Influence of Growth Velocity on the Separation of Primary Silicon in Solidified Al-Si Hypereutectic Alloy Driven by a Pulsed Electric Current
Abstract
:1. Introduction
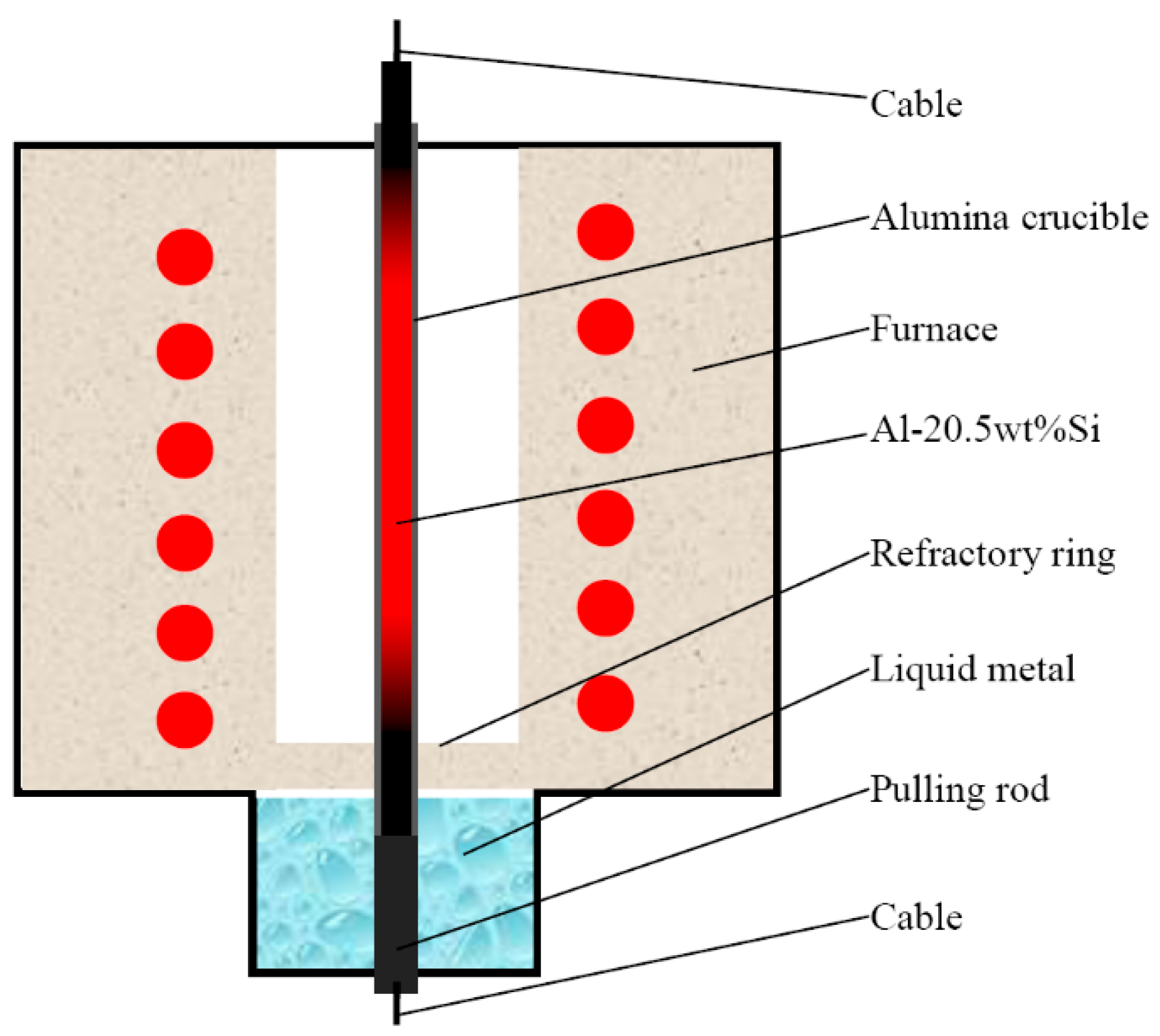
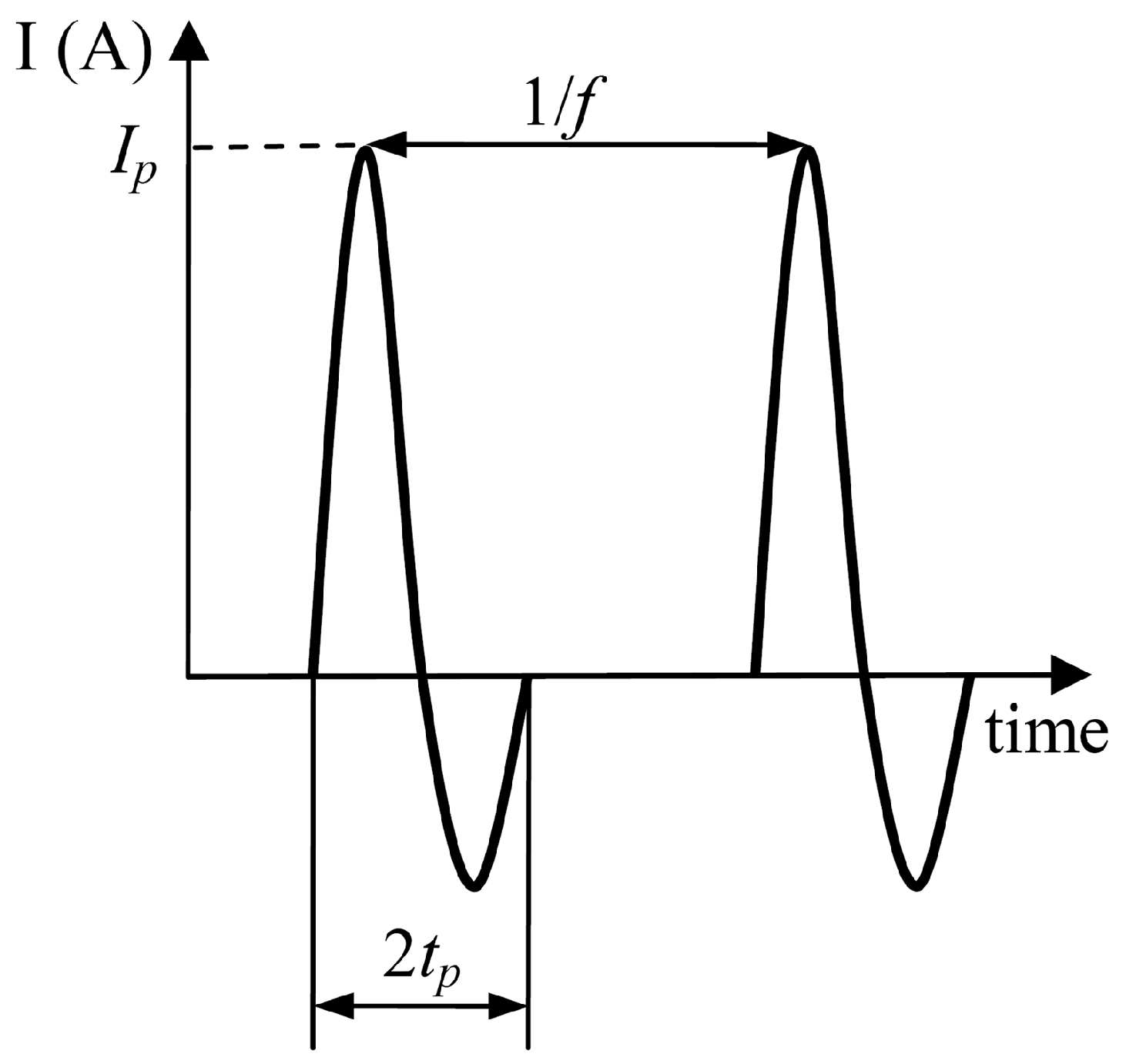
2. Materials and Methods
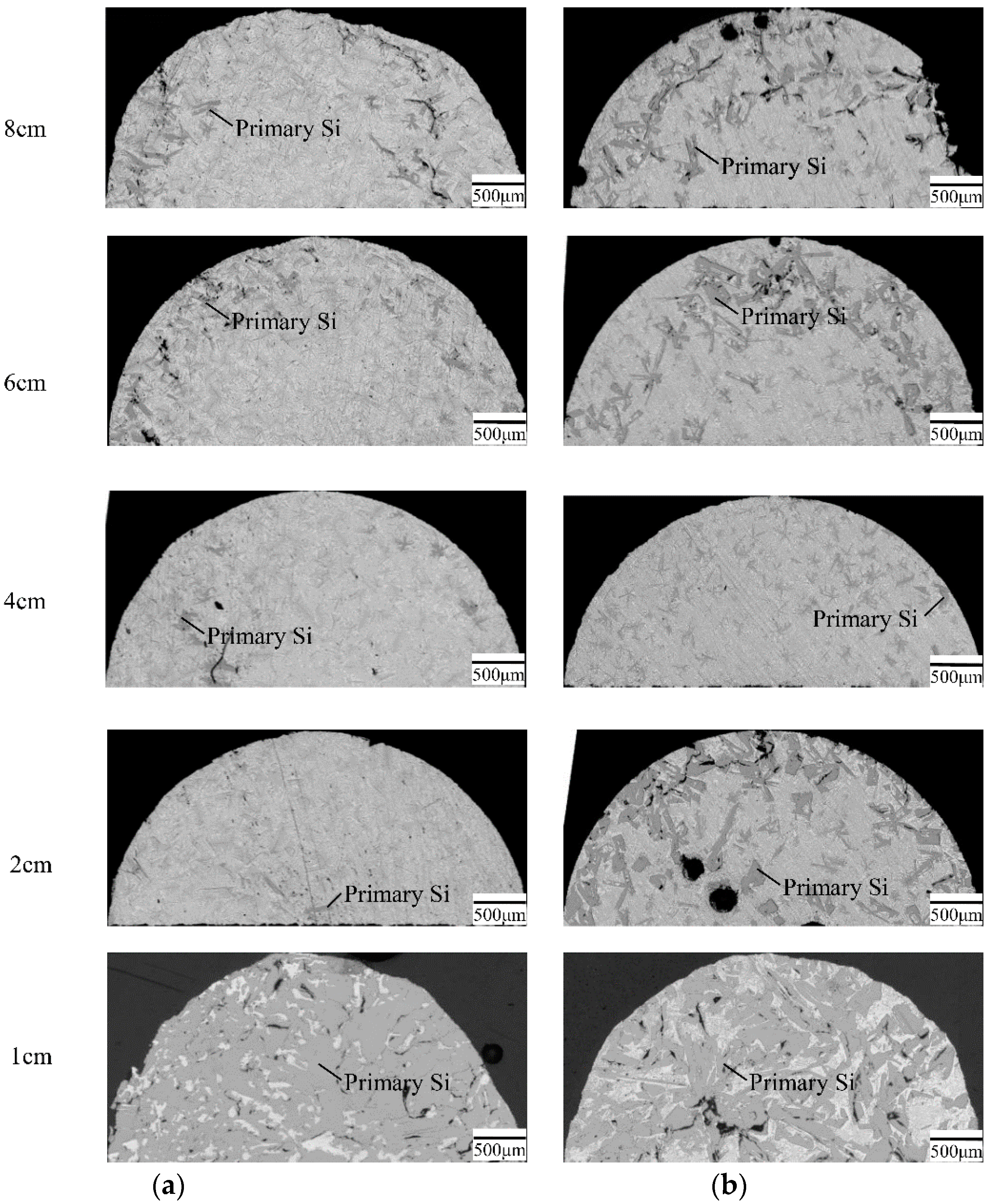
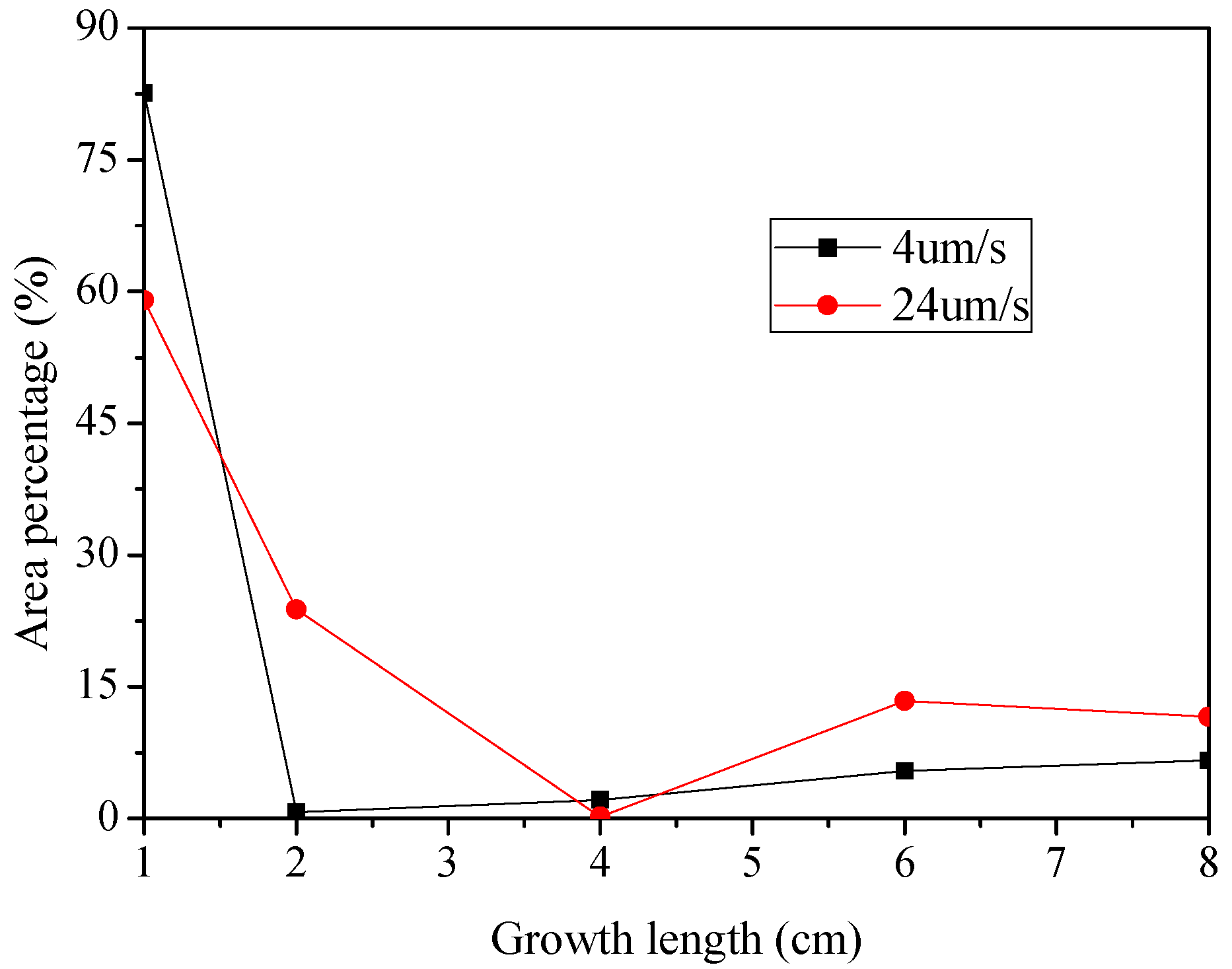
3. Results
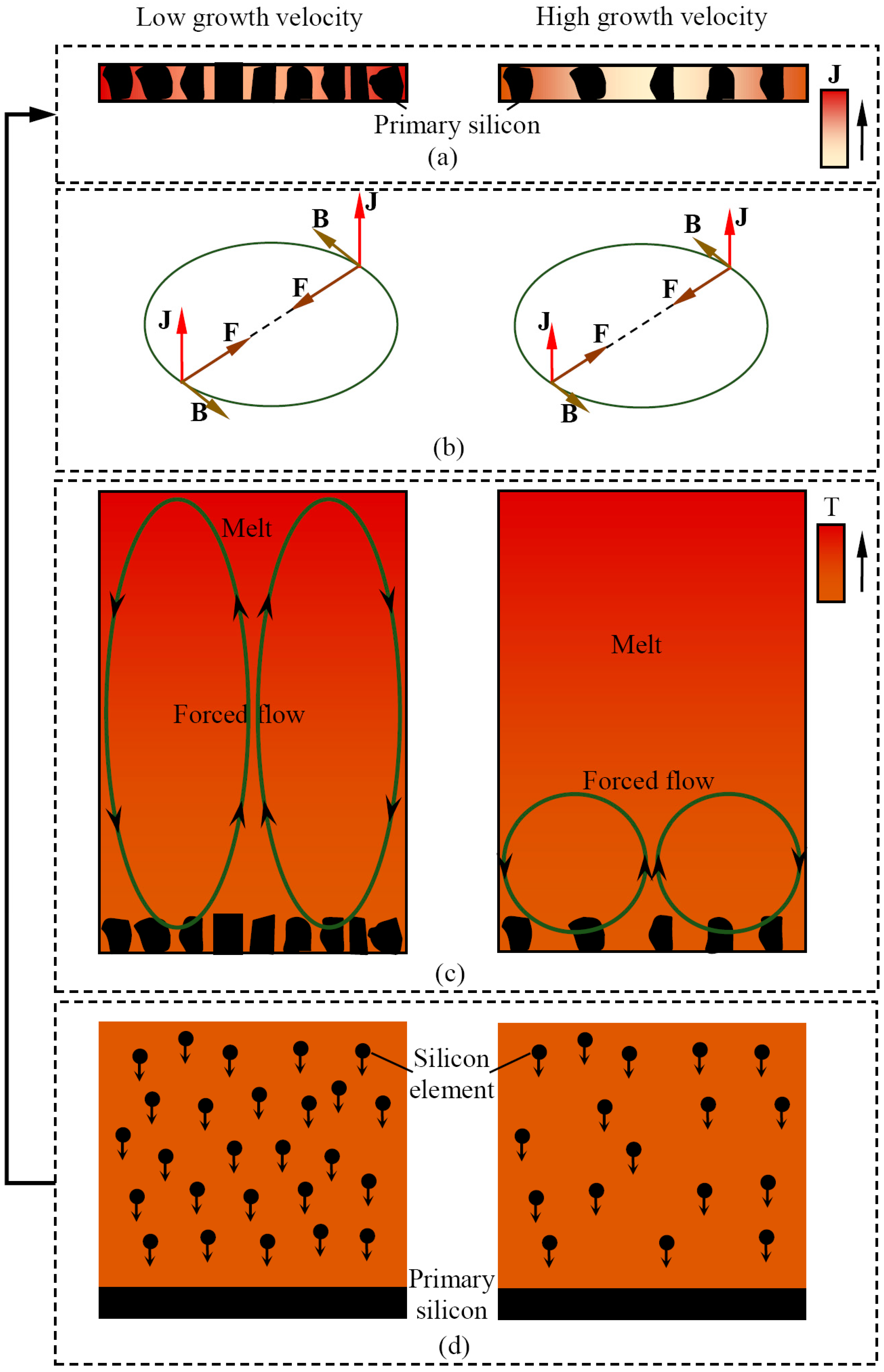
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ECP | Pulsed Electric Current |
| SG-Si | Solar Grade Silicon |
| MG-Si | Metallurgical Grade Silicon |
References
- Liu, K.; Wu, J.; Wei, K.; Ma, W.; Xie, K.; Li, S.; Yang, B.; Dai, Y. Application of molecular interaction volume model on removing impurity aluminum from metallurgical grade silicon by vacuum volatilization. Vacuum 2015, 114, 6–12. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Morita, K. Evaporation Removal of Boron from Metallurgical-Grade Silicon Using CaO-CaCl2-SiO2 Slag. Metall. Mater. Trans. B 2014, 45, 334–337. [Google Scholar] [CrossRef]
