Ceramic Materials in a Ti–C–Co–Ca3(PO4)2–Ag–Mg System Obtained by MA SHS for the Deposition of Biomedical Coatings
Abstract
:1. Introduction
2. Materials and Methods
3. Research Results and Discussion
4. Conclusions
- The study has been conducted on the influence of mechanical activation on the structural and phase transformations in the reaction mixtures in a Ti–C–Co–Ca3(PO4)2–Ag–Mg system with the content of Ag amounting to 4 and 8 at. % as well as the content of Mg amounting to 6 and 12 at. % with the MA duration varying from 0 to 11 min. It has been shown that, during the MA, the initial components undergo plastic deformation, there is a formation of lamellar composite granules, a decrease in the coherent scattering regions, a formation of an AgMg intermetallic, and a gradual increase of its content in the mixture.
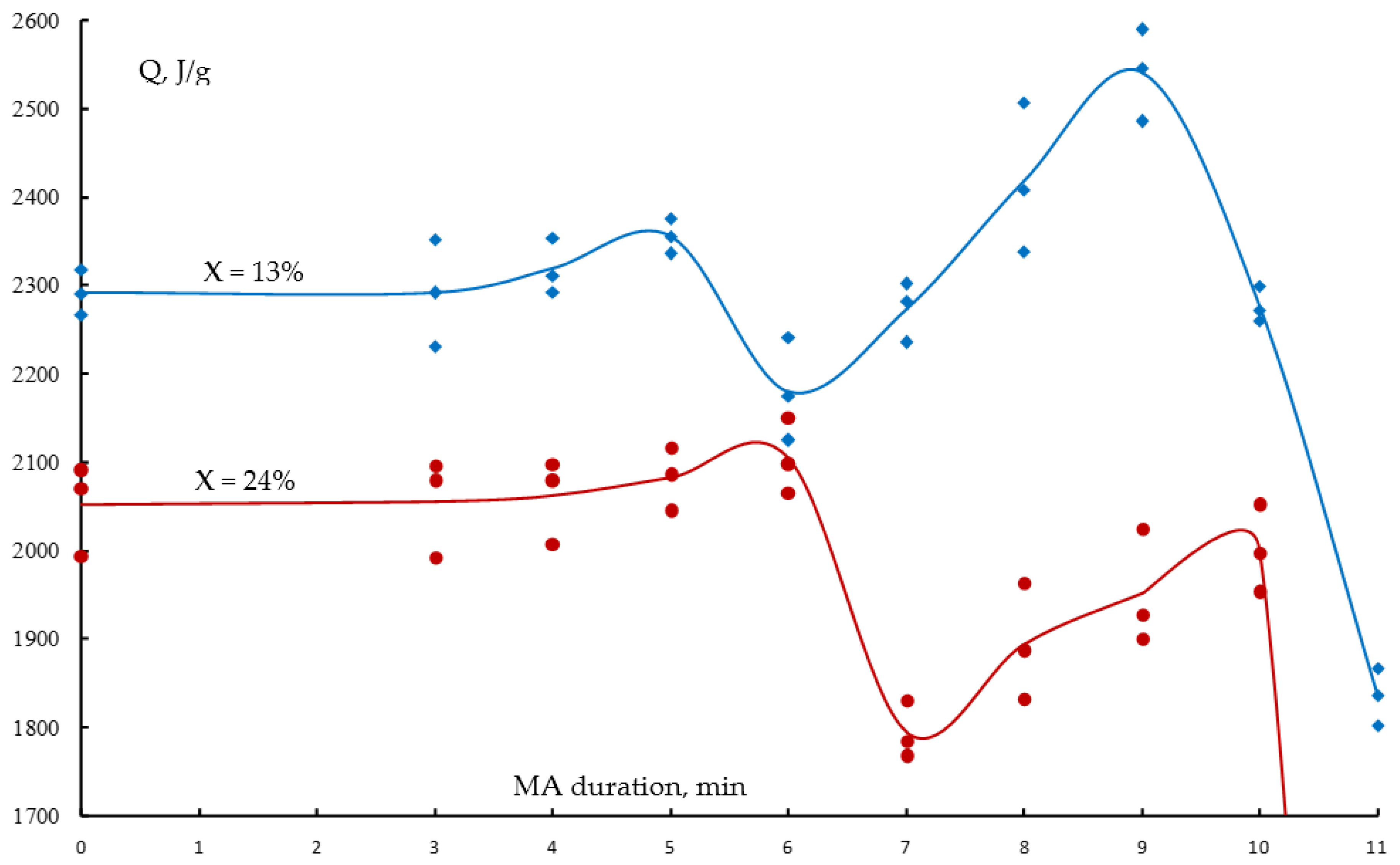
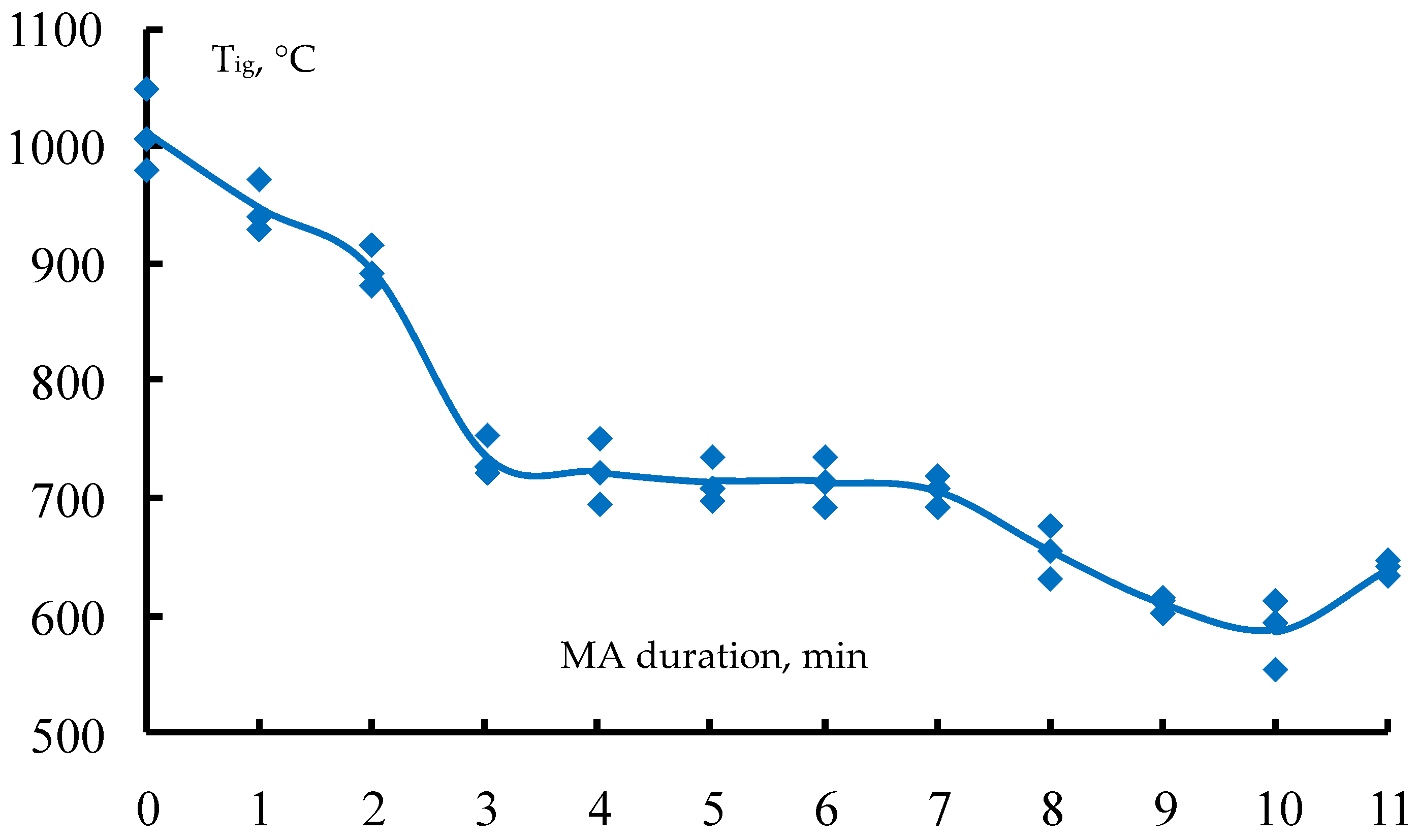
- The influence of the MA duration on the ignition temperature and heat generation during the combustion of the reaction mixtures has been investigated. The dependencies are extreme: when the MA duration increases, the starting temperature of the reaction decreases and the reaction mixture’s calorific value rises due to an accumulation of macro- and microdefects in the initial powders, and then there is a small increase of the initiation temperature and a decrease of the specific heat generation due to a partial formation of the synthesis products in the mill drum.
