Effect of Carbon Partitioning, Carbide Precipitation, and Grain Size on Brittle Fracture of Ultra-High-Strength, Low-Carbon Steel after Welding by a Quenching and Partitioning Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
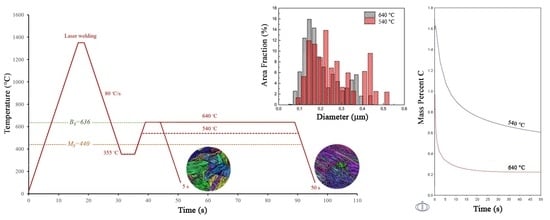
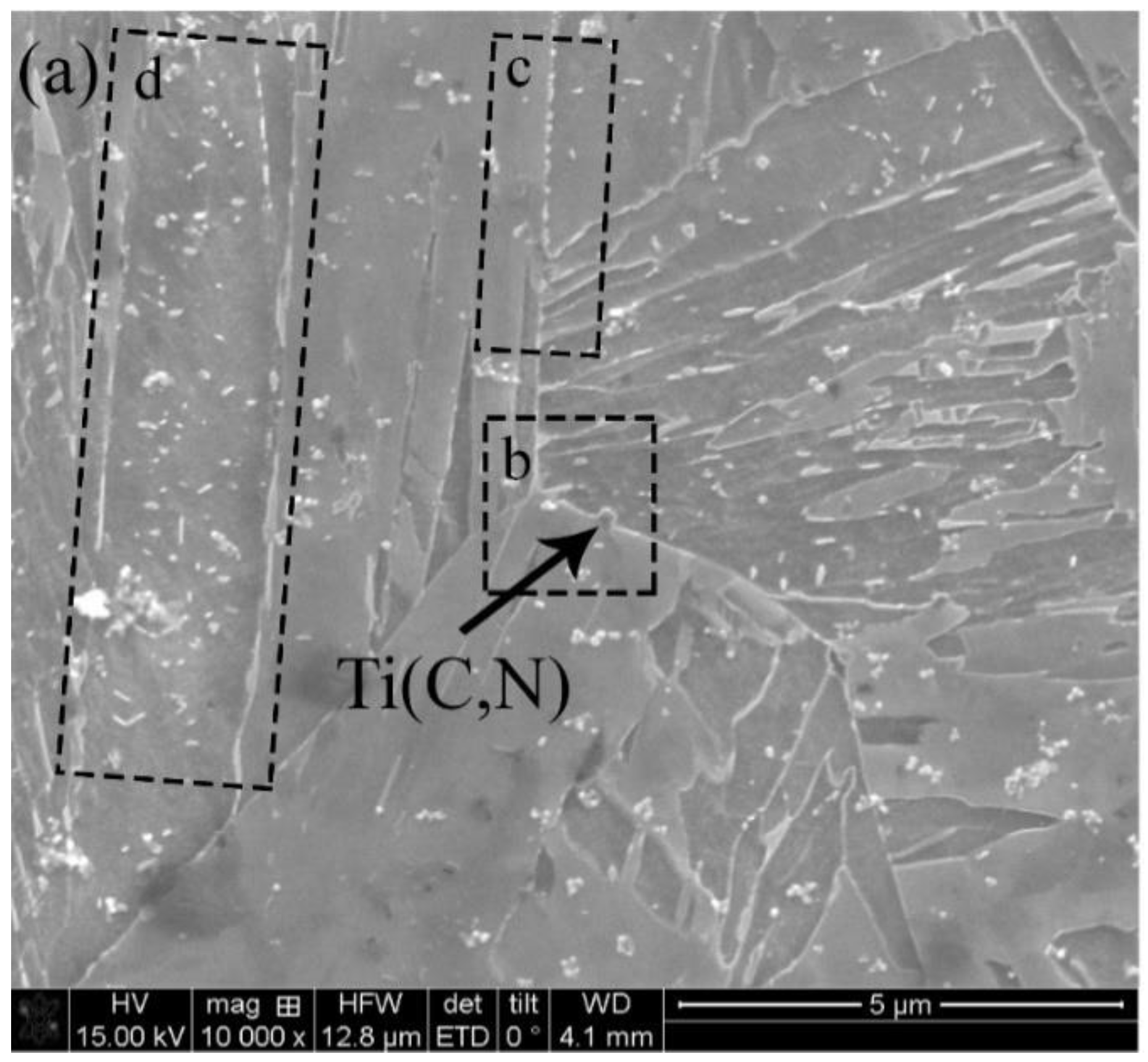
3.1. Fracture Results
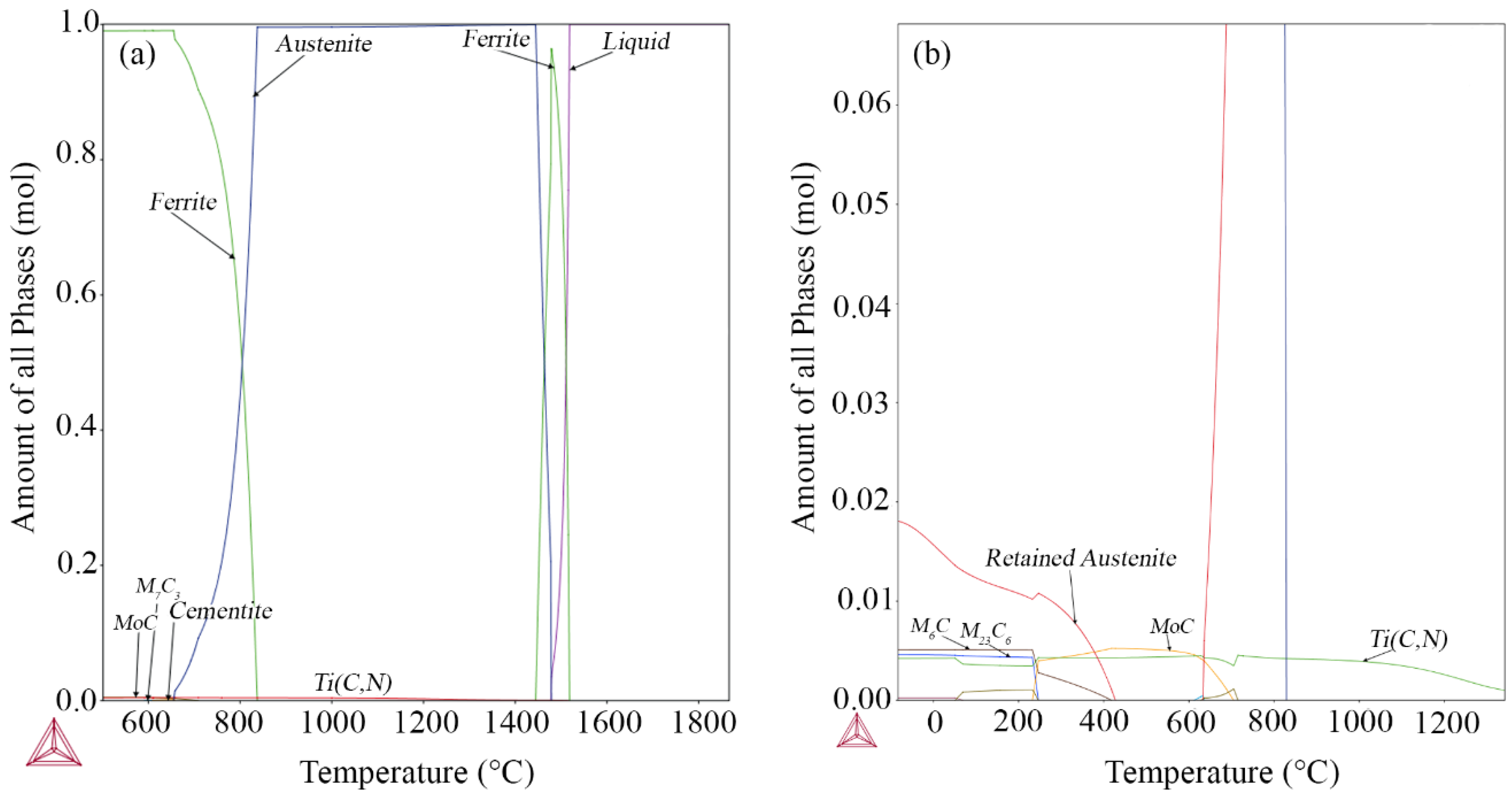
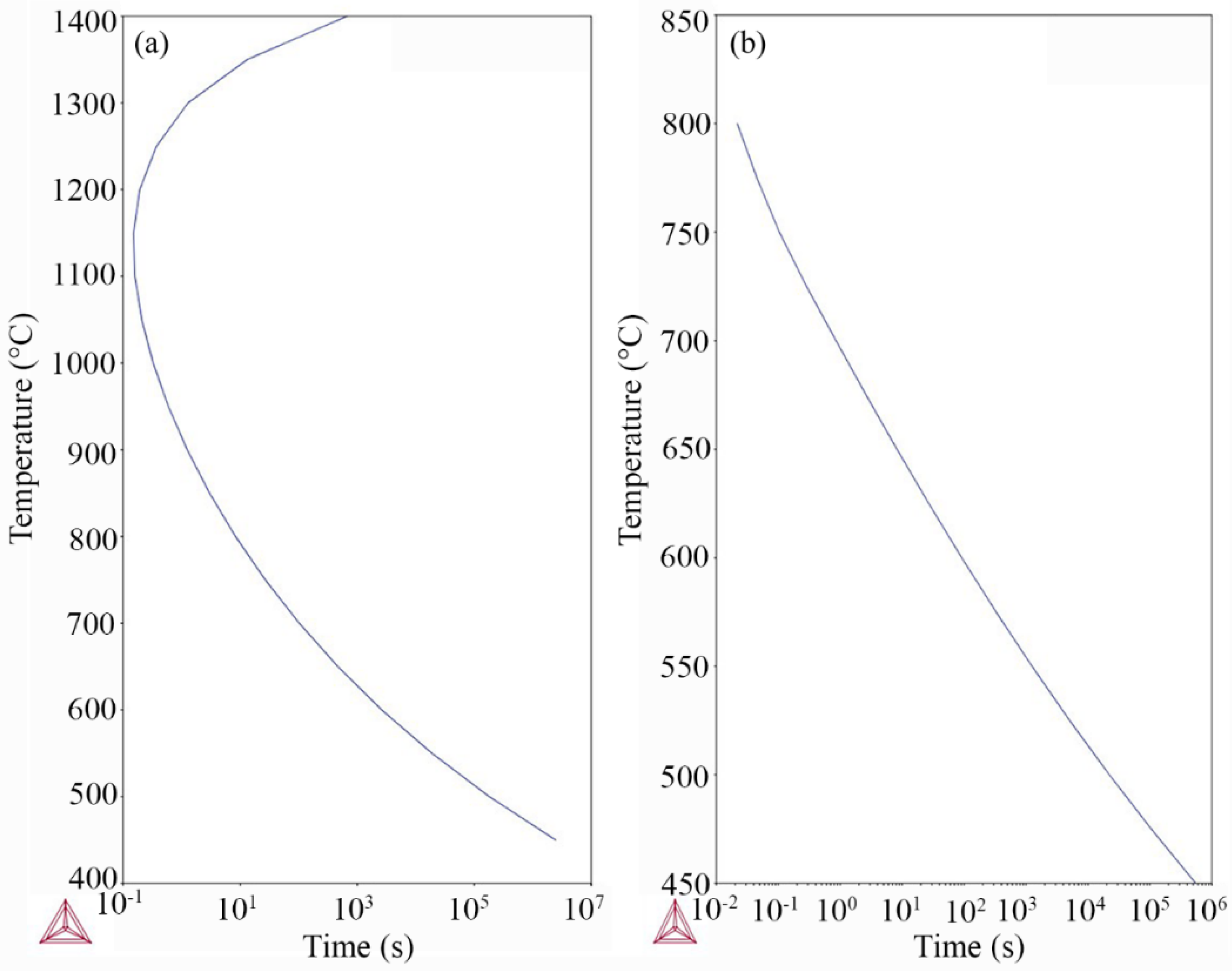
3.2. Thermodynamic Modeling
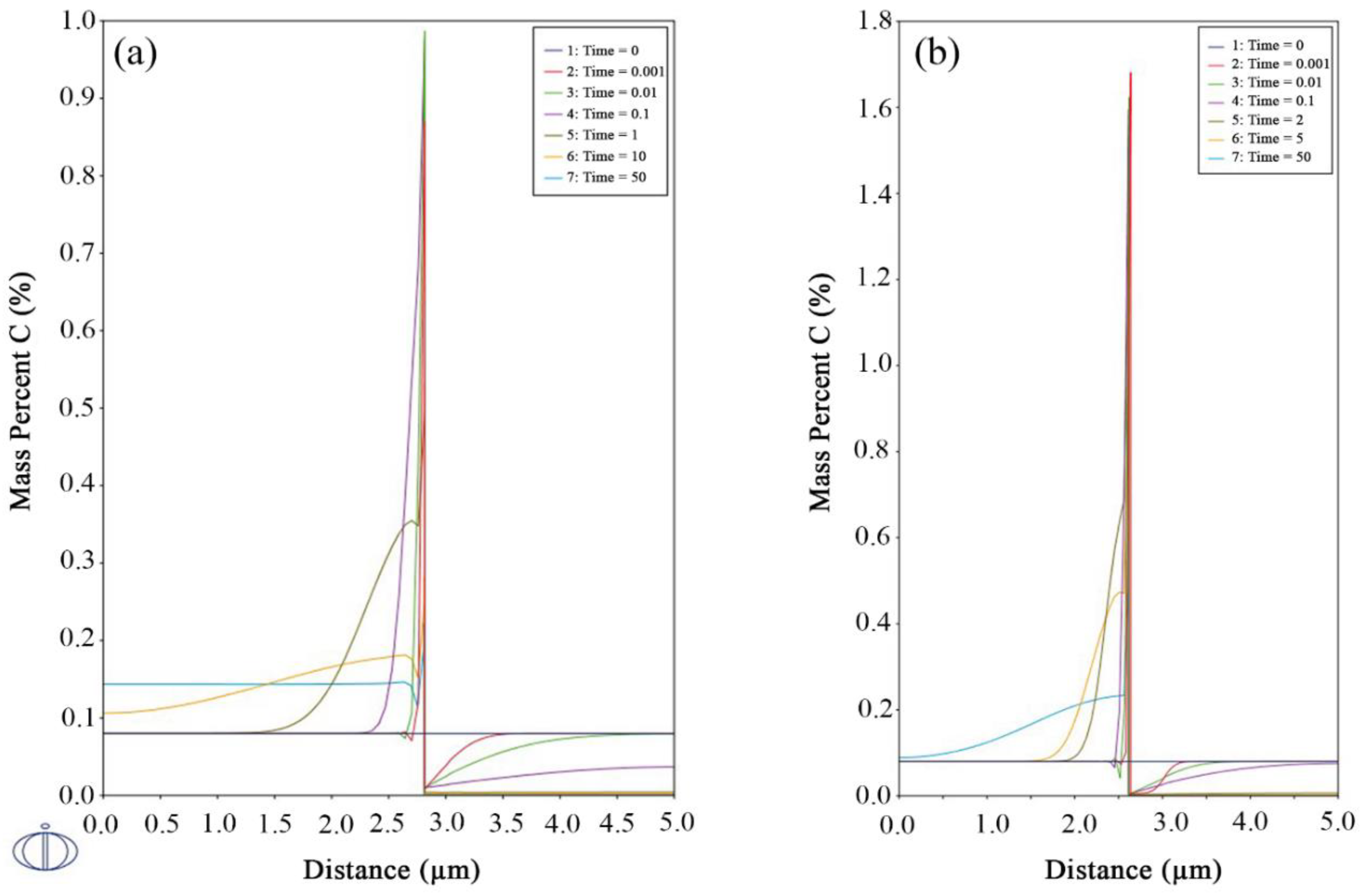
3.3. Carbon Partitioning
3.4. Kinetics of Precipitation
3.5. Grain Size Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Opbroek, E. UltraLight Steel: A Global Consortium Changes the Future of Automotive Steel; Xlibris: Bloomington, IN, USA, 2013; ISBN 1479773441. [Google Scholar]
- Shome, M.; Tumuluru, M. Welding and Joining of Advanced High Strength Steels (AHSS), 1st ed.; Woodhead Publishing: Cambridge, UK, 2015; ISBN 9780857098580. [Google Scholar]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Forouzan, F.; Vuorinen, E.; Mücklich, F. Post weld–treatment of laser welded AHSS by application of quenching and partitioning technique. Mater. Sci. Eng. A 2017, 698, 174–182. [Google Scholar] [CrossRef]
- Clarke, A.J.; Speer, J.G.; Miller, M.K.; Hackenberg, R.E.; Edmonds, D.V.; Matlock, D.K.; Rizzo, F.C.; Clarke, K.D.; De Moor, E. Carbon partitioning to austenite from martensite or bainite during the quench and partition (Q&P) process: A critical assessment. Acta Mater. 2008, 56, 16–22. