Effect of the Temperature in the Mechanical Properties of Austenite, Ferrite and Sigma Phases of Duplex Stainless Steels Using Hardness, Microhardness and Nanoindentation Techniques
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
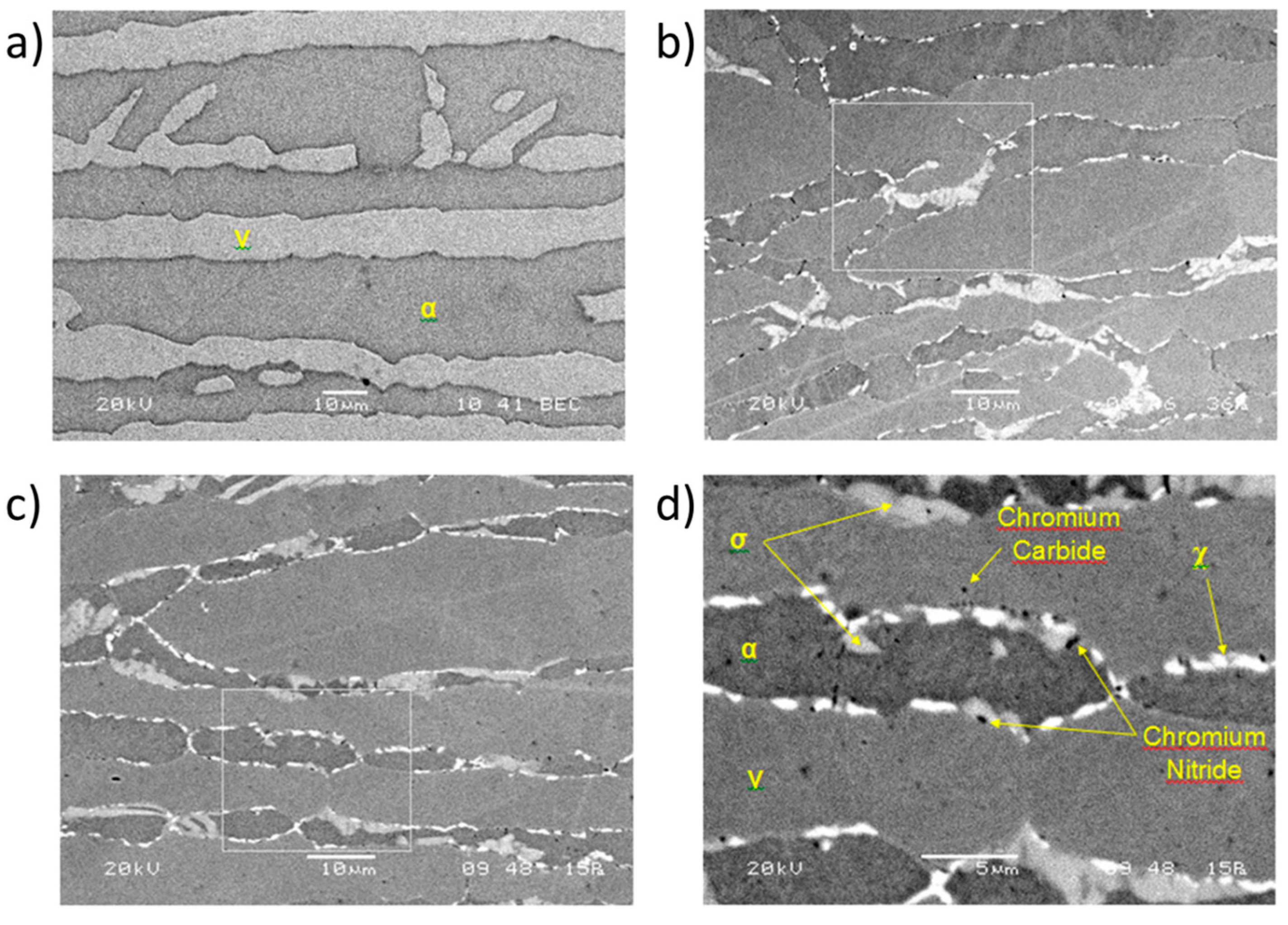
3.1. Qualitative and Quantitative Analysis of the Phases
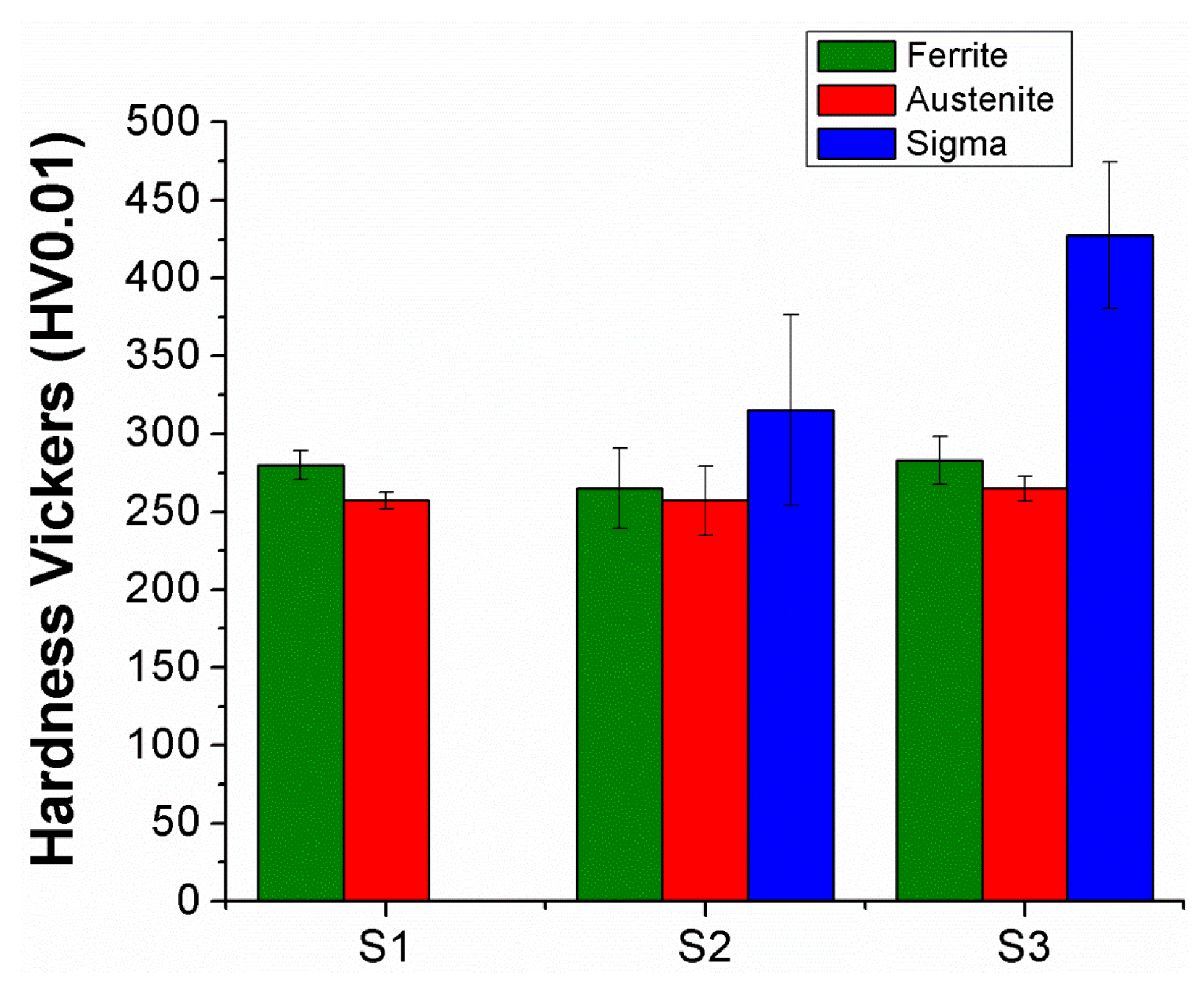
3.2. Hardness Measurements
3.3. Microhardness Measurements
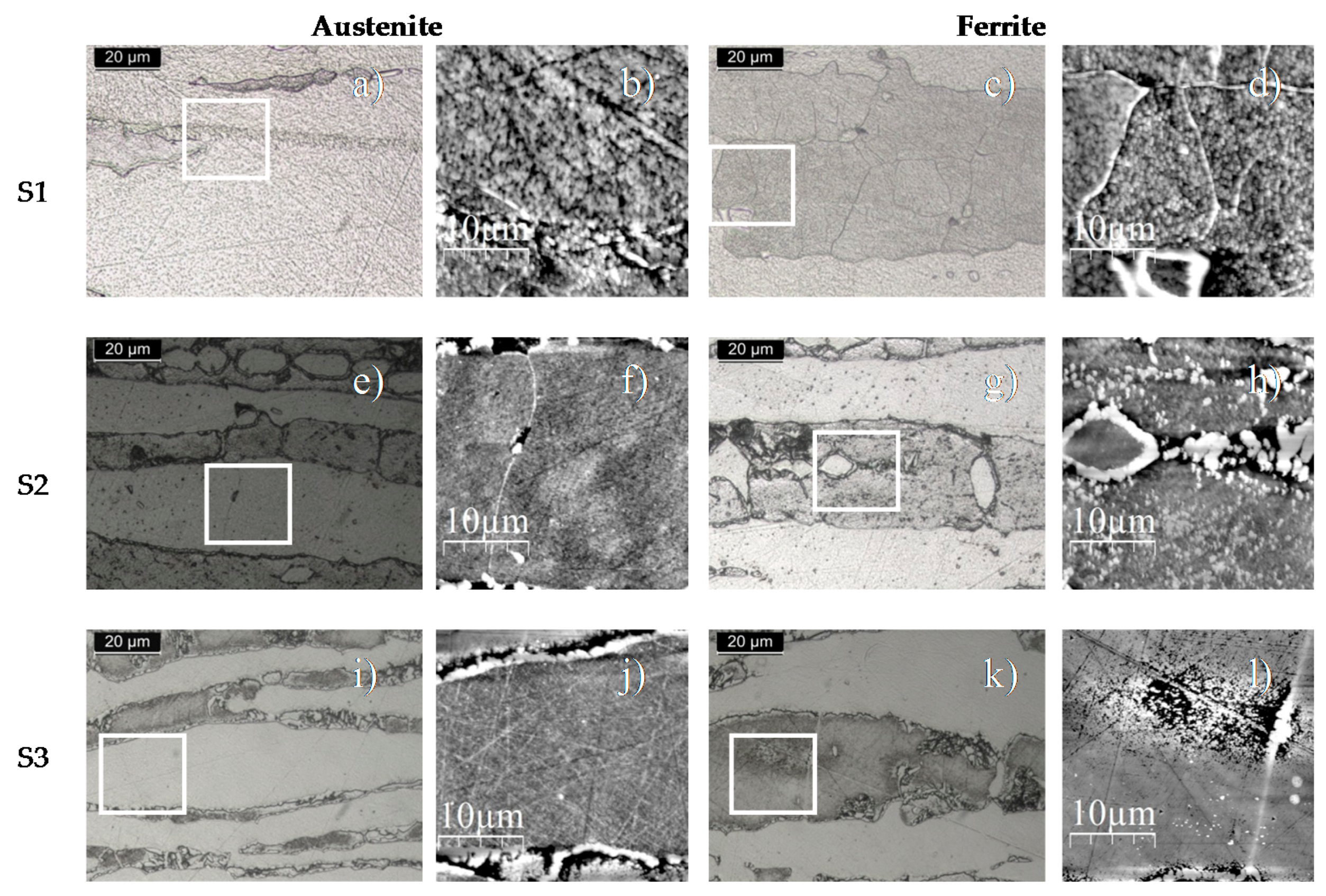
3.4. Nanoindentation Measurements
3.4.1. Nanoindentation Hardness Measurements in Austenite and Ferrite Phases
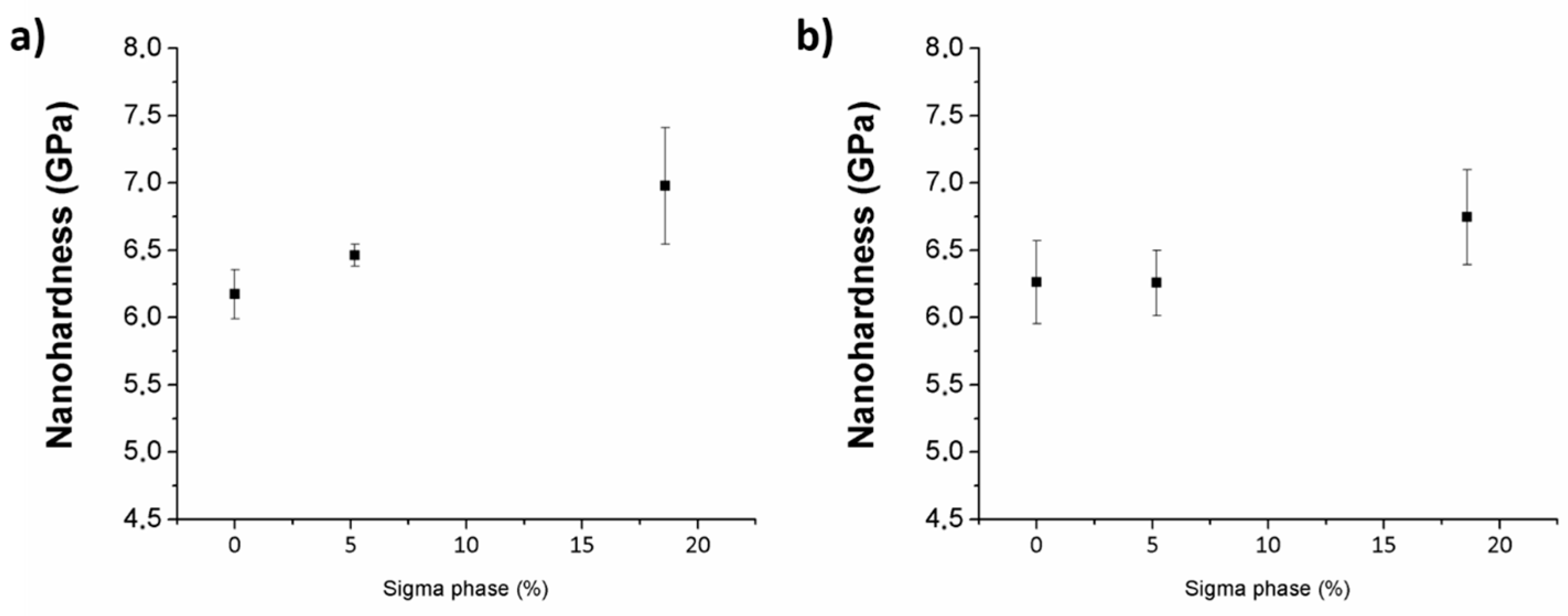
3.4.2. Nanoidentation Hardness Measurements in Sigma Phase
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Nilsson, J.-O. Super Duplex Stainless Steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Nilsson, J.O.; Wilson, A. Influence of isothermal phase transformations on toughness and pitting corrosion of super duplex stainless steel SAF 2507. Mater. Sci. Technol. 1993, 9, 545–554. [Google Scholar] [CrossRef]
- Han, Y.; Zou, D.; Yao, H.; Zhang, W.; Yu, J. Microstructural evolutions and its influence on properties of super-duplex stainless steel. Adv. Mater. Res. 2010, 97, 656–659. [Google Scholar] [CrossRef]
- Li, X.; Miodownik, A.P.; Saunders, N. Modelling of materials properties in duplex stainless steels. Mater. Sci. Technol. 2002, 18, 861–868. [Google Scholar]
- Silva, E.M.; Marinho, L.B.; Rebouças Filho, P.P.; Leite, J.P.; Leite, J.P.; Fialho, W.M.L.; de Albuquerque, V.H.C.; Tavares, J.M.R.S. Classification of induced magnetic field signals for the microstructural characterization of sigma phase in duplex stainless steels. Metals 2016, 6, 164. [Google Scholar] [CrossRef]
