Phase Transition of Peritectic Steel Q345 and Its Effect on the Equilibrium Partition Coefficients of Solutes
Abstract
:1. Introduction
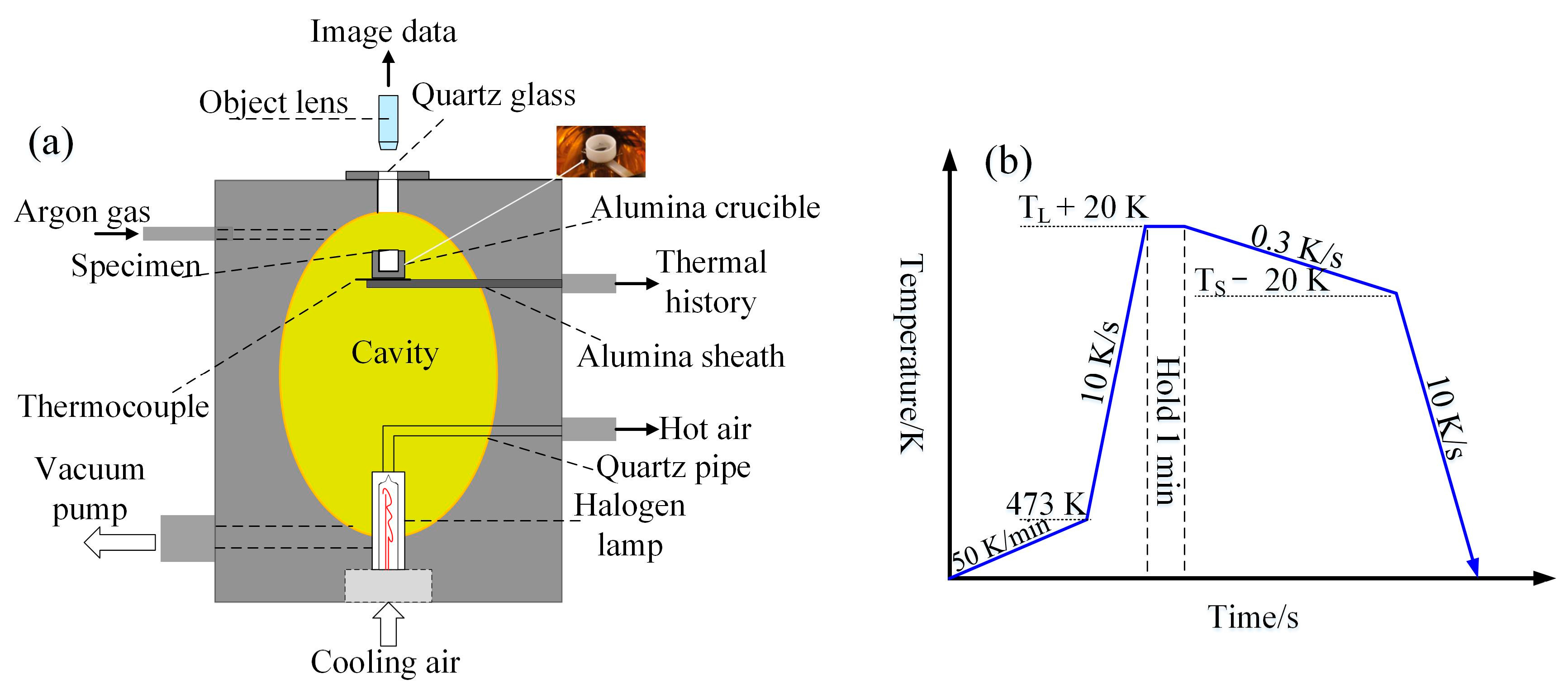
2. Experimental Procedure
3. Thermodynamic Model for the Equilibrium Partition Coefficient
3.1. Model Description
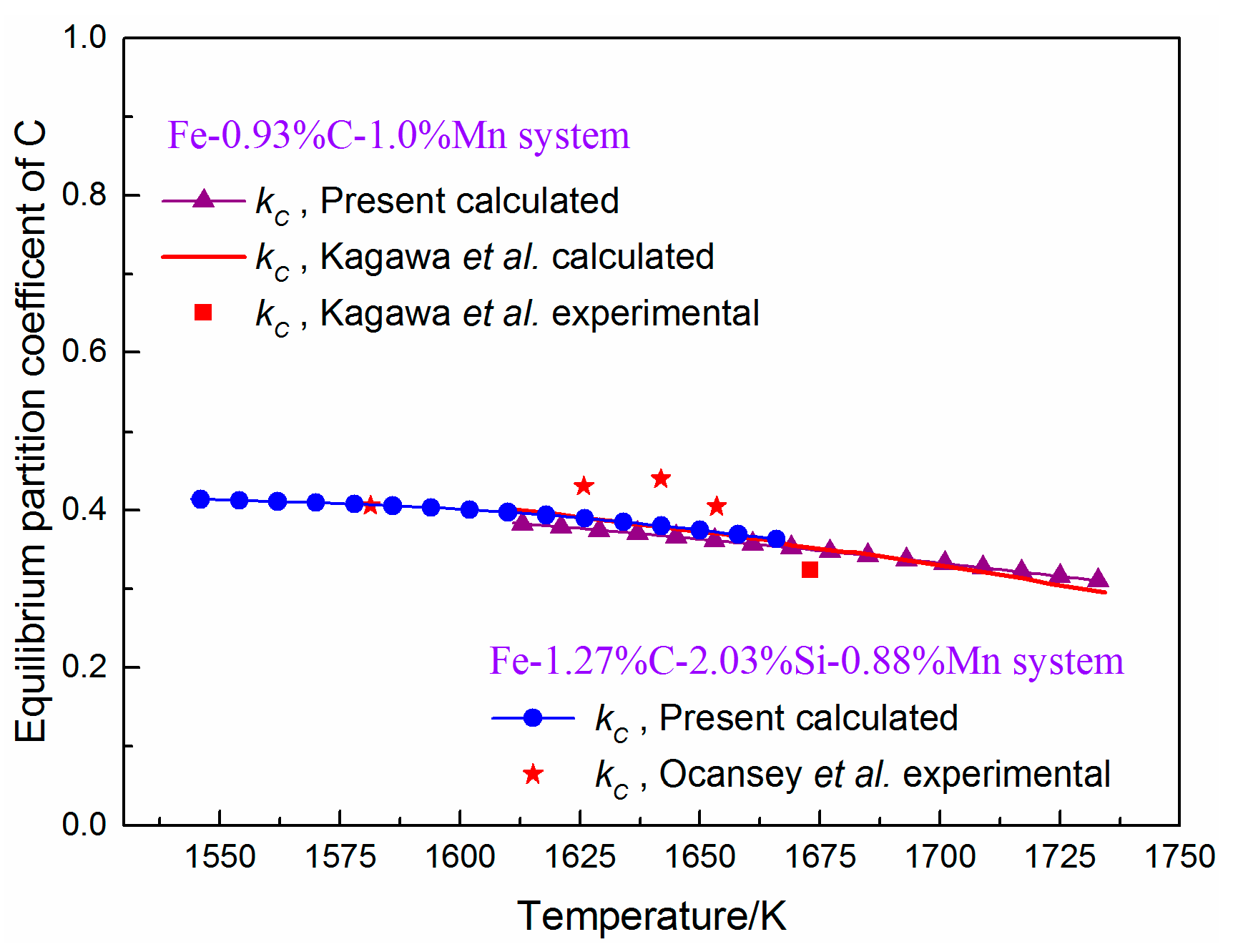
3.2. Model Verification
4. Results and Discussion
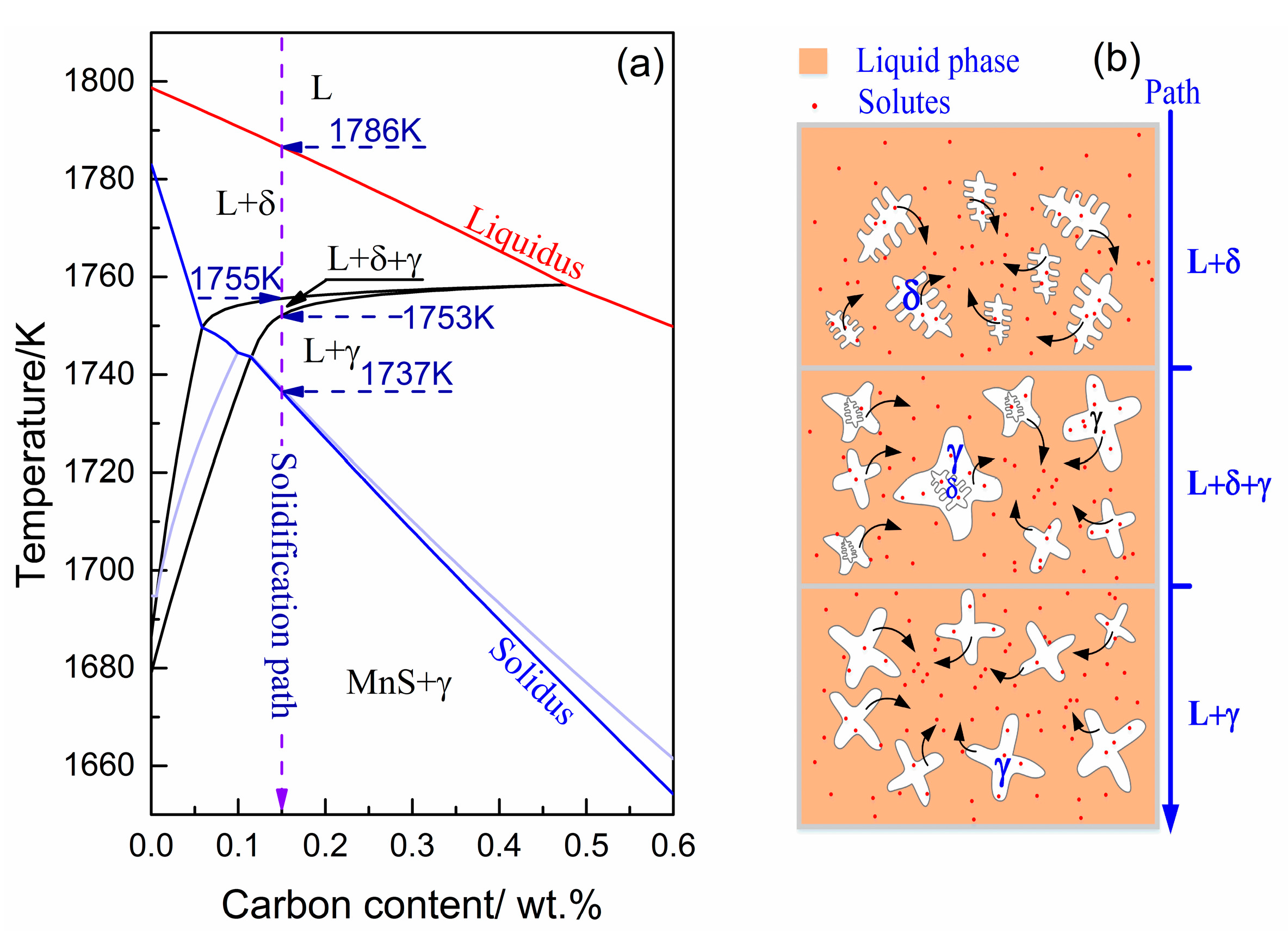
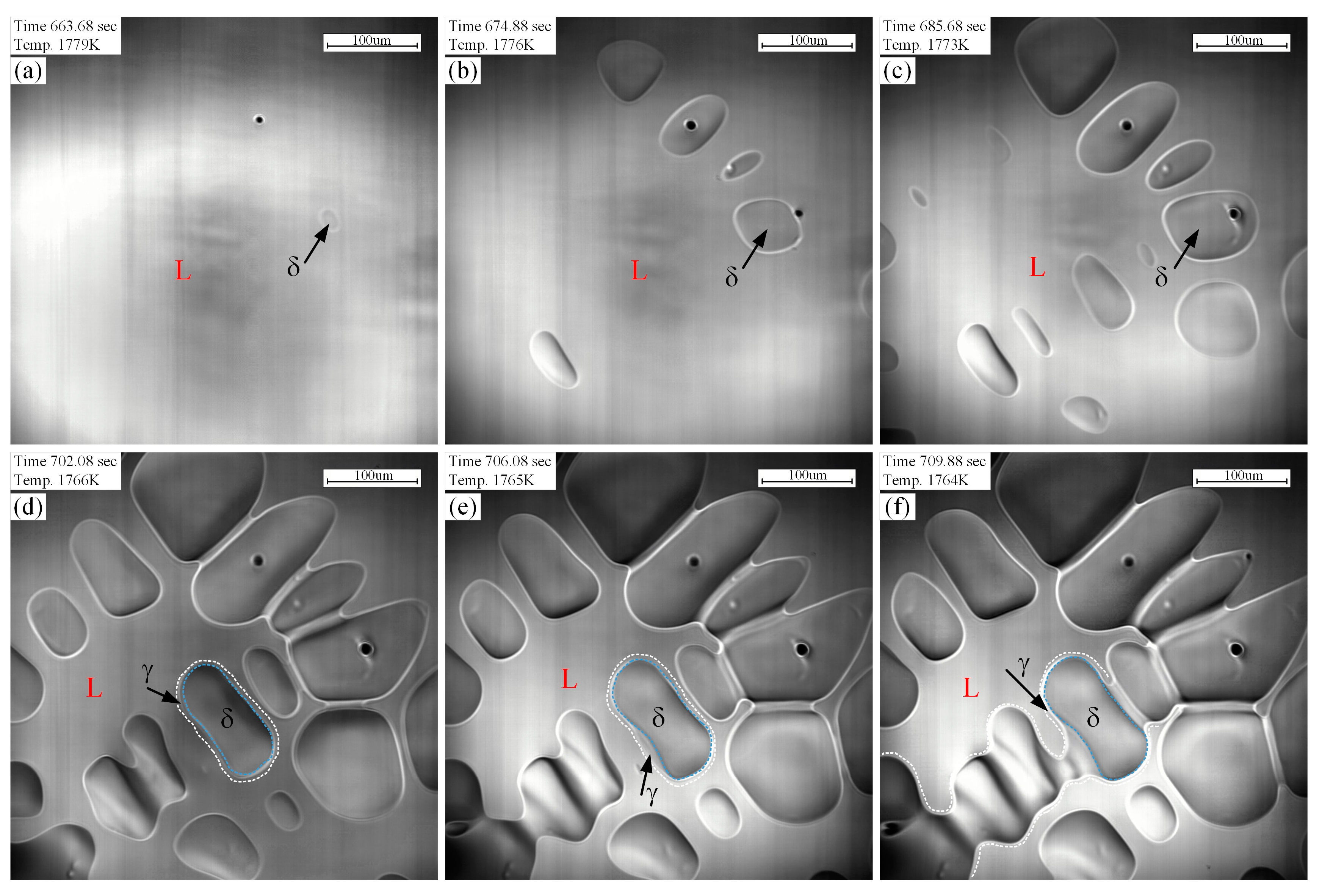
4.1. Solidification Path and Phase Transition of Peritectic Steel Q345
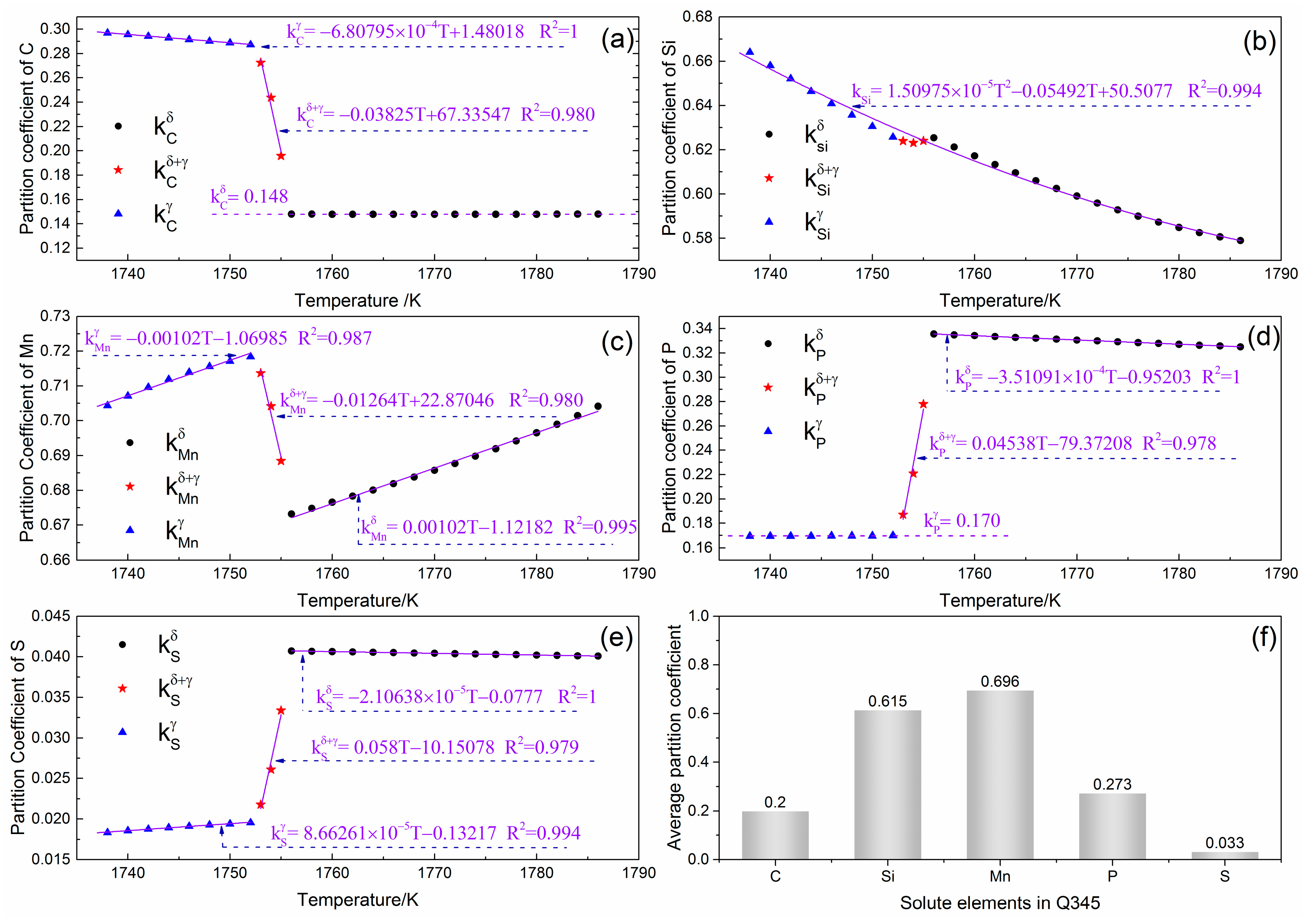
4.2. Evolution of Equilibrium Partition Coefficients during Solidification and Phase Transition
5. Conclusions
- (1)
- The L + δ, L + δ + γ, and L + γ phases coexist in sequence in the solidification path of steel Q345, which agrees well with in-situ observations.
- (2)
- The temperature dependent equilibrium partition coefficients of the solutes in Q345 were quantitatively studied, and regression formulas were established under various phase constitutions.
