Preparation and Characterization of Aminated Hydroxyethyl Cellulose-Induced Biomimetic Hydroxyapatite Coatings on the AZ31 Magnesium Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AHEC
2.3. Sample Preparation
2.4. Preparation of AHEC Films on AZ31 Surfaces
2.5. Preparation of HA Coating on AHEC/AZ31
2.6. Surface Characterization
2.7. Corrosion Evaluation
2.7.1. Electrochemical Measurements
2.7.2. Immersion Test
2.8. Cell Viability Assay and Cell Morphology Examination
3. Results and Discussion
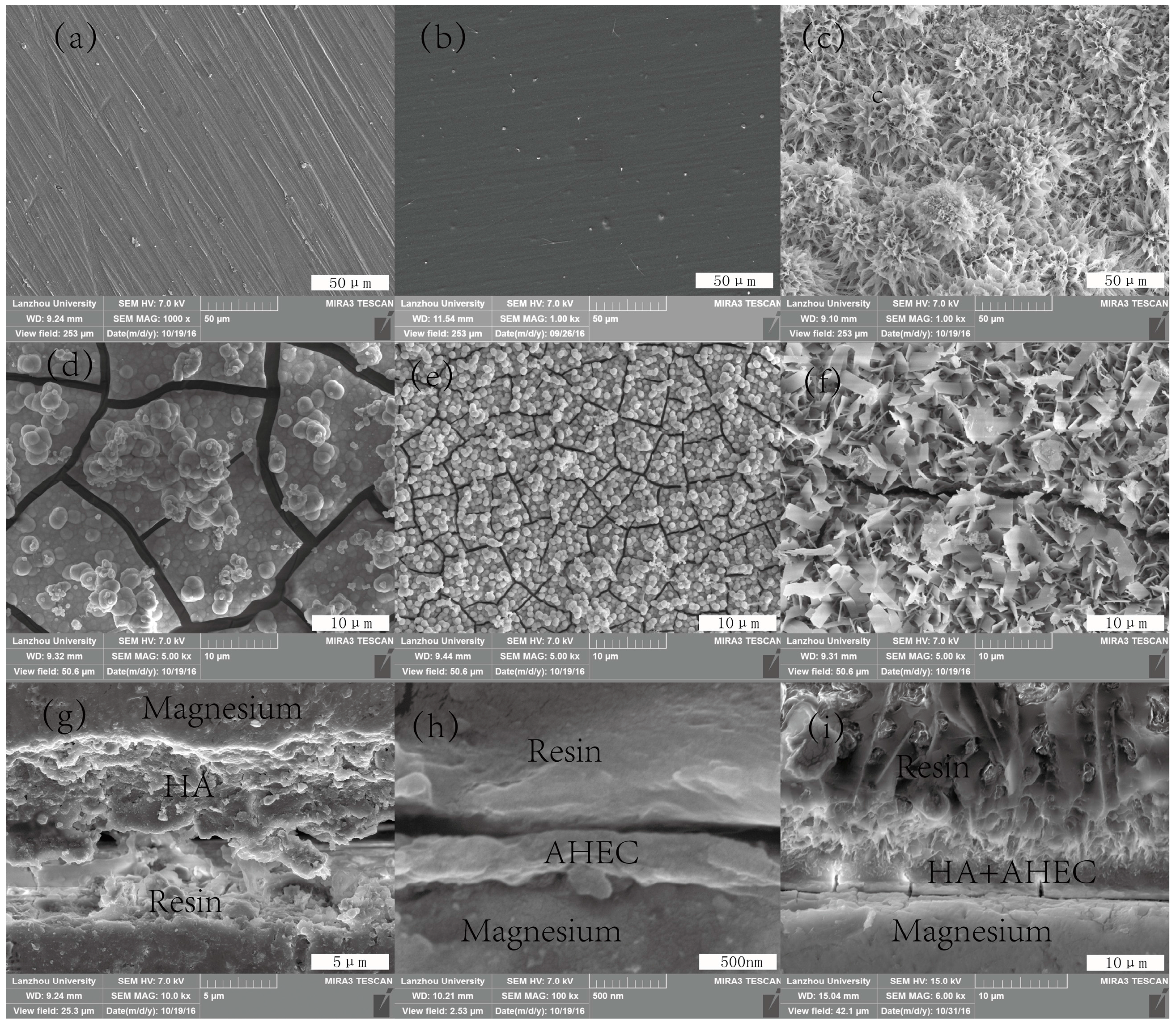
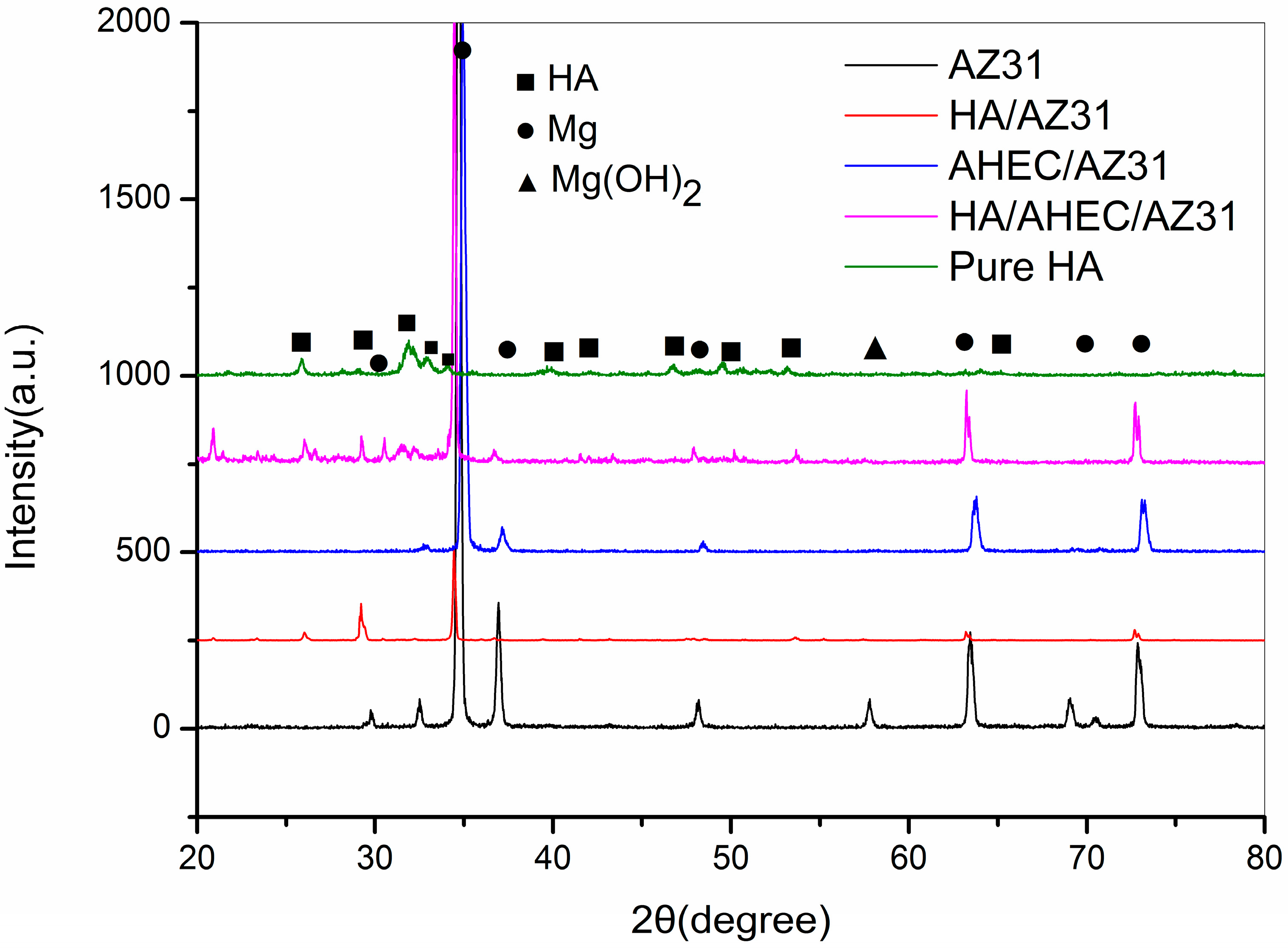
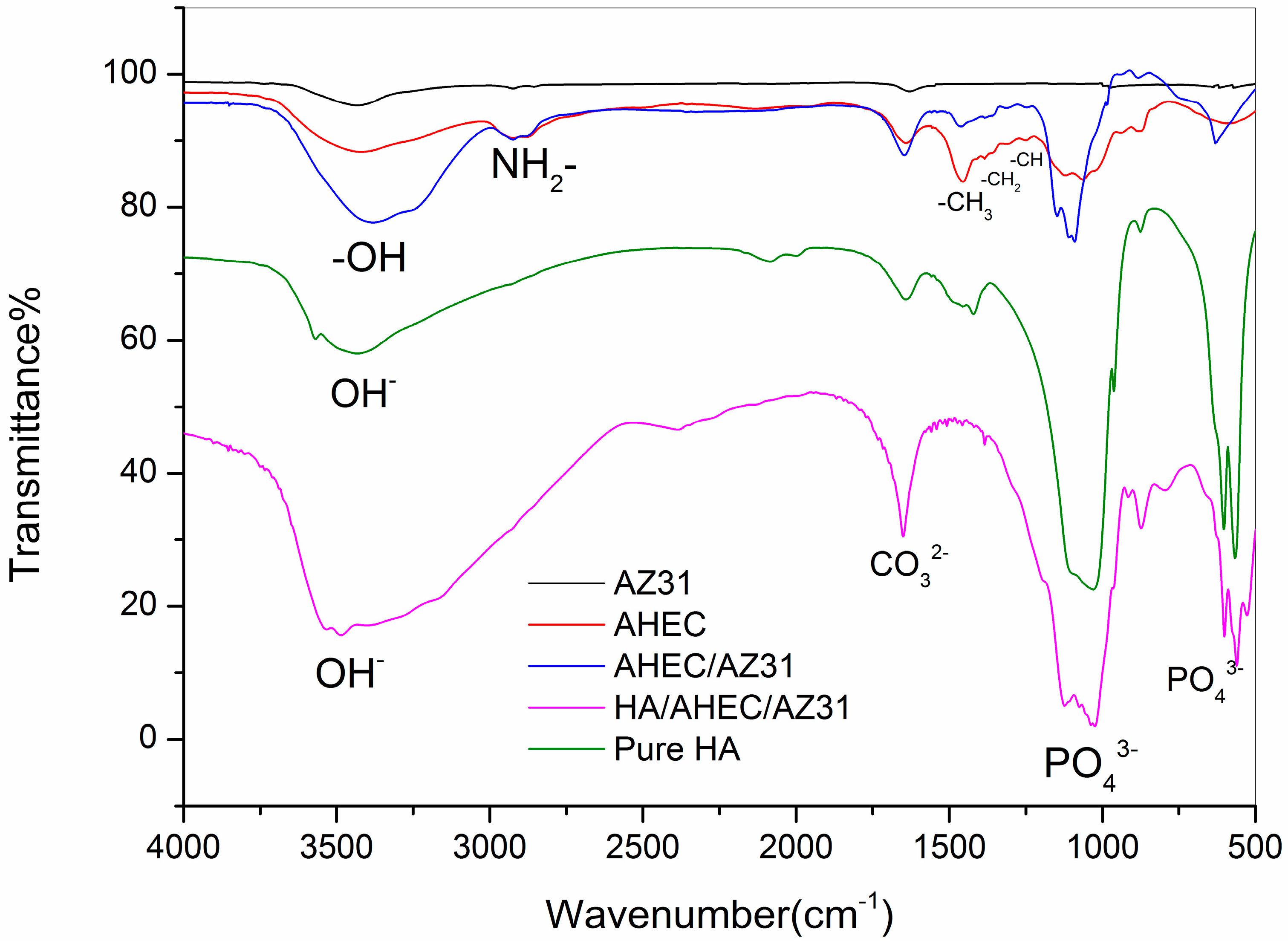
3.1. Characterization of the Samples Surfaces
3.2. Corrosion Resistance Analysis
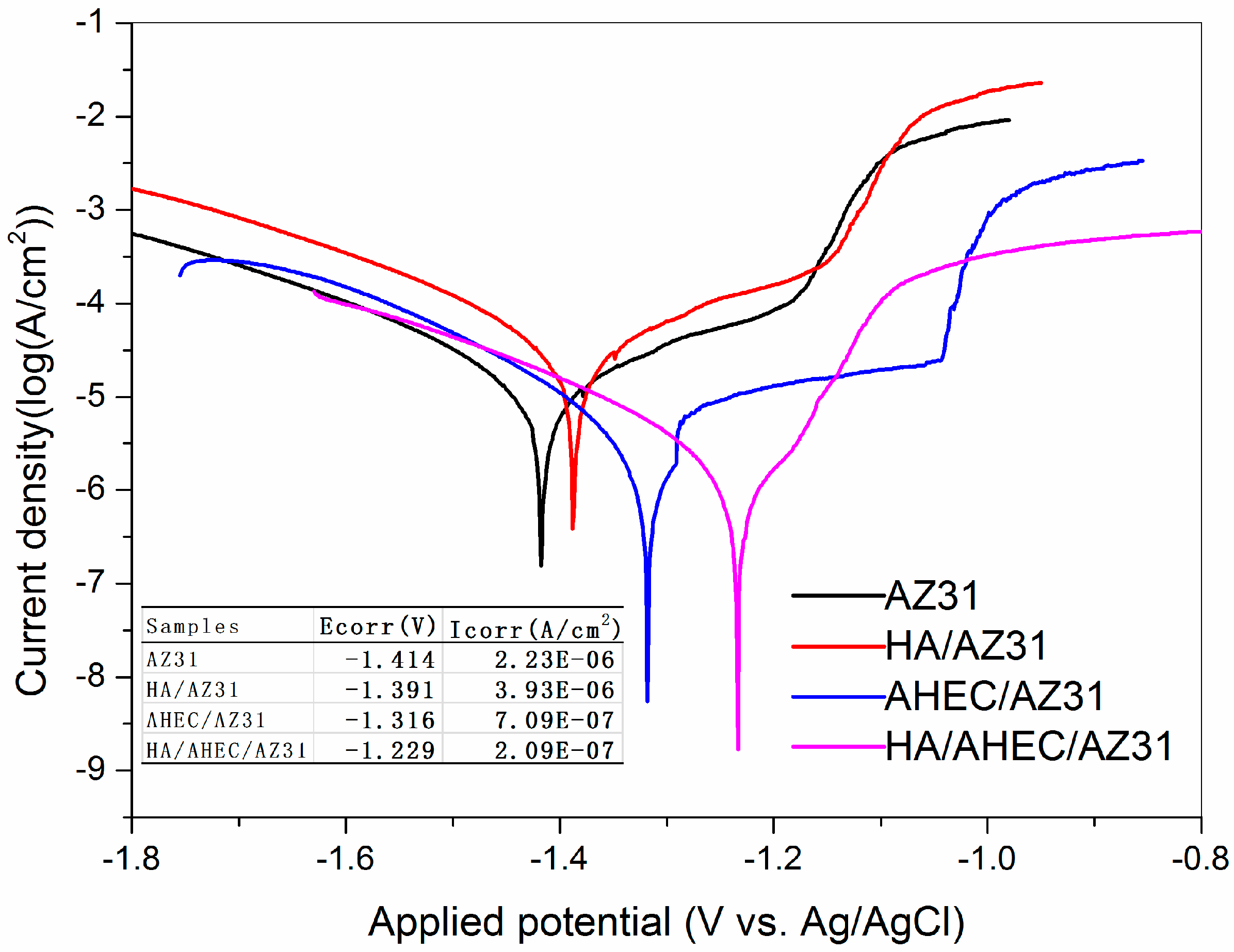
3.2.1. Polarization Studies
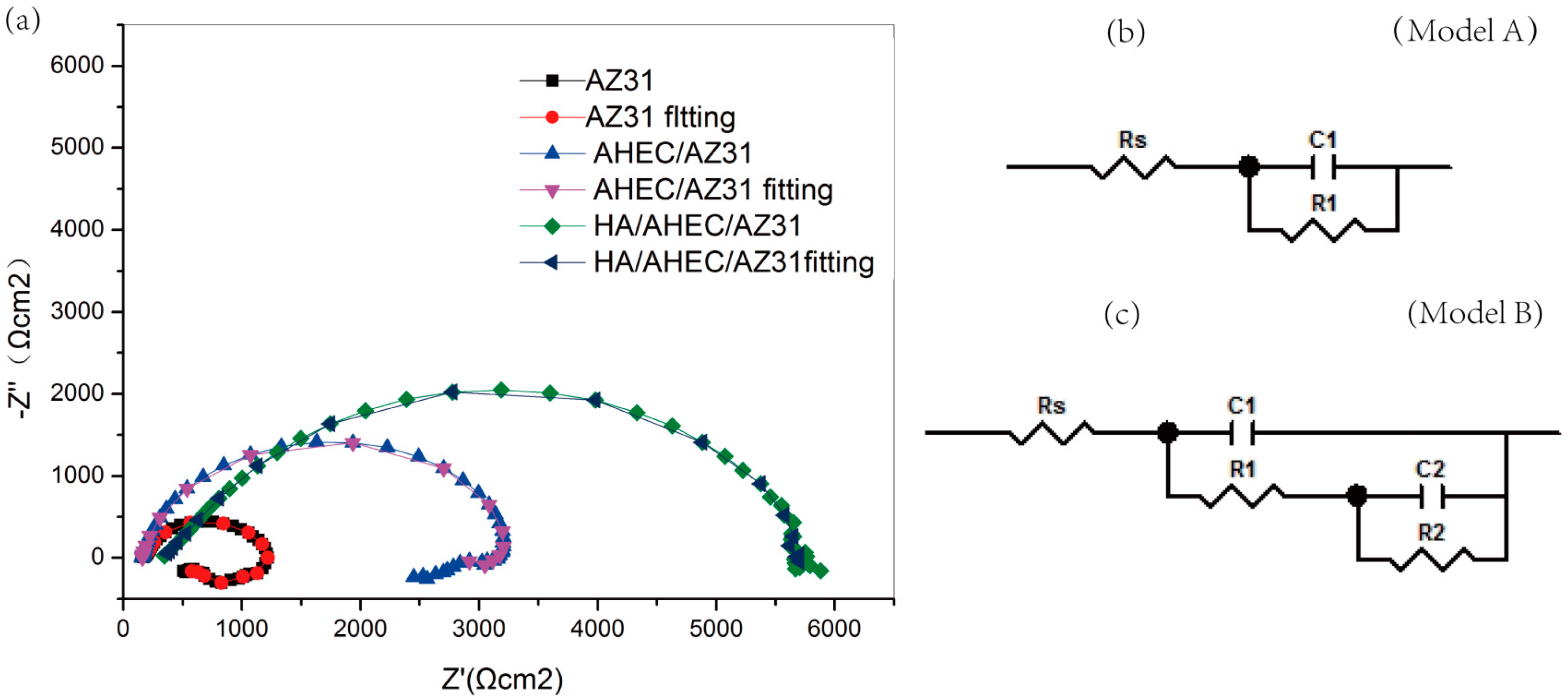
3.2.2. EIS Test
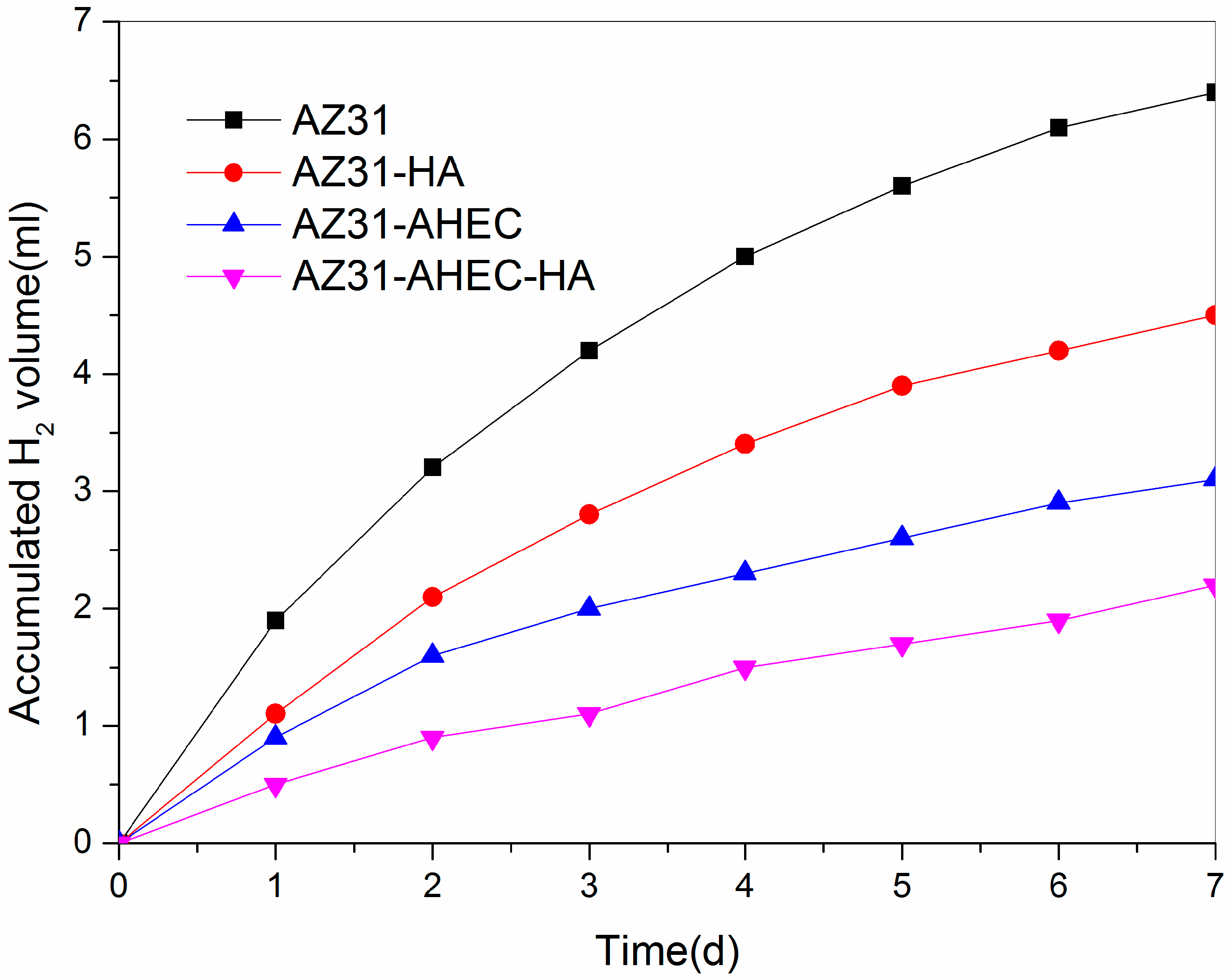
3.2.3. Immersion Test Studies
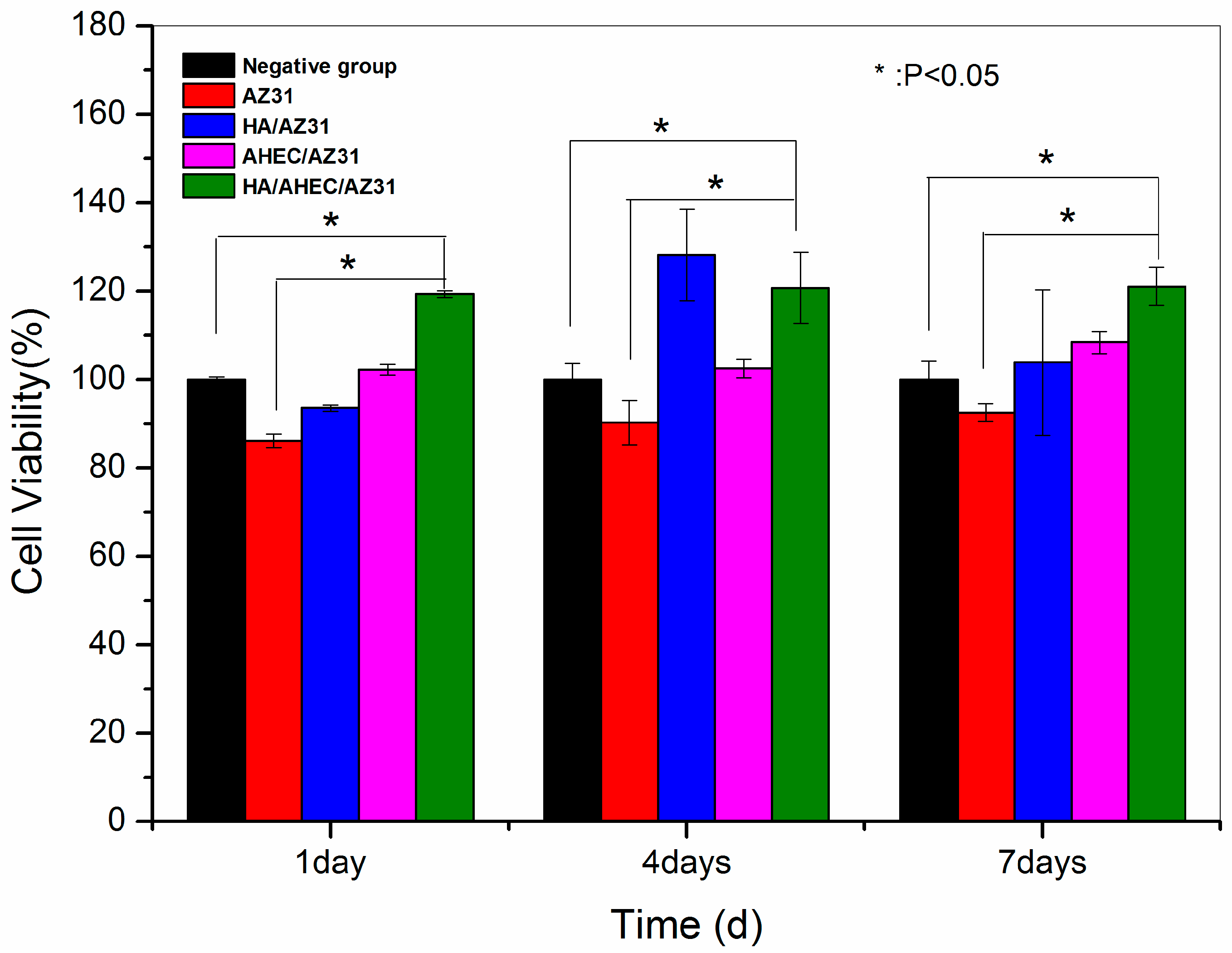
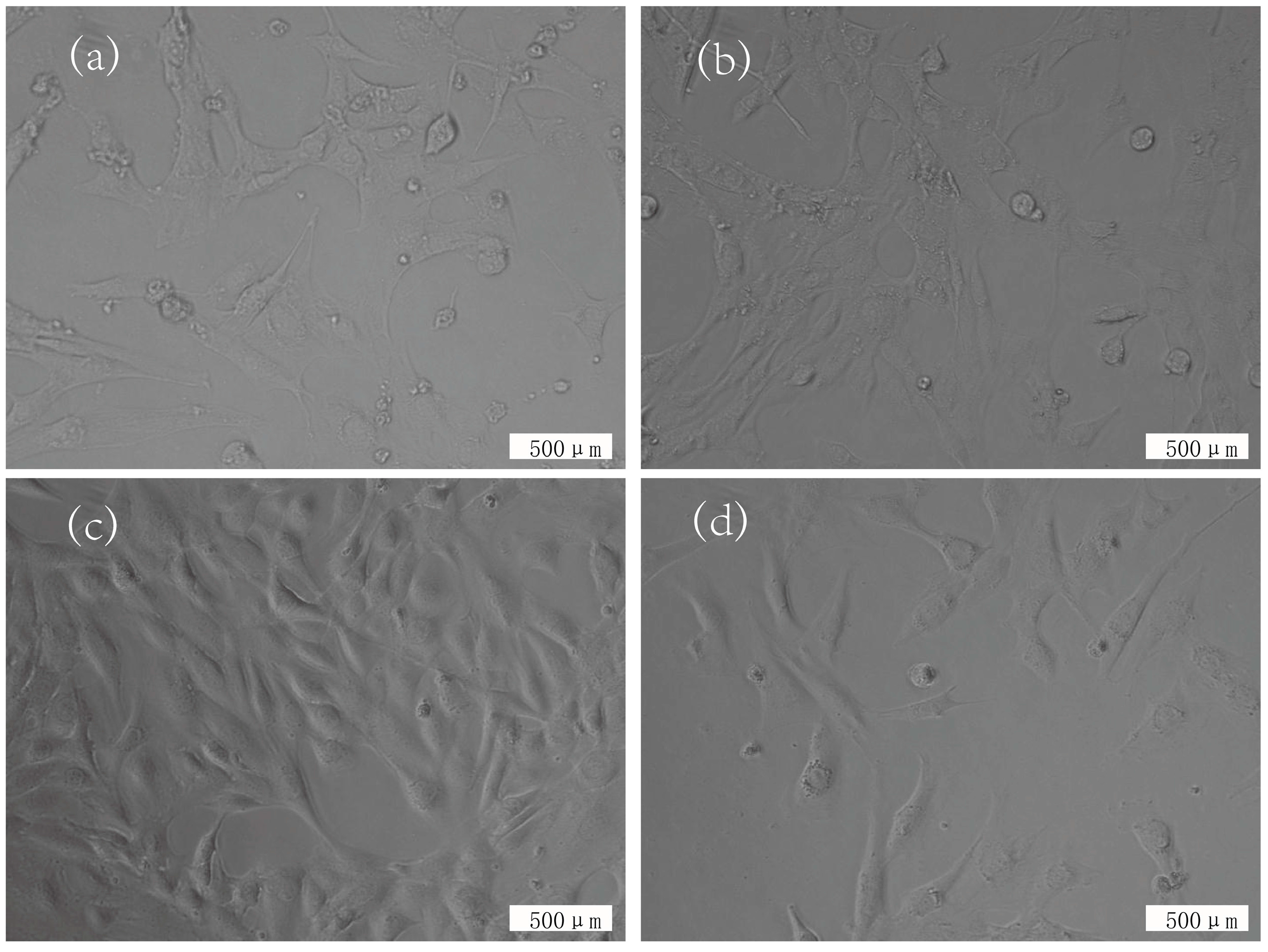
3.3. Cytotoxicity Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Brien, B.; Carroll, W. The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: A review. Acta Biomater. 2009, 5, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Sydow-Plum, G.; Tabrizian, M. Review of stent coating strategies: Clinical insights. Mater. Sci. Technol. 2013, 24, 1127–1143. [Google Scholar] [CrossRef]