- Olabi, A.G.; Lostado, R.; Benyounis, K.Y. Review of microstructures, mechanical properties, and residual stresses of ferritic and martensitic stainless-steel welded joints. Compr. Mater. Process. 2014, 6, 181–192. [Google Scholar]
- Alvarez, S.M.; Bautista, A.; Velasco, F. Corrosion behaviour of corrugated lean duplex stainless steels in simulated concrete pore solutions. Corros. Sci. 2011, 53, 1748–1755. [Google Scholar] [CrossRef]
- Olsson, J.; Snis, M. Duplex—A new generation of stainless steels for desalination plants. Desalination 2007, 205, 104–113. [Google Scholar] [CrossRef]
- Dabalà, M.; Calliari, I.; Variola, A. Corrosion behavior of a superduplex stainless steel in chloride aqueous solution. J. Mater. Eng. Perform. 2004, 13, 237–240. [Google Scholar] [CrossRef]
- Machuca, L.L.; Bailey, S.I.; Gubner, R.; Watkin, E.L.J.; Ginige, M.P.; Kaksonen, A.H. Crevice corrosion of duplex stainless steels in the presence of natural marine biofilms. NACE Int. Corros. Conf. Ser. 2012, 5, 3924–3936. [Google Scholar]
- Veljkovic, M.; Gozzi, J. Use of duplex stainless steel in economic design of a pressure vessel. J. Press. Vessel Technol. 2007, 129, 155–161. [Google Scholar] [CrossRef]
- Saithala, J.R.; Mahajanam, S.; Ubhi, H.S.; Atkinson, J. Environmental assisted cracking behavior of sigmatized super duplex stainless steel in oilfield production brine. Corrosion 2013, 69, 276–285. [Google Scholar] [CrossRef]
- Yang, S.-M.; Chen, Y.-C.; Chen, C.-H.; Huang, W.-P.; Lin, D.-Y. Microstructural characterization of δ/γ/σ/γ2/χ phases in silver-doped 2205 duplex stainless steel under 800 °C aging. J. Alloys Compd. 2015, 633, 48–53. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Kim, S.-T.; Lee, I.-S.; Kim, J.-S.; Kim, K.-T.; Park, Y.