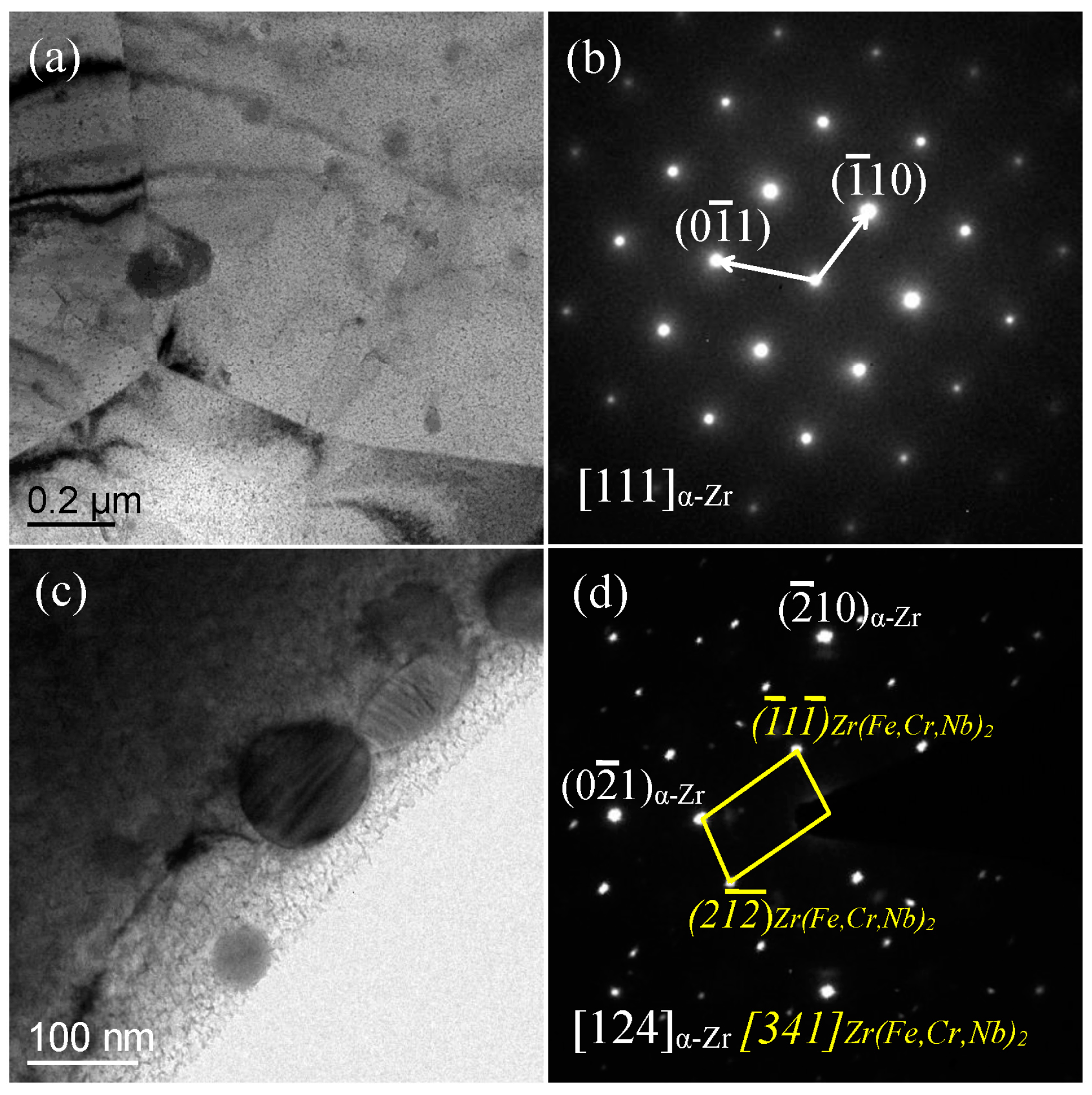
3.1. Microstructure Characterization
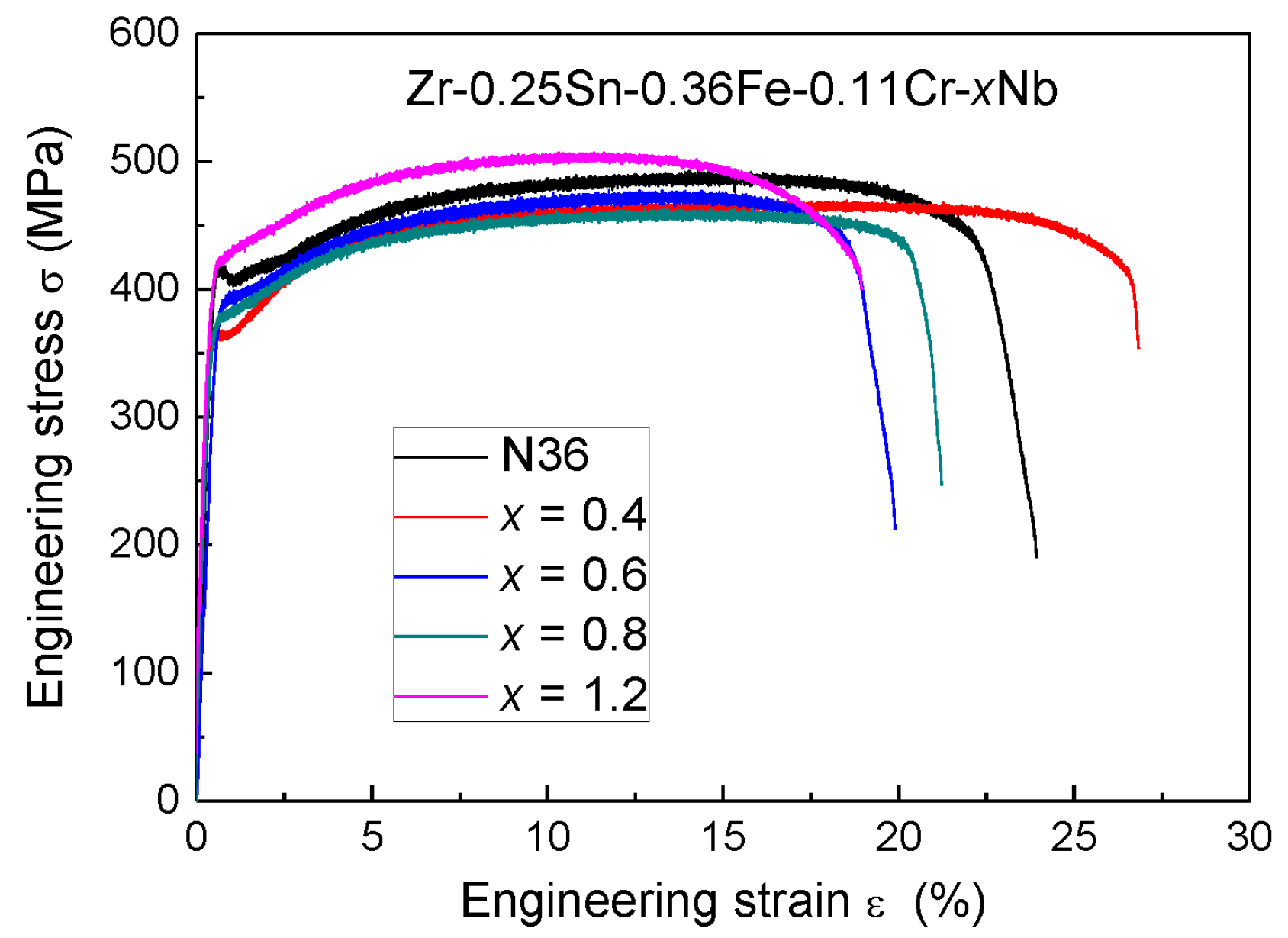
3.2. Mechanical Tensile Properties
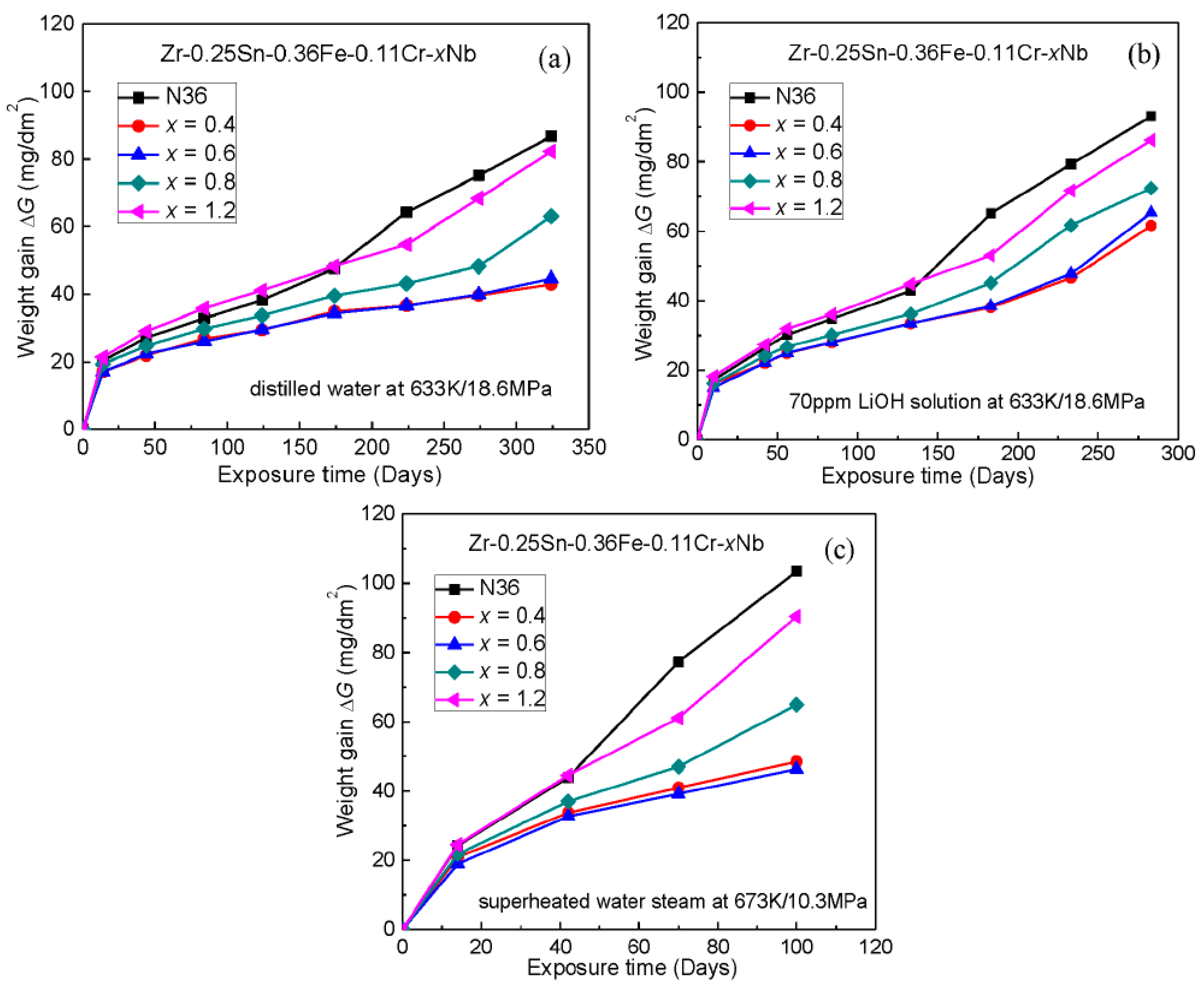
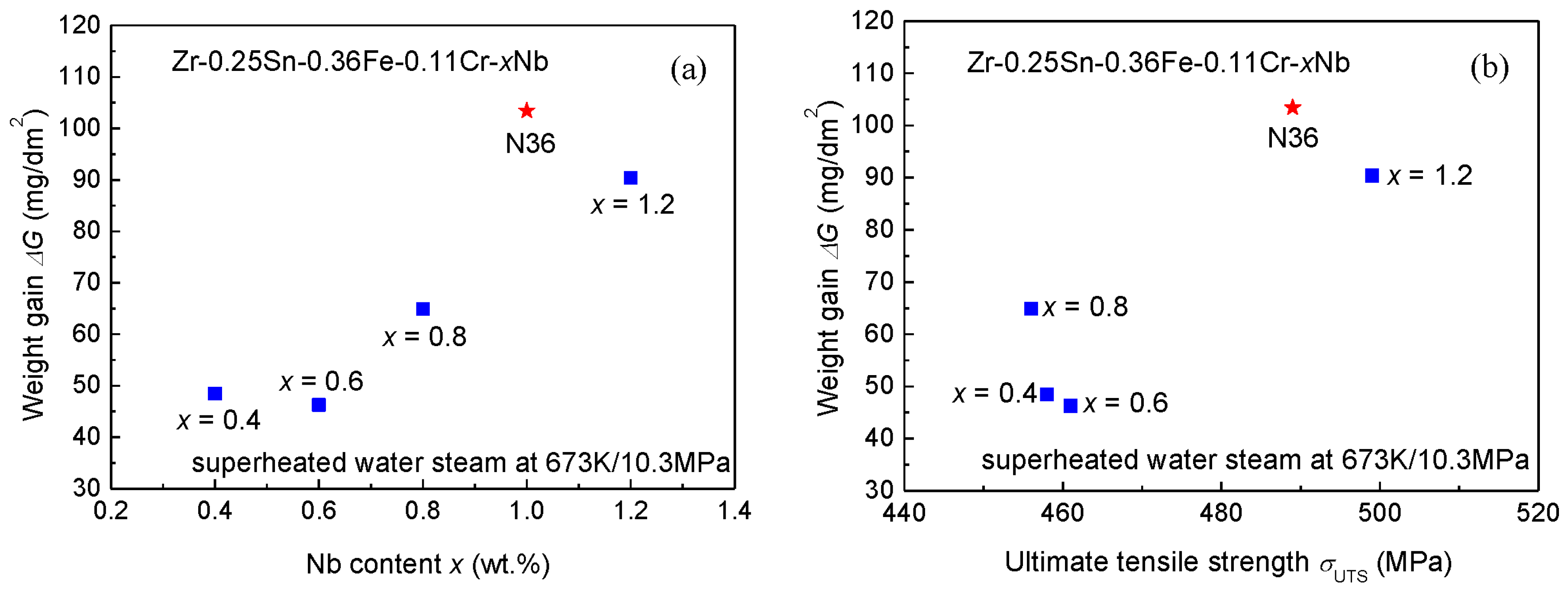
3.3. Autoclave Corrosion Resistances
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, J.-Y.; Yoo, S.J.; Choi, B.-K.; Jeong, Y.H. Corrosion and oxide characteristics of Zr-1.5Nb-0.4Sn-0.2Fe-0.1Cr alloys in 360 °C pure water and LiOH solution. J. Nucl. Mater. 2008, 373, 343–350. [Google Scholar] [CrossRef]
- Yao, M.Y.; Zhou, B.X.; Li, Q.; Liu, W.Q.; Geng, X.; Lu, Y.P. A superior corrosion behavior of Zircaloy-4 in lithiated water at 360 °C/18.6 MPa by β-quenching. J. Nucl. Mater. 2008, 374, 197–203. [Google Scholar] [CrossRef]
- Thorvaldsson, T.; Andersson, T.; Wilson, A.; Wardle, A. Correlation between 400 °C steam corrosion behavior, heat treatment, and microstructure of Zircaloy-4 tubing. In Zirconium in the Nuclear Industry: Eighth International Symposium; Van Swam, L.F.P., Eucken, C.M., Eds.; ASTM International: Baltimore, MD, USA, 1989; pp. 128–140. [Google Scholar]
- Kim, H.G.; Jeong, Y.H.; Kim, T.H. Effect of isothermal annealing on the corrosion behavior of Zr-xNb alloys. J. Nucl. Mater. 2004, 326, 125–131. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Jiang, H.; Peng, Q. Effect of heat treatment and Nb and H contents on the phase transformation of N18 and N36 zirconium alloys. J. Alloys Compd. 2008, 462, 103–108. [Google Scholar] [CrossRef]