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Sietsma, J. Microstructural Evolution of a Low–Carbon Steel during Application of Quenching and Partitioning Heat Treatments after Partial Austenitization. Metall. Mater. Trans. A 2009, 40, 46–57. [Google Scholar] [CrossRef]
- Toji, Y.; Miyamoto, G.; Raabe, D. Carbon partitioning during quenching and partitioning heat treatment accompanied by carbide precipitation. Acta Mater. 2015, 86, 137–147. [Google Scholar] [CrossRef]
- Charleux, M.; Poole, W.J.; Militzer, M.; Deschamps, A. Precipitation behavior and its effect on strengthening of an HSLA–Nb/Ti steel. Metall. Mater. Trans. A 2001, 32, 1635–1647. [Google Scholar] [CrossRef]
- Soto, R.; Saikaly, W.; Bano, X.; Issartel, C.; Rigaut, G.; Charai, A. Statistical and theoretical analysis of precipitates in dual–phase steels microalloyed with titanium and their effect on mechanical properties. Acta Mater. 1999, 47, 3475–3481. [Google Scholar] [CrossRef]
- Toji, Y.; Matsuda, H.; Herbig, M.; Choi, P.; Raabe, D. Atomic–scale analysis of carbon partitioning between martensite and austenite by atom probe tomography and correlative transmission electron microscopy. Acta Mater. 2014, 65, 215–228. [Google Scholar] [CrossRef]
- Fairchild, D.P.; Howden, D.G.; Clark, W.A.T. The mechanism of brittle fracture in a microalloyed steel: Part I. Inclusion–induced cleavage. Metall. Mater. Trans. A 2000, 31, 641–652. [Google Scholar] [CrossRef]
- Di Schino, A.; Di Nunzio, P.E. Effect of Nb microalloying on the heat affected zone microstructure of girth welded joints. Mater. Lett. 2017, 186, 86–89. [Google Scholar] [CrossRef]