-S. Effects of Cu on the precipitation of intermetallic compounds and the intergranular corrosion of hyper duplex stainless steels. Corros. Sci. 2013, 66, 217–224. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kang, K.-Y.; Kim, M.-S.; Lee, J.-M. Low-temperature mechanical behavior of super duplex stainless steel with sigma precipitation. Metals 2015, 5, 1732–1745. [Google Scholar] [CrossRef]
- Michalska, J.; Sozańska, M. Qualitative and Quantitative Analysis of σ and χ Phases in 2205 Duplex Stainless Steel. Mater. Charact. 2006, 56, 355–362. [Google Scholar] [CrossRef]
- Ferro, P.; Bonollo, F.; Timelli, G. Sigma phase precipitation modelling in a UNS S32760 superduplex stainless steel. Metall. Ital. 2012, 104, 7–12. [Google Scholar]
- Elsabbagh, F.M.; Hamouda, R.M.; Taha, M.A. On microstructure and microhardness of isothermally aged UNS S32760 and the effect on toughness and corrosion behavior. J. Mater. Eng. Perform. 2014, 23, 275–284. [Google Scholar] [CrossRef]
- Argandona, G.; Biezma, M.V.; Berrueta, J.M.; Berlanga, C.; Ruiz, A. Detection of secondary phases in UNS S32760 superduplex stainless steel by destructive and non-destructive techniques. J. Mater. Eng. Perform. 2016, 25, 5269–5279. [Google Scholar] [CrossRef]
- Llorca-Isern, N.; López-Jiménez, I.; López-Luque, H.; Biezma, M.V.; Roca, A. Study of the precipitation of secondary phases in duplex and superduplex stainless steel. Mater. Sci. Forum 2017, 879, 2537–2542. [Google Scholar] [CrossRef]
- Llorca-Isern, N.; López-Luque, H.; López-Jiménez, I.; Biezma, M.V. Identification of sigma and chi phases in duplex stainless steels. Mater. Charact. 2016, 112, 20–29. [Google Scholar] [CrossRef]