- Raman, V.; Tamilselvi, S.; Rajendran, N. Electrochemical impedance spectroscopic characterization of titanium during alkali treatment and apatite growth in simulated body fluid. Electrochim. Acta 2007, 52, 7418–7424. [Google Scholar] [CrossRef]
- Srinivasan, A.; Ranjani, P.; Rajendran, N. Electrochemical polymerization of pyrrole over AZ31 Mg alloy for biomedical applications. Electrochim. Acta 2013, 88, 310–321. [Google Scholar] [CrossRef]
- Song, G.; Song, S. A possible biodegradable magnesium implant material. Adv. Eng. Mater. 2007, 9, 298–302. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloy Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Kojima, Y. Project of platform science and technology for advanced magnesium alloys. Mater. Trans. 2001, 42, 1154–1159. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Sun, W.; Wu, T.; He, H.; Liu, G. Hydrothermal synthesis of corrosion resistant hydrotalcite conversion coating on AZ91D alloy. Mater. Lett. 2013, 106, 111–114. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Y.; Liu, X.; Liu, T.; Zou, L.; Wang, Y.; Ren, Y.; Fan, Z.; Lv, Y.; Zhang, M. In-situ hydrothermal crystallization Mg(OH)2 films on magnesium alloy AZ91 and their corrosion resistance properties. Mater. Chem. Phys. 2013, 143, 322–329. [Google Scholar] [CrossRef]
- Gopi, D.; Prakash, V.C.A.; Kavitha, L.; Kannan, S.; Bhalaji, P.R.; Shinyjoy, E.; Ferreira, J.M.F. A facile electrodeposition of hydroxyapatite onto borate passivated surgical grade stainless steel. Corros. Sci. 2011, 53, 2328–2334. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Microstructural and mechanical properties of porous biocomposite scaffolds based on polyvinyl alcohol, nano-hydroxyapatite and cellulose nanocrystals. Cellulose 2014, 21, 3409–3426. [Google Scholar] [CrossRef]
