Influence of the Heating Rate on the Foaming Behavior of Various Aluminium Alloys
Abstract
:1. Introduction
2. Experimental
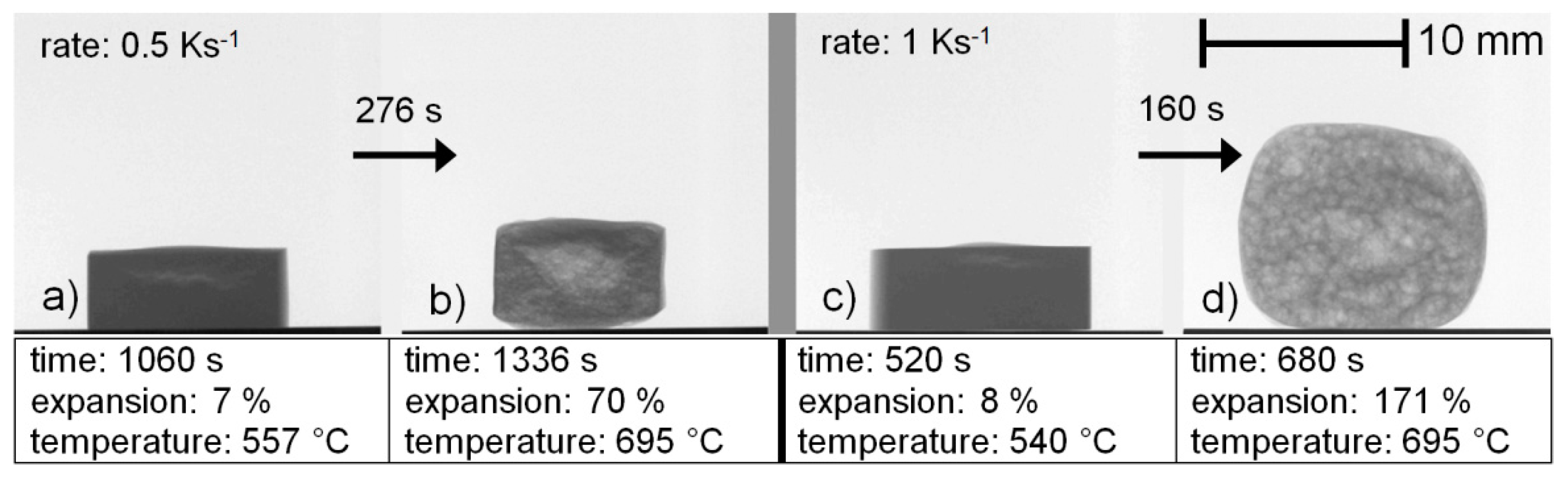
3. Results
4. Discussion
5. Conclusions
- All four alloys investigated exhibit a pronounced and non-linear dependence of expansion values on the heating rate.
- The collapse increases for all alloys when the heating rate exceeds approximately 3 K/s.
- Low heating rates lead to low expansions.
- High heating rates (<4 K/s) improve expansion only in Mg-containing alloys.