- (3)
- The solute sequence arranged in order of weakening segregation tendency in steel Q345 is S, C, P, Si, and Mn. The average equilibrium partition coefficients of these constituents are 0.696, 0.615, 0.273, 0.2, and 0.033, respectively.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sung, P.K.; Poirier, D.R. Liquid-solid partition ratios in nickel-base alloys. Metall. Mater. Trans. A 1999, 30, 2173–2181. [Google Scholar]
- Fujda, M. Centerline segregation of continuously cast slabs influence on microstructure and fracture morphology. J. Met. Mater. Miner. 2005, 15, 45–51. [Google Scholar]
- Salvino, I.M.; Ferreira, L.D.; Ferreira, A.F. Simulation of microsegregation in multicomponent alloys during solidification. Steel Res. Int. 2012, 83, 723–732. [Google Scholar] [CrossRef]
- Wang, W.L.; Zhu, M.Y.; Cai, Z.Z.; Luo, S.; Ji, C. Micro-segregation behavior of solute elements in the mushy zone of continuous casting wide-thick slab. Steel Res. Int. 2012, 83, 1152–1162. [Google Scholar] [CrossRef]
- Ghosh, A. Segregation in cast products. Sadhana 2001, 26, 5–24. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Wang, F.M.; Li, C.R.; Li, M.L.; Zhang, J.; Cui, G.J. Numerical simulation of macrosegregation in water-cooled heavy flat ingot during solidification. JOM 2014, 66, 1166–1174. [Google Scholar] [CrossRef]
- Domitner, J.; Wu, M.H.; Kharicha, A.; Ludwig, A.; Kaufmann, B.; Reiter, J.; Schaden, T. Modeling the effects of strand surface bulging and mechanical softreduction on the macrosegregation formation in steel continuous casting. Metall. Mater. Trans. A 2014, 45, 1415–1434. [Google Scholar] [CrossRef]
- Fachinotti, V.D.; Le Corre, S.; Triolet, N.; Bobadilla, M.; Bellet, M. Two-phase thermo-mechanical and macrosegregation modelling of binary alloys solidification with emphasis on the secondary cooling stage of steel slab continuous casting processes. Int. J. Numer. Methods Eng. 2006, 67, 1341–1384. [Google Scholar] [CrossRef]