- Tadic, D.; Peters, F.; Epple, M. Continuous synthesis of amorphous carbonated apatites. Biomaterials 2002, 23, 2553–2559. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, E.; Grigorescu, S.; Socol, G.; Mihailescu, I.N.; Janackovic, D.; Dindune, A.; Kanepe, Z.; Palcevskis, E.; Zdrentu, E.L.; Petrescu, S.M. Bioactive glass and hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2007, 253, 7981–7986. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Reis, R.L.; Li, P. Strontium-substituted apatite coating grown on Ti6Al4V substrate through biomimetic synthesis. J. Biomed. Mater. Res. Part B 2007, 83, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kawashita, M.; Itoh, S.; Miyamoto, K.; Takaoka, G.H. Apatite formation on titanium substrates by electrochemical deposition in metastable calcium phosphate solution. J. Mater. Sci. Mater. Med. 2008, 19, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Long, H.Y. Systematic strontium substitution in hydroxyapatite coatings on titanium via micro-arc treatment and their osteoblast/osteoclast responses. Acta Biomater. 2011, 7, 4081–4087. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Wan, Y.Z.; Tang, T.T.; Gao, C.; Dai, K.R. Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng. Part A 2009, 15, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Xiao, X.; Liu, F.; Liu, R. Chondroitin sulfate template-mediated biomimetic synthesis of nano-flake hydroxyapatite. Appl. Surf. Sci. 2008, 255, 361–364. [Google Scholar] [CrossRef]
- Garai, S.; Sinha, A. Biomimetic nanocomposites of carboxymethyl cellulose–hydroxyapatite: Novel three dimensional load bearing bone grafts. Coll. Surf. B 2014, 115, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Cellet, T.P.; Pereira, G.M.; Muniz, E.C.; Silva, R.; Rubira, A.F. Hydroxyapatite nanowhiskers embedded in chondroitin sulfate microspheres as colon targeted drug delivery system. J. Mater. Chem. B 2015, 3, 6837–6846. [Google Scholar] [CrossRef]
- Silva, R.; Pereira, G.M.; Muniz, E.C.; Rubira, A.F. Calcium carbonate crystallization on a polyethylene surface containing ultrathin layers of hydrophilic polymers. Cryst. Growth Des. 2009, 9, 3307–3312. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, J. Ultrathin cellulose film coating of porous alumina membranes for adsorption of superoxide dismutase. J. Mater. Chem. B 2013, 1, 5636–5643. [Google Scholar] [CrossRef]
- Joshi, M.K.; Tiwari, A.P.; Maharjan, B.; Won, K.S.; Kim, H.J.; Park, C.H.; Kim, C.S. Cellulose reinforced nylon-6 nanofibrous membrane: Fabrication strategies, physicochemical characterizations, wicking properties and biomimetic mineralization. Carbohydr. Polym. 