- Sabol, G.P. ZIRLOTM—An Alloy Development Success. In Zirconium in the Nuclear Industry: 14th International Symposium; Ruding, P., Kammenzind, B., Eds.; ASTM International: Bridgeport, CT, USA, 2005; Volume 2, pp. 3–24. [Google Scholar]
- Bossis, P.; Pecheur, D.; Hanifi, K.; Thomazet, J.; Blat, M. Comparison of the high burn-up corrosion on M5 and low tin Zircaloy-4. In Zirconium in the Nuclear Industry: 14th International Symposium; Ruding, P., Kammenzind, B., Eds.; ASTM International: Bridgeport, CT, USA, 2005; Volume 3, pp. 494–525. [Google Scholar]
- Shishov, V.; Peregud, M.; Nikulina, A.; Kon’kov, V.; Novikov, V.; Markelov, V.; Khokhunova, T.; Kobylyansky, G.; Novoselov, A.; Ostrovsky, Z. Structure-phase state, corrosion and irradiation properties of Zr-Nb-Fe-Sn system alloys. In Zirconium in the Nuclear Industry: 15th International Symposium; Kammenzind, B., Limbäck, M., Eds.; ASTM International: Baltimore, MD, USA, 2009; Volume 5, pp. 724–743. [Google Scholar]
- Yamate, K.; Oe, A.; Hayashi, M.; Okamoto, T.; Anada, H.; Hagi, S. Burnup extension of Japanese PWR fuels. In Proceedings of the 1997 International Topical Meeting on LWR Fuel Performance Conference, Portland, OR, USA, 2–6 March 1997; pp. 318–325. [Google Scholar]