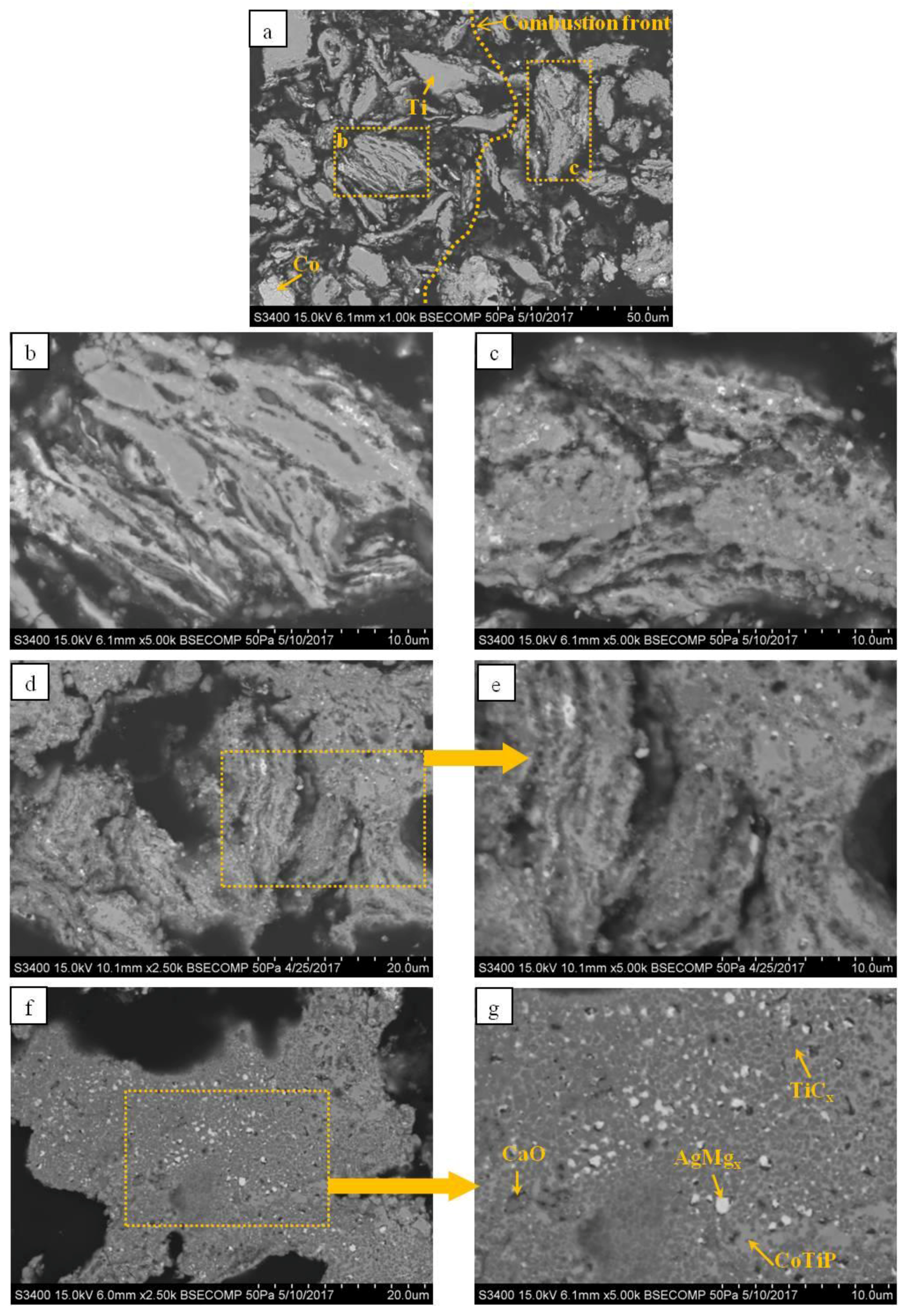
- Ceramic materials with a Co-based metallic binder have been obtained from the MA Ti–C–Co–Ca3(PO4)2–Ag–Mg mixtures using force SHS-pressing technology. The compact ceramics consist of a bound framework of nonstoichiometric TiC0.5–TiC0.6, with phases of intermetallics (TiCo and TiCo2) which are evenly distributed over the grain boundaries as well as the complex phosphide CoTiP. The introduction of Ag and Mg has resulted in the formation of a silver-based solid solution phase.