- HajyAkbary, F.; Sietsma, J.; Miyamoto, G.; Furuhara, T.; Santofimia, M.J. Interaction of carbon partitioning, carbide precipitation and bainite formation during the Q&P process in a low C steel. Acta Mater. 2016, 104, 72–83. [Google Scholar] [CrossRef]
- Clarke, A.J.; Speer, J.G.; Matlock, D.K.; Rizzo, F.C.; Edmonds, D.V.; Santofimia, M.J. Influence of carbon partitioning kinetics on final austenite fraction during quenching and partitioning. Scr. Mater. 2009, 61, 149–152. [Google Scholar] [CrossRef] [Green Version]
- Somani, M.C.; Porter, D.A.; Karjalainen, L.P.; Misra, R.D.K. On Various Aspects of Decomposition of Austenite in a High–Silicon Steel During Quenching and Partitioning. Metall. Mater. Trans. A 2014, 45, 1247–1257. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Sietsma, J. Overview of Mechanisms Involved During the Quenching and Partitioning Process in Steels. Metall. Mater. Trans. A 2011, 42, 3620–3626. [Google Scholar] [CrossRef]
- Thomas, G.A.; Speer, J.G. Interface migration during partitioning of Q&P Steel. Mater. Sci. Technol. 2014, 30, 998–1007. [Google Scholar] [CrossRef]
- De Knijf, D.; Petrov, R.; Föjer, C.; Kestens, L.A. Effect of fresh martensite on the stability of retained austenite in quenching and partitioning steel. Mater. Sci. Eng. A 2014, 615, 107–115. [Google Scholar] [CrossRef]