Author Contributions
Conflicts of Interest
References
- Duarte, I.; Banhart, J. A study of aluminium foam formation-Kinetics and microstructure. Acta Mater. 2000, 48, 2349–2362. [Google Scholar] [CrossRef]
- Garcia-Moreno, F.; Fromme, M.; Banhart, J. Real-time X-ray Radioscopy on Metallic Foams Using a Compact Micro-Focus Source. Adv. Eng. Mater. 2004, 6, 416–420. [Google Scholar] [CrossRef]
- Helwig, H.M.; Garcia-Moreno, F.; Banhart, J. A study of Mg and Cu additions on the foaming behaviour of Al-Si alloys. J. Mater. Sci. 2011, 46, 5227–5236. [Google Scholar] [CrossRef]
- Lehmhus, D. Dynamic collapse mechanisms in metal foam expansion. Adv. Eng. Mater. 2010, 12, 465–471. [Google Scholar] [CrossRef]
- Jiménez, C.; Garcia-Moreno, F.; Pfretzschner, B.; Klaus, M.; Wollgarten, M.; Zizak, I.; Schumacher, G.; Tovar, M.; Banhart, J. Decomposition of TiH2 studied in situ by synchrotron X-ray and neutron diffraction. Acta Mater. 2011, 59, 6318–6330. [Google Scholar] [CrossRef]
- Matijasevic, B.; Banhart, J. Improvement of aluminium foam technology by tailoring of blowing agent. Scr. Mater. 2006, 54, 503–508. [Google Scholar] [CrossRef]
- Jiménez, C.; Garcia-Moreno, F.; Pfretzschner, B.; Kamm, P.H.; Neu, T.R.; Klaus, M.; Genzel, C.; Hilger, A.; Manke, I.; Banhart, J. Metal foaming studied in situ by energy dispersive X-ray diffraction of synchrotron radiation, X-ray radioscopy, and optical expandometry. Adv. Eng. Mater. 2013, 15, 141–148. [Google Scholar] [CrossRef]
- Weigand, P. Untersuchung Der Einflußfaktoren Auf Die Pulvermetallurgische Herstellung Von Aluminiumschäumen; Verl. MIT: Bremen, Germany, 1999. [Google Scholar]
- Field, D.J.; Scamans, G.M.; Butler, E.P. The high temperature oxidation of Al-4.2 Wt Pct Mg alloy. Metall. Trans. A 1987, 18, 463–472. [Google Scholar]
- Kahl, W.; Fromm, E. Examination of the Strength of Oxide Skins on Aluminum Alloy Melts. Metall. Trans. B 1985, 16, 47–51. [Google Scholar] [CrossRef]
- Dudka, A.; García-Moreno, F.; Wanderka, N.; Banhart, J. Structure and distribution of oxides in aluminium foam. Acta Mater. 2008, 56, 3990–4001. [Google Scholar] [CrossRef]
- Drouzy, M.; Mascré, C. The oxidation of liquid non-ferrous metals in air or oxygen. Int. Mater. Rev. 1969, 14, 25–46. [Google Scholar] [CrossRef]
- Simančík, F.; Behulová, K.; Borš, L. Effect of ambient atmosphere on the foam expansion. In Cellular Metals and Metal Foaming Technology; Banhart, J., Ashby, M., Fleck, N., Eds.; MIT Publishing: Bremen, Germany, 2001; pp. 89–92. [Google Scholar]
- Jiménez, C. Characterization and Modification of Powders Used to Make Aluminium-Based Metal Foams. Ph.D. Thesis, Technische Universität, Berlin, Germany, 2010. [Google Scholar]
- Körner, C.; Arnold, M.; Singer, R.F. Metal foam stabilization by oxide network particles. Mater. Sci. Eng. A 2005, 396, 28–40. [Google Scholar] [CrossRef]
- Lukas, H.L.; Lebrun, N. Aluminium–Copper–Silicon. Landolt-Börnstein Group IV Phys. Chem. 2003, 11, 135–147. [Google Scholar]
- Guo, Z.Q.; Ma, D.H.; Yuan, X.G.; Dong, X. Effect of Mg Addition on the Foaming Behaviour of AlSi7 Based Alloy Prepared by Powder Metallurgy Method. Rare Met. Mater. Eng. 2016, 45, 3068–3073. [Google Scholar]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neu, T.R.; Pfretzschner, B.; García-Moreno, F.; Banhart, J. Influence of the Heating Rate on the Foaming Behavior of Various Aluminium Alloys. Metals 2017, 7, 323. https://doi.org/10.3390/met7090323
Neu TR, Pfretzschner B, García-Moreno F, Banhart J. Influence of the Heating Rate on the Foaming Behavior of Various Aluminium Alloys. Metals. 2017; 7(9):323. https://doi.org/10.3390/met7090323
Chicago/Turabian StyleNeu, Tillmann R., Beate Pfretzschner, Francisco García-Moreno, and John Banhart. 2017. "Influence of the Heating Rate on the Foaming Behavior of Various Aluminium Alloys" Metals 7, no. 9: 323. https://doi.org/10.3390/met7090323