- Atamert, S.; King, J.E. Sigma-phase formation and its prevention in duplex stainless steels. J. Mater. Sci. Lett. 1993, 12, 1144–1147. [Google Scholar] [CrossRef]
- Lee, J.; Kim, I.; Kimura, A. Application of small punch test to evaluate sigma-phase embrittlement of pressure vessel cladding material. J. Nucl. Sci. Technol. 2003, 40, 664–671. [Google Scholar] [CrossRef]
- Liu, T.; Zhiyong, R.W.; Xu, D.; Aung, A.A. Metallurgical analysis on a cracked super duplex stainless steel flange. J. Fail. Anal. Prev. 2014, 14, 470–477. [Google Scholar] [CrossRef]
- Pohl, M.; Storz, O.; Glogowski, T. Effect of Intermetallic Precipitations on the Properties of Duplex Stainless Steel. Mater. Charact. 2007, 58, 65–71. [Google Scholar] [CrossRef]
- Maehara, Y.; Koike, M.; Fujino, N.; Kunitake, T. Precipitation of sigma phase in a 25Cr-7Ni-3Mo duplex phase stainless steel. Trans. Iron Steel Inst. Jpn. 1983, 23, 240–246. [Google Scholar] [CrossRef]
- Wilms, M.E.; Gadgil, V.J.; Krougman, J.M.; Kolster, B.H. The effect of σ-phase precipitation at 800 °C on the mechanical properties of a high alloyed duplex stainless steel. Mater. High Temp. 1991, 9, 160–166. [Google Scholar] [CrossRef]
- Biezma, M.V.; Berlanga, C.; Argandona, G. Relationship between microstructure and fracture types in a UNS S32205 duplex stainless steel. Mater. Res. 2013, 16, 965–969. [Google Scholar] [CrossRef]
- Berecz, T.; Mészáros, I.; Szabó, P.J. Decomposition of the ferritic phase in isothermally aged SAF 2507 duplex stainless steel. Mater. Sci. Forum 2008, 589, 185–190. [Google Scholar] [CrossRef]
- Berecz, T.; Fazakas, É.; Mészáros, I.; Sajó, I. Decomposition kinetics of ferrite in isothermally aged SAF 2507-type duplex stainless steel. J. Mater. Eng. Perform. 2015, 24, 4777–4788. [Google Scholar] [CrossRef]