- De Knijf, D.; Puype, A.; Föjer, C.; Petrov, R. The influence of ultra–fast annealing prior to quenching and partitioning on the microstructure and mechanical properties. Mater. Sci. Eng. A 2015, 627, 182–190. [Google Scholar] [CrossRef]
- De Diego-Calderón, I.; Sabirov, I.; Molina-Aldareguia, J.M.; Föjer, C.; Thiessen, R.; Petrov, R.H. Microstructural design in quenched and partitioned (Q&P) steels to improve their fracture properties. Mater. Sci. Eng. A 2016, 657, 136–146. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Shi, J.; Hui, W.; Dong, H. Effect of microstructural refinement on the toughness of low carbon martensitic steel. Scr. Mater. 2008, 58, 492–495. [Google Scholar] [CrossRef]
- Forouzan, F.; Gunasekaran, S.; Hedayati, A.; Vuorinen, E.; Mucklich, F. Microstructure analysis and mechanical properties of Low alloy High strength Quenched and Partitioned Steel. DiVA 2016, 258, 574–578. [Google Scholar] [CrossRef]
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.F.; Sundman, B. Thermo-Calc & DICTRA, Computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar]
- Thermo-Calc Software TCFE9 Steels/Fe-alloys Database. Available online: https://www.thermocalc.com/products-services/databases/thermodynamic/ (accessed on 20 September 2018).
- A.B. MOBFE3: TCS Steels/Fe–Alloys Mobility Database. Thermo–Calc Software. Available online: https://www.thermocalc.com/products-services/databases/mobility/ (accessed on 20 September 2018).
- Bhadeshia, H.; Honeycombe, R. Microstructure and properties. In Steels: Microstructure and Properties, 3rd ed.; Butterworth–Heinemann: Cambridge, UK, 2011; ISBN1 0080462928. ISBN2 9780080462929. [Google Scholar]