- Electrodes for pulsed electrospark deposition of biocompatible and bioactive coatings, including ones with an antibacterial effect, have been made from the activated Ti–C–Co–Ca3(PO4)2–Ag–Mg reaction mixtures.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agarwal, R.; García, A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015, 94, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Williams, R.; Aojula, A.; O’Neill, J.; Trzińscka, Z.; Grover, L.; Scott, R.A.H.; Peacock, A.F.A.; Logan, A.; Stamboulis, A.; et al. Peptide aptamers: Novel coatings for orthopaedic implants. Mater. Sci. Eng. C 2015, 54, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Ho, S.C.; Chang, C.H.; Chen, C.C.; Say, W.C. Influence of roughness on in-vivo properties of titanium implant surface and their electrochemical behavior. Surf. Coat. Technol. 2016, 302, 215–226. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Batenina, I.V.; Yadroitsev, I.A.; Ryashin, N.S.; Kiryukhantsev-Korneev, P.V.; Kudryashov, A.E.; Sheveyko, A.N.; Zhitnyak, I.Y.; Gloushankova, N.A.; Smurov, I.Y.; et al. A new combined approach to metal-ceramic implants with controllable surface topography, chemistry, blind porosity, and wettability. Surf. Coat. Technol. 2012, 208, 14–23. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Batenina, I.V.; Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Kuptsov, K.A.; Zhitnyak, I.Y.; Anisimova, N.Y.; Gloushankova, N.A. Ag-and Cu-doped multifunctional bioactive nanostructured TiCaPCON films. Appl. Surf. Sci. 2013, 285, 331–343. [Google Scholar] [CrossRef]
- Sánchez-López, J.C.; Abad, M.D.; Carvalho, I.; Galindo, R.E.; Benito, N.; Ribeiro, S.; Henriques, M.; Cavaleiro, A.; Carvalho, S. Influence of silver content on the tribomechanical behavior on Ag–TiCN bioactive coatings. Surf. Coat. Technol. 2012, 206, 2192–2198. [Google Scholar] [CrossRef] [Green Version]
- Litovchenko, N.V.; Potanin, A.Y.; Zamulaeva, E.I.; Sukhorukova, I.V.; Pogozhev, Y.S.; Gloushankova, N.A.; Ignatov, S.G.; Levashov, E.A.; Shtansky, D.V. Combustion synthesis of Ti–C–Co–Ca3(PO4)2-Ag-Mg electrodes and their utilization for pulsed electrospark deposition of bioactive coatings having an antibacterial effect. Surf. Coat. Technol. 2017, 309, 75–85. [Google Scholar] [CrossRef]
- Feng, H.; Wang, G.; Wu, G.; Jin, W.; Wu, H.; Chu, P.K. Plasma and ion-beam modification of metallic biomaterials for improved anti-bacterial properties. Surf. Coat. Technol. 2016, 306, 140–146. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Ivanov, E.; Boldyrev, V.V. The science and technology of mechanical alloying. Mater. Sci. Eng. A 2001, 304–306, 151–158. [Google Scholar] [CrossRef]