- Zhou, B.X.; Yao, M.Y.; Li, Z.K.; Wang, X.M.; Zhou, J.; Long, C.S.; Liu, Q.; Luan, B.F. Optimization of N18 zirconium alloy for fuel cladding of water reactors. J. Mater. Sci. Technol. 2012, 28, 606–613. [Google Scholar] [CrossRef]
- Wei, J.; Frankel, P.; Polatidis, E.; Blat, M.; Ambard, A.; Comstock, R.; Hallstadius, L.; Hudson, D.; Smith, G.; Grovenor, C. The effect of Sn on autoclave corrosion performance and corrosion mechanisms in Zr-Sn-Nb alloys. Acta Mater. 2013, 61, 4200–4214. [Google Scholar] [CrossRef]
- Chun, Y.B.; Hwang, S.K.; Kim, M.H.; Kwun, S.I.; Kim, Y.S. Effect of Mo on recrystallization characteristics of Zr-Nb-(Sn)-Mo experimental alloys. J. Nucl. Mater. 1999, 265, 28–37. [Google Scholar] [CrossRef]
- Shishov, V.N.; Peregud, M.M.; Nikulina, A.V.; Pimenov, Y.V.; Kobylyansky, G.P.; Novoselov, A.E.; Ostrovsky, Z.E.; Obukhov, A.V. Influence of Structure—Phase State of Nb Containing Zr Alloys on Irradiation-Induced Growth. In Zirconium in the Nuclear Industry: 14th International Symposium; Ruding, P., Kammenzind, B., Eds.; ASTM International: Bridgeport, CT, USA, 2005; Volume 2, pp. 1–18. [Google Scholar]
- Kim, Y.-S.; Kim, S.-K.; Bang, J.-G.; Jung, Y.-H. Effects of Sn and Nb on massive hydriding kinetics of Zr-XSn-YNb alloy. J. Nucl. Mater. 2000, 279, 335–343. [Google Scholar] [CrossRef]
- Wadekar, S.; Raman, V.; Banerjee, S.; Asundi, M. Structure-property correlation of Zr-base alloys. J. Nucl. Mater. 1988, 151, 162–171. [Google Scholar] [CrossRef]