- Nishikawa, A.S.; Santofimia, M.J.; Sietsma, J.; Goldenstein, H. Influence of bainite reaction on the kinetics of carbon redistribution during the Quenching and Partitioning process. Acta Mater. 2018, 142, 142–151. [Google Scholar] [CrossRef]
- Payson, P.; Savage, C.H. Martensite reactions in alloy steels. Trans. ASM 1944, 33, 261–280. [Google Scholar]
- Grange, R.A.; Stewart, H.M. The temperature range of martensite formation. Trans. AIME 1946, 167, 467–501. [Google Scholar]
- Van Bohemen, S. Bainite and martensite start temperature calculated with exponential carbon dependence. Mater. Sci. Technol. 2012, 28, 487–495. [Google Scholar] [CrossRef]
- Nehrenberg, A.E. Contribution to Discussion on Grange and Stewart. Trans. Am. Inst. Min. Met. Eng. 1946, 167, 494–498. [Google Scholar]
- Haynes, A.G.; Steven, W. The temperature of formation of martensite and bainite in low–alloy steel. J. Iron Steel Inst. 1956, 183, 349–359. [Google Scholar]
- Andrews, K.W. Empirical formulae for the calculation of some transformation temperatures. J. Iron Steel Inst. 1965, 203, 721–727. [Google Scholar]
- Wu, R.; Li, J.; Li, W.; Wu, X.; Jin, X.; Zhou, S.; Wang, L. Effect of metastable austenite on fracture resistance of quenched and partitioned (Q&P) sheet steels. Mater. Sci. Eng. A 2016, 657, 57–63. [Google Scholar] [CrossRef]
- Gustafson, Å. Coarsening of TiC in austenitic stainless steel-experiments and simulations in comparison. Mater. Sci. Eng. A 2000, 287, 52–58. [Google Scholar] [CrossRef]