- Chandra, K.; Kain, V.; Bhutani, V.; Raja, V.S.; Tewari, R.; Dey, G.K.; Chakravartty, J.K. Low temperature thermal aging of austenitic stainless steel welds: Kinetics and effects on mechanical properties. Mater. Sci. Eng. A 2012, 534, 163–175. [Google Scholar] [CrossRef]
- Morris, D. The influence of sigma phase on creep ductility in type 316 stainless steel. Scr. Metall. 1979, 13, 1195–1196. [Google Scholar] [CrossRef]
- May, V.E. Sigma phase embrittlement of austenitic stainless steel FCCU regenerator internals. Mater. Perform. 1985, 24, 18–22. [Google Scholar]
- Perron, A.; Toffolon-Masclet, C.; Ledoux, X.; Buy, F.; Guilbert, T.; Urvoy, S.; Bosonnet, S.; Marini, B.; Cortial, F.; Texier, G.; et al. Understanding sigma-phase precipitation in a stabilized austenitic stainless steel (316Nb) through complementary CALPHAD-based and experimental investigations. Acta Mater. 2014, 79, 16–29. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Nanoindentation in materials research: Past, present, and future. MRS Bull. 2010, 35, 897–907. [Google Scholar] [CrossRef]
- Furnémont, Q.; Kempf, M.; Jacques, P.J.; Göken, M.; Delannay, F. On the measurement of the nanohardness of the constitutive phases of trip-assisted multiphase steels. Mater. Sci. Eng. A 2002, 328, 26–32. [Google Scholar] [CrossRef]
- Wang, X.F.; Yang, X.P.; Guo, Z.D.; Zhou, Y.C.; Song, H.W. Nanoindentation characterization of mechanical properties of ferrite and austenite in duplex stainless steel. Adv. Mater. Process. 2007, 26, 1165–1171. [Google Scholar] [CrossRef]
- El Mehtedi, M.; Spigarelli, S.; Ricci, P.; Paternoster, C.; Quadrini, E. Mechanical characterization of phases in duplex 2205 stainless steel by nanoindentation technique. Metall. Ital. 2010, 102, 11–16. [Google Scholar]