- Long, M.J.; Chen, D.F. Study on mitigating center macro-segregation during steel continuous casting process. Steel Res. Int. 2011, 82, 847–856. [Google Scholar] [CrossRef]
- Valdes, J.; Shang, S.L.; Liu, Z.K.; King, P.; Liu, X.B. Quenching differential thermal analysis and thermodynamic calculation to determine partition coefficients of solute elements in simplified Ni-base superalloys. Metall. Mater. Trans A 2010, 41, 487–498. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Pikunov, M.V.; Cheverikin, V.V. The partition coefficients of components in Cu-Ni-Mn alloys. Metall. Mater. Trans. A 2015, 46, 843–850. [Google Scholar] [CrossRef]
- Ocansey, P.M.; Poirier, D.R. Equilibrium partition ratios of C, Mn, and Si in a high carbon steel. Mater. Sci. Eng. A 1996, 211, 10–14. [Google Scholar] [CrossRef]
- Kagawa, A.; Iwata, K.; Nofal, A.A.; Okamoto, T. Theoretical evaluation of equilibrium partition-coefficients of solute elements in Fe-C-base quaternary and multicomponent systems. Mater. Sci. Technol. 1985, 1, 678–683. [Google Scholar] [CrossRef]
- Schneider, M.C.; Beckermann, C. Formation of macrosegregation by multicomponent thermosolutal convection during the solidification of steel. Metall. Mater. Trans. A 1995, 26, 2373–2388. [Google Scholar] [CrossRef]
- Yang, H.L.; Zhao, L.G.; Zhang, X.Z.; Deng, K.W.; Li, W.C.; Gan, Y. Mathematical simulation on coupled flow, heat, and solute transport in slab continuous casting process. Metall. Mater. Trans. B 1998, 29, 1345–1356. [Google Scholar] [CrossRef]
- Felicelli, S.D.; Poirier, D.R.; Giamei, A.F.; Heinrich, J.C. Simulating convection and macrosegregation in superalloys. JOM 1997, 49, 21–25. [Google Scholar] [CrossRef]
- Aboutalebi, M.R.; Hasan, M.; Guthrie, R.I.L. Coupled turbulent flow, heat, and solute transport in continuous casting processes. Metall. Mater. Trans. B 1995, 26, 731–744. [Google Scholar] [CrossRef]
- Li, J.; Wu, M.H.; Ludwig, A.; Kharicha, A. Simulation of macrosegregation in a 2.45-ton steel ingot using a three-phase mixed columnar-equiaxed model. Int. J. Heat Mass Transf. 2014, 72, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. Factsage thermochemical software and databases. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Wang, Y.N.; Bao, Y.P.; Wang, M.; Zhang, L.C.; Chen, Y.N. Basic research on precipitation and control of BN inclusions in steel. Metall. Mater. Trans. B 2013, 44, 1144–1154. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Long, M.; Cao, J.; Chen, D.; Liu, T.; Dong, Z. Phase Transition of Peritectic Steel Q345 and Its Effect on the Equilibrium Partition Coefficients of Solutes. Metals 2017, 7, 288. https://doi.org/10.3390/met7080288
Chen H, Long M, Cao J, Chen D, Liu T, Dong Z. Phase Transition of Peritectic Steel Q345 and Its Effect on the Equilibrium Partition Coefficients of Solutes. Metals. 2017; 7(8):288. https://doi.org/10.3390/met7080288
Chicago/Turabian StyleChen, Huabiao, Mujun Long, Junsheng Cao, Dengfu Chen, Tao Liu, and Zhihua Dong. 2017. "Phase Transition of Peritectic Steel Q345 and Its Effect on the Equilibrium Partition Coefficients of Solutes" Metals 7, no. 8: 288. https://doi.org/10.3390/met7080288