- Pêcheur, D. Oxidation of β-Nb and Zr(Fe,V)2 precipitates in oxide films formed on advanced Zr-based alloys. J. Nucl. Mater. 2000, 278, 195–201. [Google Scholar] [CrossRef]
- Steinbrück, M.; Böttcher, M. Air oxidation of Zircaloy-4, M5® and ZIRLO™ cladding alloys at high temperatures. J. Nucl. Mater. 2011, 414, 276–285. [Google Scholar] [CrossRef]
- Shen, H.; Peng, S.; Xiang, X.; Naab, F.; Sun, K.; Zu, X. Proton irradiation effects on the precipitate in a Zr-1.6Sn-0.6Nb-0.2Fe-0.1Cr alloy. J. Nucl. Mater. 2014, 452, 335–342. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Zhou, B.; Yan, Q.; Yao, M. Effect of heat treatment on the microstructure and corrosion resistance of a Zr-Sn-Nb-Fe-Cr alloy. J. Nucl. Mater. 2005, 341, 97–102. [Google Scholar] [CrossRef]
- Hong, H.S.; Moon, J.S.; Kim, S.J.; Lee, K.S. Investigation on the oxidation characteristics of copper-added modified Zircaloy-4 alloys in pressurized water at 360 °C. J. Nucl. Mater. 2001, 297, 113–119. [Google Scholar] [CrossRef]
- Lelievre, G.; Tessier, C.; Iltis, X.; Berthier, B.; Lefebvre, F. Impact of intermetallic precipitates on hydrogen distribution in the oxide layers formed on zirconium alloys in a steam atmosphere: A 2D (3He, p)α nuclear analysis study in microbeam mode. J. Alloys Compd. 1998, 268, 308–317. [Google Scholar] [CrossRef]