- Gadelrab, K.R.; Li, G.; Chiesa, M.; Souier, T. Local characterization of austenite and ferrite phases in duplex stainless steel using MFM and nanoindentation. J. Mater. Res. 2012, 27, 1573–1579. [Google Scholar] [CrossRef]
- Guo, L.Q.; Lin, M.C.; Qiao, L.J.; Volinsky, A.A. Ferrite and austenite phase identification in duplex stainless steel using SPM techniques. Appl. Surf. Sci. 2013, 287, 499–501. [Google Scholar] [CrossRef]
- Ohmura, T.; Tsuzaki, K.; Sawada, K.; Kimura, K. Inhomogeneous nano-mechanical properties in the multi-phase microstructure of long-term aged type 316 stainless steel. J. Mater. Res. 2006, 21, 1229–1236. [Google Scholar] [CrossRef]
- Ohmura, T.; Sawada, K.; Kimura, K.; Tsuzaki, K. Alteration in nanohardness of matrix phase associated with precipitation during long-term aging of type 316 stainless steel. Mater. Sci. Eng. A 2008, 489, 85–92. [Google Scholar] [CrossRef]
- Peguet, L.; Gaugain, A. Localized corrosion resistance of duplex stainless steels: Methodology and properties; a review paper. Rev. Metall. 2011, 108, 231–243. [Google Scholar] [CrossRef]
- Fang, Y.L.; Liu, Z.Y.; Xue, W.Y.; Song, H.M.; Jiang, L.Z. Precipitation of secondary phases in lean duplex stainless steel 2101 during isothermal ageing. ISIJ Int. 2010, 50, 286–293. [Google Scholar] [CrossRef]
- Guimarães, A.A.; Mei, P.R. Precipitation of carbides and sigma phase in AISI type 446 stainless steel under working conditions. J. Mater. Process. Technol. 2004, 155, 1681–1689. [Google Scholar] [CrossRef]
- Walter, C.; Mitterer, C. 3D versus 2D finite element simulation of the effect of surface roughness on nanoindentation of hard coatings. Surf. Coat. Technol. 2009, 203, 3286–3290. [Google Scholar] [CrossRef]