- Díaz–Fuentes, M.; Iza–Mendia, A.; Gutiérrez, I. Analysis of different acicular ferrite microstructures in low–carbon steels by electron backscattered diffraction. Study of their toughness behavior. Metall. Mater. Trans. A 2003, 34, 2505–2516. [Google Scholar] [CrossRef]
- Rodriguez–Ibabe, J. The Role of Microstructure in Toughness Behaviour of Microalloyed Steels. Mater. Sci. Forum. 1998, 284–286, 51–62. [Google Scholar] [CrossRef]
- Wang, M.; Hell, J.; Tasan, C.C. Martensite size effects on damage in quenching and partitioning steels. Scr. Mater. 2017, 138, 1–5. [Google Scholar] [CrossRef]
- Verbeken, K.; Barbé, L.; Raabe, D. Evaluation of the crystallographic orientation relationships between FCC and BCC phases in TRIP steels. ISIJ Int. 2009, 49, 1601–1609. [Google Scholar] [CrossRef]
- Morito, S.; Tanaka, H.; Konishi, R.; Furuhara, T.; Maki, T. The morphology and crystallography of lath martensite in Fe–C alloys. Acta Mater. 2003, 51, 1789–1799. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels. In Theory and Practice, 3rd ed.; Maney Publishing: Cambridge, UK, 2015; ISBN1 1909662747. ISBN2 9781909662742. [Google Scholar]
- Kawata, H.; Sakamoto, K.; Moritani, T.; Morito, S.; Furuhara, T.; Maki, T. Crystallography of ausformed upper bainite structure in Fe–9Ni–C alloys. Mater. Sci. Eng. A 2006, 438, 140–144. [Google Scholar] [CrossRef]







| C | Si | Mn | P | S | Al | Ti | Mo | Cr | Ni | Cu | V | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.082 | 0.23 | 1.79 | 0.008 | 0.001 | 0.038 | 0.184 | 0.503 | 0.064 | 0.296 | 0.016 | 0.012 | 0.004 | Balance |