- Toffolon-Masclet, C.; Brachet, J.-C.; Jago, G. Studies of second phase particles in different zirconium alloys using extractive carbon replica and an electrolytic anodic dissolution procedure. J. Nucl. Mater. 2002, 305, 224–231. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, H.G.; Kim, T.H. Effect of β phase, precipitate and Nb-concentration in matrix on corrosion and oxide characteristics of Zr-xNb alloys. J. Nucl. Mater. 2003, 317, 1–12. [Google Scholar] [CrossRef]
- Shishov, V.N. The evolution of microstructure and deformation stability in Zr-Nb-(Sn, Fe) alloys under neutron irradiation. In Zirconium in the Nuclear Industry: 16th International Symposium; Limbäck, M., Barbéris, P., Eds.; ASTM International: Baltimore, MD, USA, 2012; Volume 7, pp. 200–213. [Google Scholar]
- Waseda, Y.; Matsubara, E.; Shinoda, K. X-ray Diffraction Crystallography; Springer: Heidelberg, Germany, 2011; pp. 107–127. [Google Scholar]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Russell, R.B. Coefficients of thermal expansion for zirconium. J. Metals 1954, 6, 1045–1052. [Google Scholar]
- Curtis, R.E.; Dressier, G. Effect of thermomechanical processing and heat treatment on the properties of Zr-3Nb-lSn strip and tubing. In Zirconium in the Nuclear Applications; Schemel, J.H., Rosenbaum, H.S., Eds.; ASTM International: Harrisburg, PA, USA, 1974; Volume 551, pp. 104–128. [Google Scholar]

| Compositions | Lattice Constants | σYS (MPa) | σUTS (MPa) | δ (%) | ||
|---|---|---|---|---|---|---|
| (wt %) | (at %) | a (nm) | c (nm) | |||