| Cr | Ni | Mn | Mo | Si | Cu | W | N | C | P | S |
|---|---|---|---|---|---|---|---|---|---|---|
| 25.52 | 7.33 | 0.63 | 3.56 | 0.44 | 0.74 | 0.51 | 0.257 | 0.019 | 0.022 | <0.003 |
| Sample Identification | Time (min) | Austenite | Ferrite | Sigma |
|---|---|---|---|---|
| S1 | 0 | 43.0 ± 1.8 | 57.0 ± 1.8 | 0 ± 0.0 |
| S2 | 21 | 44.9 ± 2.9 | 49.9 ± 3.2 | 5.2 ± 0.8 |
| S3 | 25 | 41.2 ± 3.9 | 40.2 ± 2.9 | 18.6 ± 1.8 |
| Thermal Treatment at 850 °C (min) | Phase | Si | Cr | Fe | Ni | Mo | W |
|---|---|---|---|---|---|---|---|
| S2 (21 min) | σ | 0.46 | 28.72 | 59.44 | 5.64 | 4.85 | 0.88 |
| S3 (25 min) | σ | 0.36 | 30.73 | 56.52 | 4.72 | 6.59 | 0.56 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argandoña, G.; Palacio, J.F.; Berlanga, C.; Biezma, M.V.; Rivero, P.J.; Peña, J.; Rodriguez, R. Effect of the Temperature in the Mechanical Properties of Austenite, Ferrite and Sigma Phases of Duplex Stainless Steels Using Hardness, Microhardness and Nanoindentation Techniques. Metals 2017, 7, 219. https://doi.org/10.3390/met7060219
Argandoña G, Palacio JF, Berlanga C, Biezma MV, Rivero PJ, Peña J, Rodriguez R. Effect of the Temperature in the Mechanical Properties of Austenite, Ferrite and Sigma Phases of Duplex Stainless Steels Using Hardness, Microhardness and Nanoindentation Techniques. Metals. 2017; 7(6):219. https://doi.org/10.3390/met7060219
Chicago/Turabian StyleArgandoña, Gorka, José F. Palacio, Carlos Berlanga, María V. Biezma, Pedro J. Rivero, Julio Peña, and Rafael Rodriguez. 2017. "Effect of the Temperature in the Mechanical Properties of Austenite, Ferrite and Sigma Phases of Duplex Stainless Steels Using Hardness, Microhardness and Nanoindentation Techniques" Metals 7, no. 6: 219. https://doi.org/10.3390/met7060219