2016, 147, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Q.; Yue, Y.; Zhang, Q. Application of rod-shaped cellulose nanocrystals in polyacrylamide hydrogels. J. Coll. Interface Sci. 2011, 353, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bayol, E.; Gürten, A.A.; Dursun, M.; Kayakırılmaz, K. Adsorption behavior and inhibition corrosion effect of sodium carboxymethyl cellulose on mild steel in acidic medium. Acta Phys. Chim. Sin. 2008, 24, 2236–2242. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Udosoro, I.I.; Udoh, A.P. Inhibitive and adsorption behaviour of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corros. Sci. 2010, 52, 1317–1325. [Google Scholar] [CrossRef]
- Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. Physicochemical studies of glucose, gellan gum, and hydroxypropyl cellulose—Inhibition of cast iron corrosion. Carbohydr. Polym. 2013, 95, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Arukalam, I.O.; Madufor, I.C.; Ogbobe, O.; Oguzie, E.E. Adsorption and inhibitive properties of hydroxypropyl methylcellulose on the acid corrosion of mild steel. Int. J. Appl. Sci. Eng. Res. 2013, 2, 613–629. [Google Scholar]
- Sangeetha, Y.; Meenakshi, S.; Sundaram, C.S. Corrosion inhibition of aminated hydroxyl ethyl cellulose on mild steel in acidic condition. Carbohydr. Polym. 2016, 150, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Liesiene, J.; Kazlauske, J. Functionalization of cellulose: Synthesis of water-soluble cationic cellulose derivatives. Cellul. Chem. Technol. 2013, 47, 515–525. [Google Scholar]