| Sample Code | QT (°C) | PT (°C) | Pt (s) | A80 (%) | A5 (%) | Rp0.2 (MPa) | Rm (MPa) | Energy (J/cm2) |
|---|---|---|---|---|---|---|---|---|
| S(540,5) | 355 | 540 | 5 | 4 | 6 | 1005 | 1025 | 109 |
| S(640, 50) | 355 | 640 | 50 | 6 | 8 | 1034 | 1051 | 4 |
| Carbon Diffusion Coefficient | 440 °C | 540 °C | 640 °C |
|---|---|---|---|
| Dcγ | 3.30 × 10−16 | 7.12 × 10−14 | 2.82 × 10−21 |
| Dcα | 8.54 × 10−13 | 4.49 × 10−17 | 1.22 × 10−18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forouzan, F.; Guitar, M.A.; Vuorinen, E.; Mücklich, F. Effect of Carbon Partitioning, Carbide Precipitation, and Grain Size on Brittle Fracture of Ultra-High-Strength, Low-Carbon Steel after Welding by a Quenching and Partitioning Process. Metals 2018, 8, 747. https://doi.org/10.3390/met8100747
Forouzan F, Guitar MA, Vuorinen E, Mücklich F. Effect of Carbon Partitioning, Carbide Precipitation, and Grain Size on Brittle Fracture of Ultra-High-Strength, Low-Carbon Steel after Welding by a Quenching and Partitioning Process. Metals. 2018; 8(10):747. https://doi.org/10.3390/met8100747
Chicago/Turabian StyleForouzan, Farnoosh, M. Agustina Guitar, Esa Vuorinen, and Frank Mücklich. 2018. "Effect of Carbon Partitioning, Carbide Precipitation, and Grain Size on Brittle Fracture of Ultra-High-Strength, Low-Carbon Steel after Welding by a Quenching and Partitioning Process" Metals 8, no. 10: 747. https://doi.org/10.3390/met8100747