- Levashov, E.A.; Kurbatkina, V.V.; Rogachev, A.S.; Kochetov, N.A. Mechanoactivation of SHS system and processes. Int. J. SHS 2007, 16, 46–50. [Google Scholar] [CrossRef]
- Potanin, A.Y.; Zvyagintseva, N.V.; Pogozhev, Y.S.; Levashov, E.A.; Rupasov, S.I.; Shtansky, D.V.; Kochetov, N.A.; Kovalev, D.Y. Silicon carbide ceramics SHS-produced from mechanoactivated Si–C–B mixtures. Int. J. SHS 2015, 24, 119–127. [Google Scholar] [CrossRef]
- Eremina, E.N.; Kurbatkina, V.V.; Levashov, E.A.; Rogachev, A.S.; Kochetov, N.A. Obtaining the composite MoB material by means of force SHS compacting with preliminary mechanical activation of Mo–10%B mixture. Chem. Sustain. Dev. 2005, 13, 197–204. [Google Scholar]
- Patsera, E.I.; Kurbatkina, V.V.; Levashov, E.A.; Kochetov, N.A.; Rogachev, A.S.; Umarov, L.M. SHS in mechanically activated Cr–B and Ti–Cr–B blends: Role of gas-transport reactions. Int. J. SHS 2012, 21, 110–116. [Google Scholar] [CrossRef]
- Patsera, E.I.; Levashov, E.A.; Kurbatkina, V.V.; Kovalev, D.Y. Production of ultra-high temperature carbide (Ta,Zr)C by self-propagating high-temperature synthesis of mechanically activated mixtures. Ceram. Int. 2015, 41, 8885–8893. [Google Scholar] [CrossRef]
- Loginov, P.A.; Levashov, E.A.; Potanin, A.Y.; Kudryashov, A.E.; Manakova, O.S.; Shvyndina, N.V.; Sukhorukova, I.V. Sintered Ti–Ti3P–CaO electrodes and their application for pulsed electrospark treatment of titanium. Ceram. Int. 2016, 42, 7043–7053. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Shkodich, N.F.; Vadchenko, S.G.; Baras, F.; Kovalev, D.Y.; Rouvimov, S.; Nepapushev, A.A.; Mukasyan, A.S. Influence of the high energy ball milling on structure and reactivity of the Ni + Al powder mixture. J. Alloys Compd. 2013, 577, 600–605. [Google Scholar] [CrossRef]
- Loginov, P.A.; Levashov, E.A.; Kurbatkina, V.V.; Zaitsev, A.A.; Sidorenko, D.A. Evolution of the microstructure of Cu–Fe–Co–Ni powder mixtures upon mechanical alloying. Powder Technol. 2015, 276, 166–174. [Google Scholar] [CrossRef]
- Yoon, B.; Lee, S.H.; Lee, H. Low-temperature densification of nano Si–C powder containing Al–C additives prepared by high-energy ball-milling. Ceram. Int. 2017, 43, 12–19. [Google Scholar] [CrossRef]