- Cui, W.; Beniash, E.; Gawalt, E.; Xu, Z.; Sfeir, C. Biomimetic coating of magnesium alloy for enhanced corrosion resistance and calcium phosphate deposition. Acta Biomater. 2013, 9, 8650–8659. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhong, M.; Zheng, C.; Cao, L.; Wang, D.; Wang, L.; Liang, J.; Cao, B. Preparation and characterization of dopamine-induced biomimetic hydroxyapatite coatings on the AZ31 magnesium alloy. Surf. Coat. Technol. 2015, 281, 82–88. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Jabbarzare, S.; Iqbal, N.; Kadir, M.R. Deposition of nanostructured fluorine-doped hydroxyapatite–polycaprolactone duplex coating to enhance the mechanical properties and corrosion resistance of Mg alloy. Mater. Sci. Eng. C 2015, 60, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Han, E.-H.; Shan, D.; Yim, C.D.; You, B.S. The effect of Zn concentration on the corrosion behavior of Mg–xZn alloys. Corros. Sci. 2012, 65, 322–330. [Google Scholar] [CrossRef]
- Fragal, E.H.; Cellet, T.S.; Fragal, V.H.; Companhoni, M.V.; Ueda-Nakamura, T.; Muniz, E.C.; Silva, R.; Rubira, A.F. Hybrid materials for bone tissue engineering from biomimetic growth of hydroxyapatite on cellulose nanowhiskers. Carbohydr. Polym. 2016, 152, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, D.; Almora-Barrios, N.; Leeuw, N.H.; Gervais, C.; Bonhomme, C.; Mauri, F.; Chrzanowski, W.; Knowles, J.C.; Newport, R.J.; Wong, A.; et al. Magnesium incorporation into hydroxyapatite. Biomaterials 2011, 32, 1826–1837. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Daroonparvar, M.; Saud, S.N.; Abdul-Kadir, M.R. Bi-layer nano-TiO2/FHA composite coatings on Mg–Zn–Ce alloy prepared by combined physical vapour deposition and electrochemical deposition methods. Vacuum 2014, 110, 127–135. [Google Scholar] [CrossRef]
- Jia, X.; Xu, M.; Wang, Y.; Ran, D.; Yang, S.; Zhang, M. Polydopamine-based molecular imprinting on silica-modified magnetic nanoparticles for recognition and separation of bovine hemoglobin. Analyst 2013, 138, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Hydroxyapatite, a biomaterial: Its chemical synthesis, characterization and study of biocompatibility prepared from shell of garden snail, Helix aspersa. Bull. Mater. Sci. 2012, 35, 1031–1038. [Google Scholar] [CrossRef]
- Sureshkumar, M.; Lee, C.K. Polydopamine coated magnetic-chitin (MCT) particles as a new matrix for enzyme immobilization. Carbohydr. Polym. 2011, 84, 775–780. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, H.; Nabiyouni, M.; Bhaduri, S.B. Rapid coating of AZ31 magnesium alloy with calcium deficient hydroxyapatite using microwave energy. Mater. Sci. Eng. C 2015, 49, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Fekry, A.M.; Ameer, M.A. Corrosion inhibition of mild steel in acidic media using newly synthesized heterocyclic organic molecules. Int. J. Hydrog. Energy 2010, 35, 7641–7651. [Google Scholar] [CrossRef]