| Zr-0.25Sn-0.36Fe-0.11Cr-0.40Nb (Z-0.4Nb) | Zr98.63Sn0.20Nb0.39Fe0.59Cr0.20 | 0.3228 ± 0.0001 | 0.5137 ± 0.0001 | 365 | 458 | 26.5 |
| Zr-0.25Sn-0.36Fe-0.11Cr-0.60Nb (Z-0.6Nb) | Zr98.44Sn0.20Nb0.59Fe0.59Cr0.20 | 0.3229 ± 0.0003 | 0.5137 ± 0.0001 | 380 | 461 | 18.6 |
| Zr-0.25Sn-0.36Fe-0.11Cr-0.80Nb (Z-0.8Nb) | Zr98.24Sn0.20Nb0.78Fe0.59Cr0.20 | 0.3229 ± 0.0002 | 0.5146 ± 0.0001 | 373 | 456 | 20.2 |
| Zr-0.25Sn-0.36Fe-0.11Cr-1.20Nb (Z-1.2Nb) | Zr97.85Sn0.20Nb1.17Fe0.59Cr0.20 | 0.3229 ± 0.0003 | 0.5146 ± 0.0001 | 425 | 499 | 18.4 |
| Zr-1.0Sn-0.25Fe-1.0Nb (N36 (Ref.)) | Zr97.85Sn0.78Nb0.98Fe0.39 | 0.3226 ± 0.0003 | 0.5137 ± 0.0001 | 422 | 489 | 22.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Jiang, B.; Pang, C.; Dai, X.; Sun, Y.; Liao, W.; Wang, Q.; Dong, C. New Low-Sn Zr Cladding Alloys with Excellent Autoclave Corrosion Resistance and High Strength. Metals 2017, 7, 144. https://doi.org/10.3390/met7040144
Zhang R, Jiang B, Pang C, Dai X, Sun Y, Liao W, Wang Q, Dong C. New Low-Sn Zr Cladding Alloys with Excellent Autoclave Corrosion Resistance and High Strength. Metals. 2017; 7(4):144. https://doi.org/10.3390/met7040144
Chicago/Turabian StyleZhang, Ruiqian, Beibei Jiang, Chang Pang, Xun Dai, Yongduo Sun, Wei Liao, Qing Wang, and Chuang Dong. 2017. "New Low-Sn Zr Cladding Alloys with Excellent Autoclave Corrosion Resistance and High Strength" Metals 7, no. 4: 144. https://doi.org/10.3390/met7040144