- Liu, S.; Huang, Z.-L.; Liu, G.; Yang, G.-B. Preparing nano-crystalline rare earth doped WC/Co powder by high energy ball milling. Int. J. Refract. Met. Hard Mater. 2006, 24, 461–464. [Google Scholar]
- Zovas, P.E.; German, R.M.; Hwang, K.S.; Li, C.J. Activated and liquid-phase sintering–progress and problems. J. Met. 1983, 35, 28–33. [Google Scholar] [CrossRef]
- German, M.R.; Pavan, S.; Seong, J.P. Review: Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- Fu, Z.; Koc, R. Processing and characterization of TiB2–TiNiFeCrCoAl high-entropy alloy composite. J. Am. Ceram. Soc. 2017, 100, 2803–2813. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Shamanian, M. Synthesis of Mo5SiB2 based nanocomposites by mechanical alloying and subsequent heat treatment. Mater. Sci. Eng. A 2011, 528, 3295–3301. [Google Scholar] [CrossRef]
- Zakeri, M.; Ramezani, M. Synthesis of MoSi2–TiC nanocomposite powder via mechanical alloying and subsequent annealing. Ceram. Int. 2012, 38, 1353–1357. [Google Scholar] [CrossRef]
- Suryanarayana, C. Structure and properties of ultrafine-grained MoSi2 + Si3N4 composites synthesized by mechanical alloying. Mater. Sci. Eng. A 2008, 479, 23–30. [Google Scholar] [CrossRef]
- Sheveyko, A.N.; Manakova, O.S.; Zamulaeva, E.I.; Kudryashov, A.E.; Potanin, A.Y.; Sukhorukova, I.V.; Zhitnyak, I.Y.; Gloushankova, N.A.; Levashov, E.A.; Shtansky, D.V. Structural transformations in TiC–CaO–Ti3PO(x)–(Ag2Ca) electrodes and biocompatible TiCaPCO(N)–(Ag) coatings during pulsed electrospark deposition. Surf. Coat. Technol. 2016, 302, 327–335. [Google Scholar] [CrossRef]
- Levashov, E.A.; Pogozhev, Y.S.; Potanin, A.Y.; Kochetov, N.A.; Kovalev, D.Y.; Shvyndina, N.V.; Sviridova, T.A. Self-propagating high-temperature synthesis of advanced ceramics in the Mo-Si-B system: Kinetics and mechanism of combustion and structure formation. Ceram. Int. 2014, 40, 6541–6552. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Shelekhov, E.V.; Sviridova, T.A. Programs for X-ray analysis of polycrystals. Met. Sci. Heat Treat. 2000, 42, 309–313. [Google Scholar] [CrossRef]
- Urretavizcaya, G.; Chávez, A.C.S.; Castro, F.J. Hydrogen absorption and desorption in the Mg–Ag system. J. Alloys Compd. 2014, 611, 202–209. [Google Scholar] [CrossRef]
- Bae, J.; Ida, Y.; Sekine, K.; Kawano, F.; Hamada, K. Effects of high-energy ball-milling on injectability and strength of β-tricalcium-phosphate cement. J. Mech. Behav. Biomed. Mater. 2015, 47, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Aoki, K.; Himuro, Y.; Ohnuma, I.; Kainuma, R.; Ishida, K. Phase equilibria in the Co-Ti portion of the Co-Al-Ti ternary system. J. Phase Equilib. 2001, 22, 219–226. [Google Scholar] [CrossRef]