- Hu, J.M.; Zhang, J.Q.; Cao, C.N. Determination of water uptake and diffusion of Cl− ion in epoxy primer on aluminum alloys in NaCl solution by electrochemical impedance spectroscopy. Prog. Org. Coat. 2003, 46, 273–279. [Google Scholar] [CrossRef]
- Fekry, A.M.; El-Sherif, R.M. Electrochemical corrosion behavior of magnesium and titanium alloys in simulated body fluid. Electrochim. Acta 2009, 54, 7280–7285. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Xu, Y.; Sun, J.; Yang, L.; Guo, C.; Liang, J.; Cao, B. Preparation and Characterization of Aminated Hydroxyethyl Cellulose-Induced Biomimetic Hydroxyapatite Coatings on the AZ31 Magnesium Alloy. Metals 2017, 7, 214. https://doi.org/10.3390/met7060214
Zhu B, Xu Y, Sun J, Yang L, Guo C, Liang J, Cao B. Preparation and Characterization of Aminated Hydroxyethyl Cellulose-Induced Biomimetic Hydroxyapatite Coatings on the AZ31 Magnesium Alloy. Metals. 2017; 7(6):214. https://doi.org/10.3390/met7060214
Chicago/Turabian StyleZhu, Bowu, Yimeng Xu, Jing Sun, Lei Yang, Changgang Guo, Jun Liang, and Baocheng Cao. 2017. "Preparation and Characterization of Aminated Hydroxyethyl Cellulose-Induced Biomimetic Hydroxyapatite Coatings on the AZ31 Magnesium Alloy" Metals 7, no. 6: 214. https://doi.org/10.3390/met7060214
APA StyleZhu, B., Xu, Y., Sun, J., Yang, L., Guo, C., Liang, J., & Cao, B. (2017). Preparation and Characterization of Aminated Hydroxyethyl Cellulose-Induced Biomimetic Hydroxyapatite Coatings on the AZ31 Magnesium Alloy. Metals, 7(6), 214. https://doi.org/10.3390/met7060214