- Ding, Z.; Ma, W.; Wei, K.; Wu, J.; Zhou, Y.; Xie, K. Boron removal from metallurgical-grade silicon using lithium containing slag. J. Non-Cryst. Solids 2012, 358, 2708–2712. [Google Scholar] [CrossRef]
- Tan, Y.; Ren, S.; Shi, S.; Wen, S.; Jiang, D.; Dong, W.; Ji, M.; Sun, S. Removal of aluminum and calcium in multicrystalline silicon by vacuum induction melting and directional solidification. Vacuum 2014, 99, 272–276. [Google Scholar] [CrossRef]
- Liu, T.; Dong, Z.; Zhao, Y.; Wang, J.; Chen, T.; Xie, H.; Li, J.; Ni, H.; Huo, D. Purification of metallurgical silicon through directional solidification in a large cold crucible. J. Cryst. Growth 2012, 355, 145–150. [Google Scholar] [CrossRef]
- Alemany, C.; Trassy, C.; Pateyron, B.; Li, K.I.; Delannoy, Y. Refining of metallurgical-grade silicon by inductive plasma. Sol. Energy Mater. Sol. Cells 2002, 72, 41–48. [Google Scholar] [CrossRef]
- Tan, Y.; Guo, X.; Shi, S.; Dong, W.; Jiang, D. Study on the removal process of phosphorus from silicon by electron beam melting. Vacuum 2013, 93, 65–70. [Google Scholar] [CrossRef]
- Pires, J.C.S.; Otubo, J.; Braga, A.F.B.; Mei, P.R. The purification of metallurgical grade silicon by electron beam melting. J. Mater. Process. Technol. 2005, 169, 16–20. [Google Scholar] [CrossRef]
- Wang, P.; Lu, H.; Lai, Y. Control of silicon solidification and the impurities from an Al-Si melt. J. Cryst. Growth 2014, 390, 96–100. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z. Thermodynamic evaluation of segregation behaviors of metallic impurities in metallurgical grade silicon during Al-Si solvent refining process. J. Cryst. Growth 2014, 394, 18–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Zhong, H.; Xu, Z.; Li, L.; Gong, Y.; Miao, X.; Song, C.; Zhai, Q. Comparative