- Potanin, A.Y.; Levashov, E.A.; Pogozhev, Y.S.; Shvindina, N.V.; Kovalev, D.Y. The features of combustion and structure formation of ceramic materials in the TiC–Ti3POx–CaO system. Ceram. Int. 2015, 41, 8177–8185. [Google Scholar] [CrossRef]
- Zueva, L.V.; Gusev, A.I. Effect of nonstoichiometry and ordering on the period of the basis structure of cubic titanium carbide. Phys. Solid State 1999, 41, 1032–1038. [Google Scholar] [CrossRef]
- Levashov, E.A.; Kudryashov, A.E.; Pogozhev, Y.S.; Vakaev, P.V.; Sviridova, T.A.; Zamulaeva, E.I.; Milonich, S.; Todorovich, M. An investigation of the influence of the parameters of pulse discharges on mass transfer, structure, composition, and properties of TiC-NiAl-based electrical spark coatings modified by nanodispersed components. Russ. J. Non-Ferr. Met. 2004, 45, 32–40. [Google Scholar]
- Levashov, E.A.; Vakaev, P.V.; Zamulaeva, E.I.; Kudryashov, A.E.; Pogozhev, Y.S.; Shtansky, D.V.; Voevodin, A.A.; Sanz, A. Nanoparticle dispersion-strengthened coatings and electrode materials for electrospark deposition. Thin Solid Films 2006, 515, 1161–1165. [Google Scholar] [CrossRef]
- Levashov, E.A.; Kudryashov, A.E.; Pogozhev, Y.S.; Vakaev, P.V.; Zamulaeva, E.I.; Sviridova, T.A. Specific features of formation of nanostructured electrospark protective coatings on the OT4–1 titanium alloy with the use of electrode materials of the TiC-Ti3AlC2 system disperse-strengthened by nanoparticles. Russ. J. Non-Ferr. Met. 2007, 48, 368–378. [Google Scholar] [CrossRef]

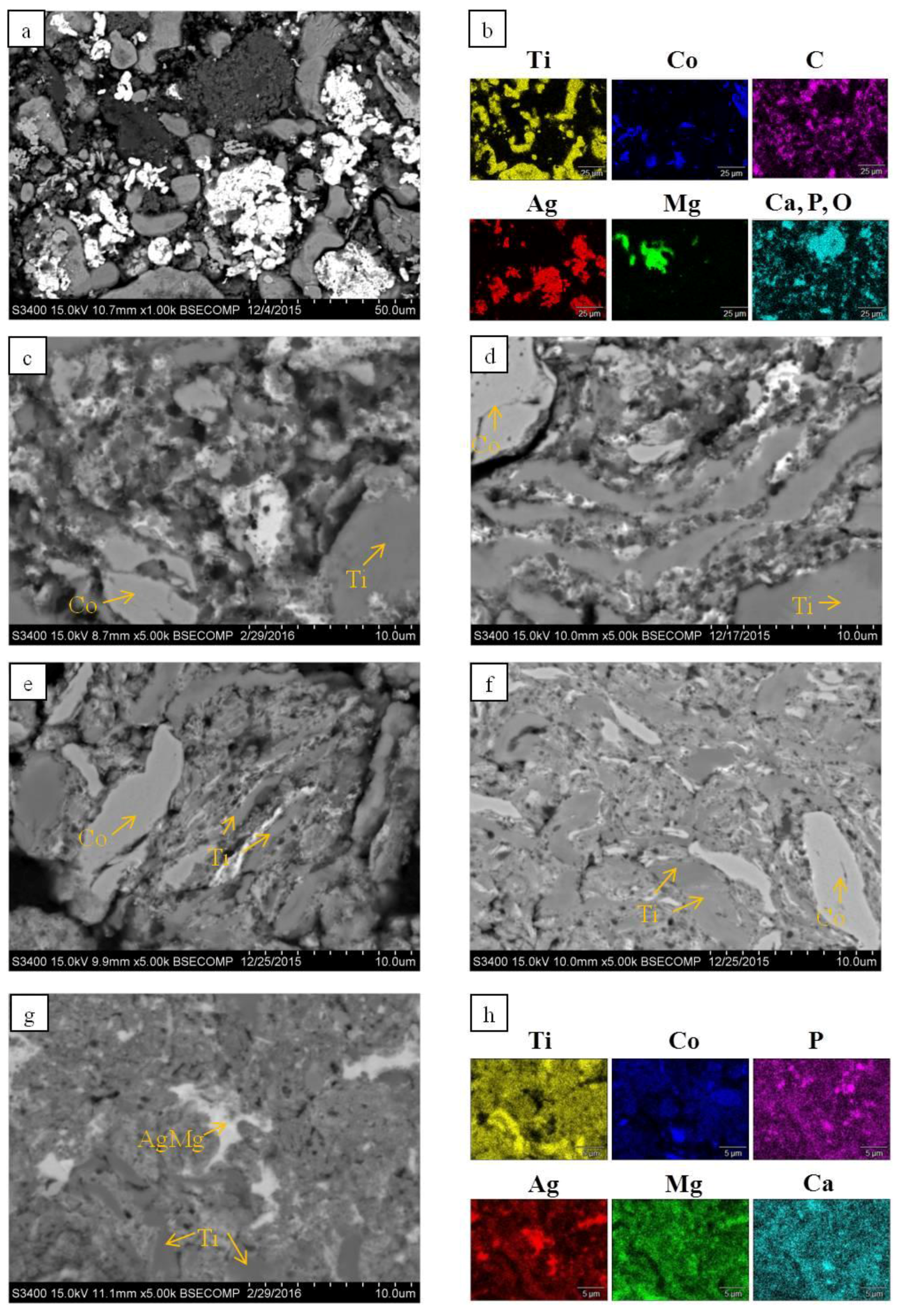
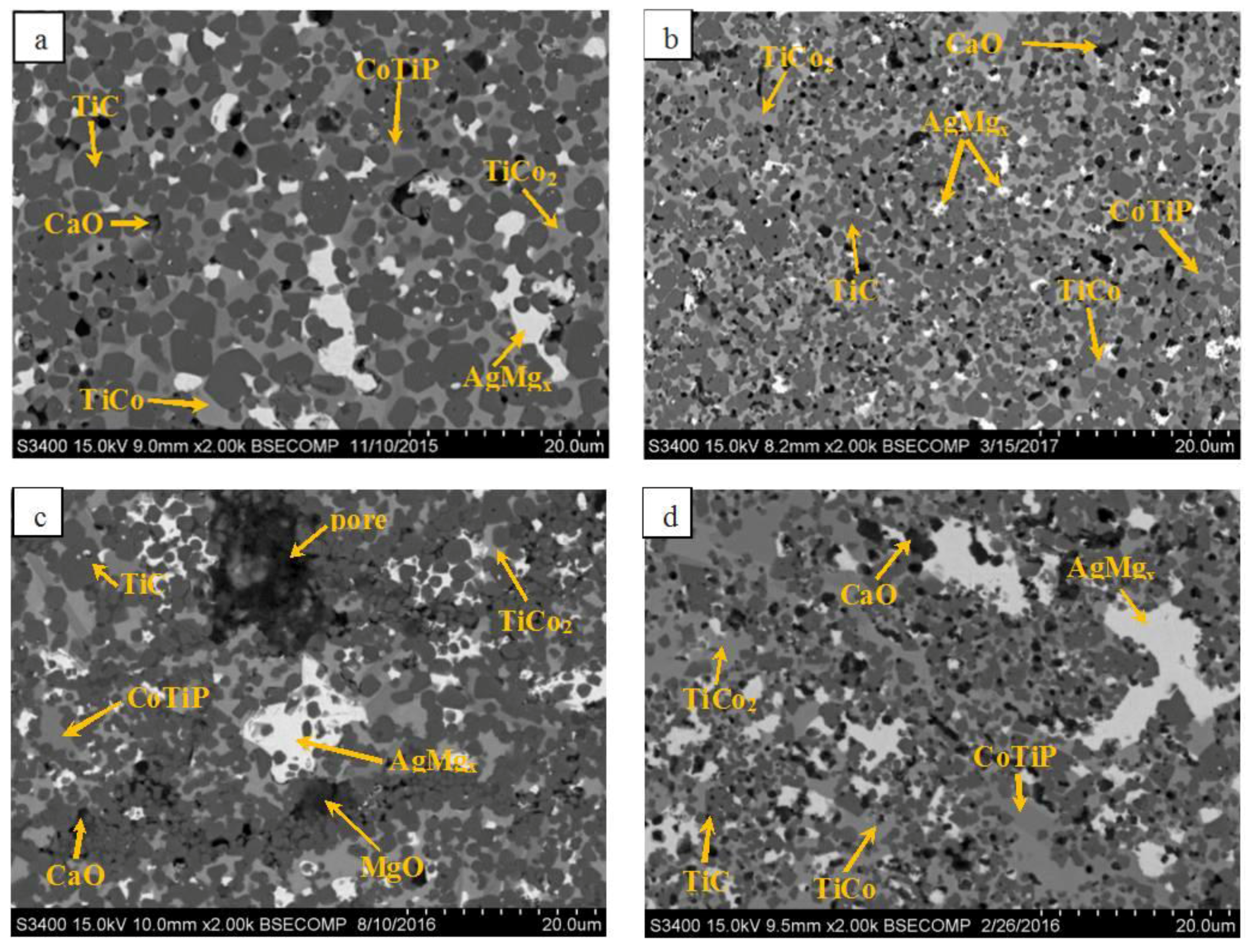
| Phase | α-Ti (hP2/1) | Ag (cF4/1) | TiH2 (cF12/1) | Mg (hP2/1) | Ca3(PO4)2 (hR92/2) | α-Co (hP2/1) | AgMg (cP2/1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MA Duration, min | wt. % | CSR, Å | ε, % | wt. % | CSR, Å | ε, % | wt. % | wt. % | wt. % | wt. % | wt. % |
| 3 | 39 | 551 ± 50 | 0.27 ± 0.03 | 23 | 232 ± 30 | 0.21 ± 0.02 | 5 | 7 | 11 | 15 | – |
| 5 | 49 | 420 ± 50 | 0.27 ± 0.03 | 19 | 166 ± 30 | 0.21 ± 0.02 | 4 | 3 | 9 | 12 | 4 |
| 6 | 55 | 405 ± 50 | 0.28 ± 0.03 | 16 | 166 ± 30 | 0.25 ± 0.03 | 4 | – | 9 | 11 | 5 |
| 7 | 56 | 374 ± 40 | 0.29 ± 0.03 | 12 | 155 ± 20 | 0.40 ± 0.04 | 5 | – | 6 | 8 | 13 |
| 9 | 59 | 366 ± 40 | 0.30 ± 0.03 | 11 | 152 ± 20 | 0.51 ± 0.05 | 5 | – | 5 | 7 | 13 |