Study on the Grain Refinement of Al-Si Alloy Solidified under the Impact of Pulsed Electric Current and Travelling Magnetic Field. Metals 2016, 6, 170. [Google Scholar] [CrossRef]
- Zhang, Y.; Räbiger, D.; Eckert, S. Solidification of pure aluminium affected by a pulsed electrical field and electromagnetic stirring. J. Mater. Sci. 2016, 51, 2153–2159. [Google Scholar] [CrossRef]
- Jie, J.; Zou, Q.; Sun, J.; Lu, Y.; Wang, T.; Li, T. Separation mechanism of the primary Si phase from the hypereutectic Al-Si alloy using a rotating magnetic field during solidification. Acta Mater. 2014, 72, 57–66. [Google Scholar] [CrossRef]
- Zou, Q.; Jie, J.; Sun, J.; Wang, T.; Cao, Z.; Li, T. Effect of Si content on separation and purification of the primary Si phase from hypereutectic Al-Si alloy using rotating magnetic field. Sep. Purif. Technol. 2015, 142, 101–107. [Google Scholar] [CrossRef]
- Li, J.; Ni, P.; Wang, L.; Tan, Y. Influence of direct electric current on solidification process of Al-Si alloy. Mater. Sci. Semicond. Process. 2017, 61, 79–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, X.; Shen, Z.; Han, Q.; Song, C.; Zhai, Q. Macro segregation formation mechanism of the primary silicon phase in directionally solidified Al-Si hypereutectic alloys under the impact of electric currents. Acta Mater. 2015, 97, 357–366. [Google Scholar] [CrossRef]
- Zhang, L.C.; Das, J.; Lu, H.B.; Duhamel, C.; Calin, M.; Eckert, J. High strength Ti-Fe-Sn ultrafine composites with large plasticity. Scr. Mater. 2007, 57, 101–104. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Liu, Y.; Cao, G.; Zhang, L.C. Phase transition, microstructural evolution and mechanical properties of Ti-Nb-Fe alloys induced by Fe addition. Mater. Des. 2016, 97, 279–286. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ye, C.; Xu, Y.; Zhong, H.; Chen, X.; Miao, X.; Song, C.; Zhai, Q. Influence of Growth Velocity on the Separation of Primary Silicon in Solidified Al-Si Hypereutectic Alloy Driven by a Pulsed Electric Current. Metals 2017, 7, 184. https://doi.org/10.3390/met7060184
Zhang Y, Ye C, Xu Y, Zhong H, Chen X, Miao X, Song C, Zhai Q. Influence of Growth Velocity on the Separation of Primary Silicon in Solidified Al-Si Hypereutectic Alloy Driven by a Pulsed Electric Current. Metals. 2017; 7(6):184. https://doi.org/10.3390/met7060184
Chicago/Turabian StyleZhang, Yunhu, Chunyang Ye, Yanyi Xu, Honggang Zhong, Xiangru Chen, Xincheng Miao, Changjiang Song, and Qijie Zhai. 2017. "Influence of Growth Velocity on the Separation of Primary Silicon in Solidified Al-Si Hypereutectic Alloy Driven by a Pulsed Electric Current" Metals 7, no. 6: 184. https://doi.org/10.3390/met7060184