| 10 | 61 | 364 ± 40 | 0.33 ± 0.03 | 8 | 142 ± 20 | 0.57 ± 0.06 | 4 | – | 6 | 3 | 18 |
| 11 | Mechanochemical synthesis (Refer to Figure 1c) | ||||||||||
| MA Duration, min | 5 | 7 | 9 | |||
|---|---|---|---|---|---|---|
| Phase (Pearson Symbol) | wt. % | Lattice Parameter, Å | wt. % | Lattice Parameter, Å | wt. % | Lattice Parameter, Å |
| TiC (cF8/2) | 49 | a = 4.311 | 43 | a = 4.309 | 44 | a = 4.310 |
| AgMgx (cF4/1) | 18 | a = 4.099 | 17 | a = 4.112 | 18 | a = 4.117 |
| CaO (cF8/2) | 6 | a = 4.803 | 7 | a = 4.805 | 6 | a = 4.807 |
| TiCo2 (cF24/1) | 5 | a = 6.682 | 12 | a = 6.708 | 11 | a = 6.711 |
| TiCo (cP2/1) | 4 | a = 2.981 | 13 | a = 2.969 | 13 | a = 2.968 |
| TiCo3 (cP4/2) | 10 | a = 3.618 | – | – | ||
| CoTiP (oP12/2) | 8 | – | 8 | – | 8 | – |
| Mixture Composition and Mixing Type | Phase (Pearson Symbol) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TiC (cF8/2) | TiCo (cP2/1) | TiCo2 (cF24/1) | CoTiP (oP12/2) | AgMgx (cF4/1) | CaO (cF8/2) | MgO (cF8/2) | ||||||||
| wt. % | Lattice Parameter, Å | wt. % | Lattice Parameter, Å | wt. % | Lattice Parameter, Å | wt. % | Lattice Parameter, Å | wt. % | Lattice Parameter, Å | wt. % | Lattice Parameter, Å | wt. % | Lattice Parameter, Å | |
| a (BRM) | 61 | a = 4.309 | 20 | a = 2.965 | – | 10 | a = 6.033 b = 3.557 c = 6.875 | 7 | a = 4.107 | 2 | a = 4.802 | – | ||
| b (5 min of MA) | 60 | a = 4.313 | 10 | a = 2.969 | 8 | a = 6.714 | 9 | a = 6.033 b = 3.562 c = 6.864 | 10 | a = 4.105 | 3 | a = 4.804 | – | |
| c (9 min of MA) | 50 | a = 4.313 | – | 18 | a = 6.699 | 6 | – | 15 | a = 4.104 | 6 | a = 4.801 | 5 | a = 4.213 | |
| d (5 min of MA) | 44 | a = 4.312 | 20 | a = 2.970 | 7 | a = 6.712 | 10 | a = 6.018 b = 3.549 c = 6.877 | 16 | a = 4.117 | 3 | a = 4.804 | – | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potanin, A.; Pogozhev, Y.; Novikov, A.; Levashov, E. Ceramic Materials in a Ti–C–Co–Ca3(PO4)2–Ag–Mg System Obtained by MA SHS for the Deposition of Biomedical Coatings. Metals 2017, 7, 378. https://doi.org/10.3390/met7090378
Potanin A, Pogozhev Y, Novikov A, Levashov E. Ceramic Materials in a Ti–C–Co–Ca3(PO4)2–Ag–Mg System Obtained by MA SHS for the Deposition of Biomedical Coatings. Metals. 2017; 7(9):378. https://doi.org/10.3390/met7090378
Chicago/Turabian StylePotanin, Artem, Yury Pogozhev, Alexander Novikov, and Evgeny Levashov. 2017. "Ceramic Materials in a Ti–C–Co–Ca3(PO4)2–Ag–Mg System Obtained by MA SHS for the Deposition of Biomedical Coatings" Metals 7, no. 9: 378. https://doi.org/10.3390/met7090378




