Microstructure and Mechanical Behavior of Al-Mg Composites Synthesized by Reactive Sintering
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
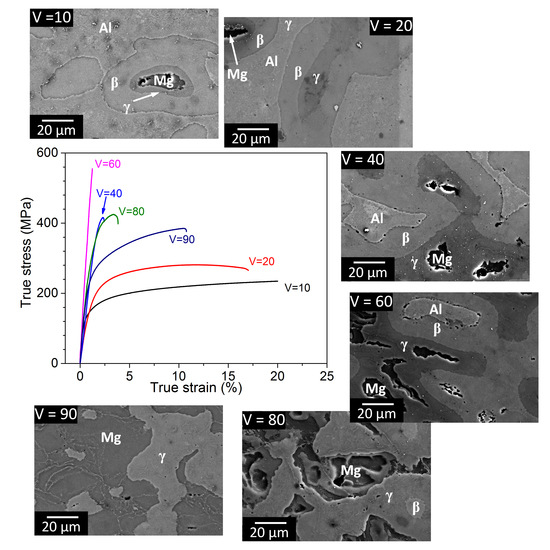
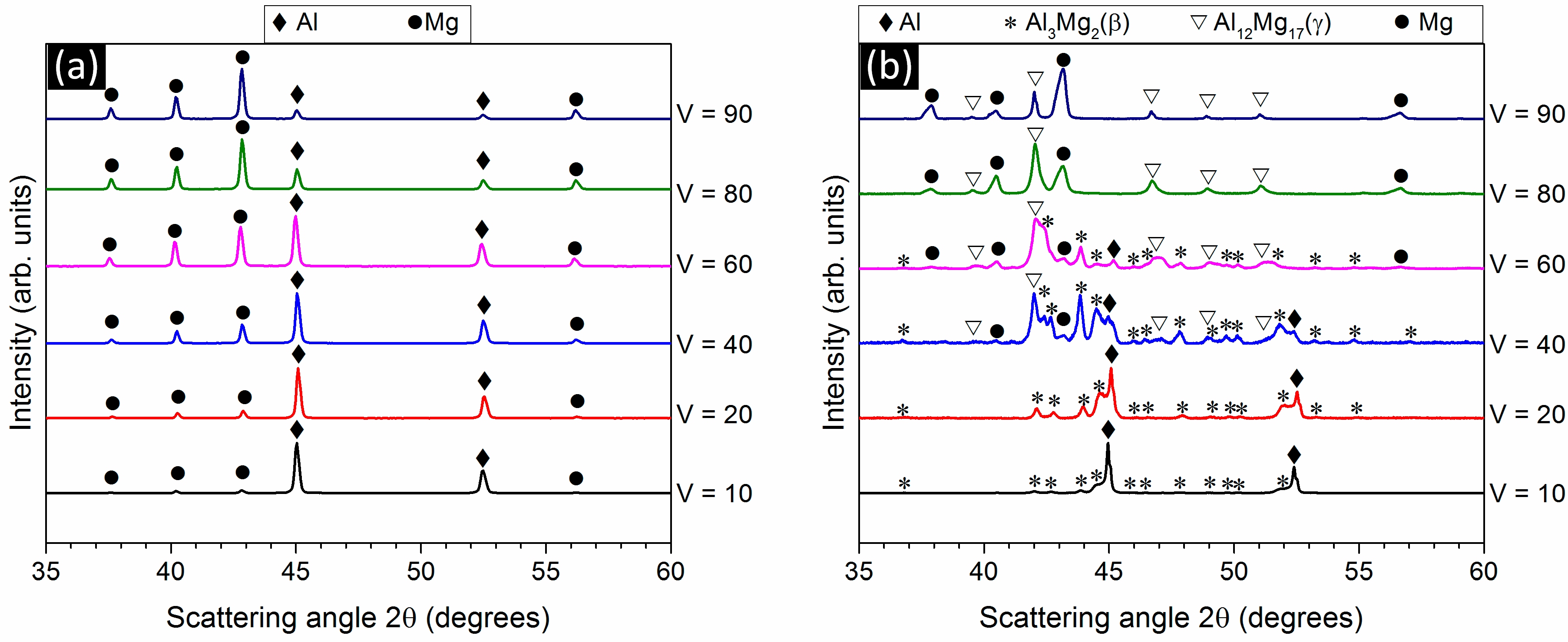
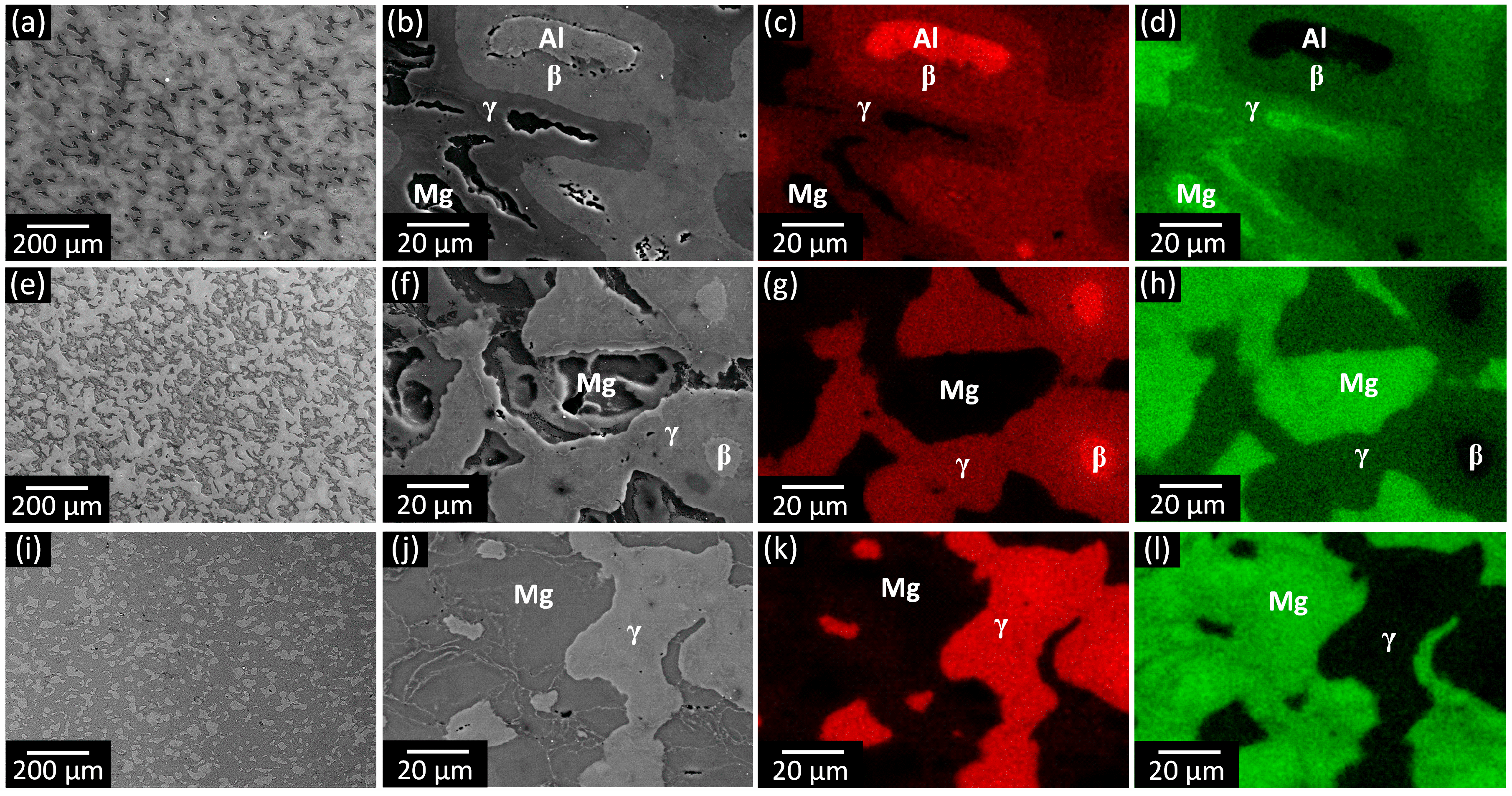
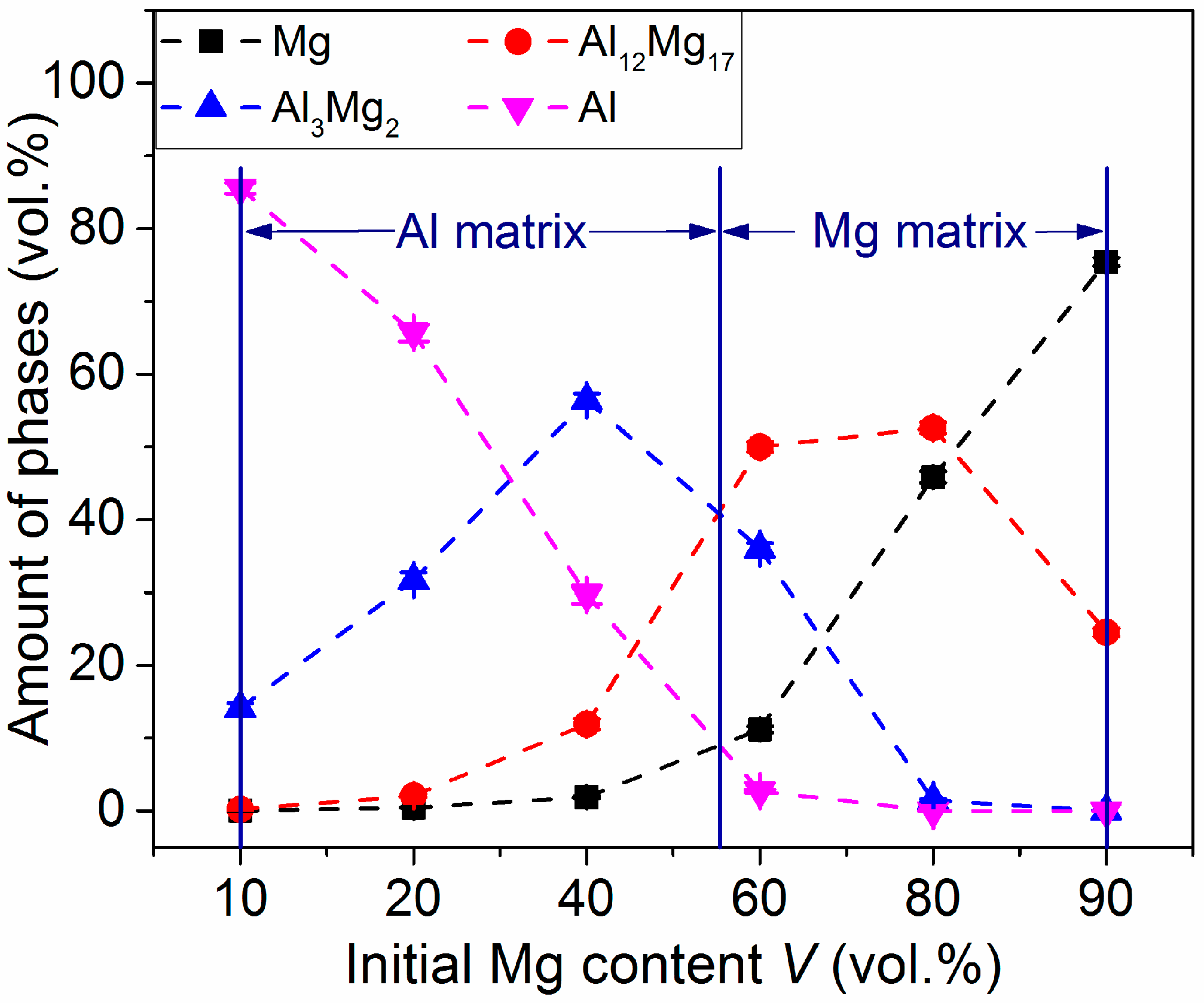
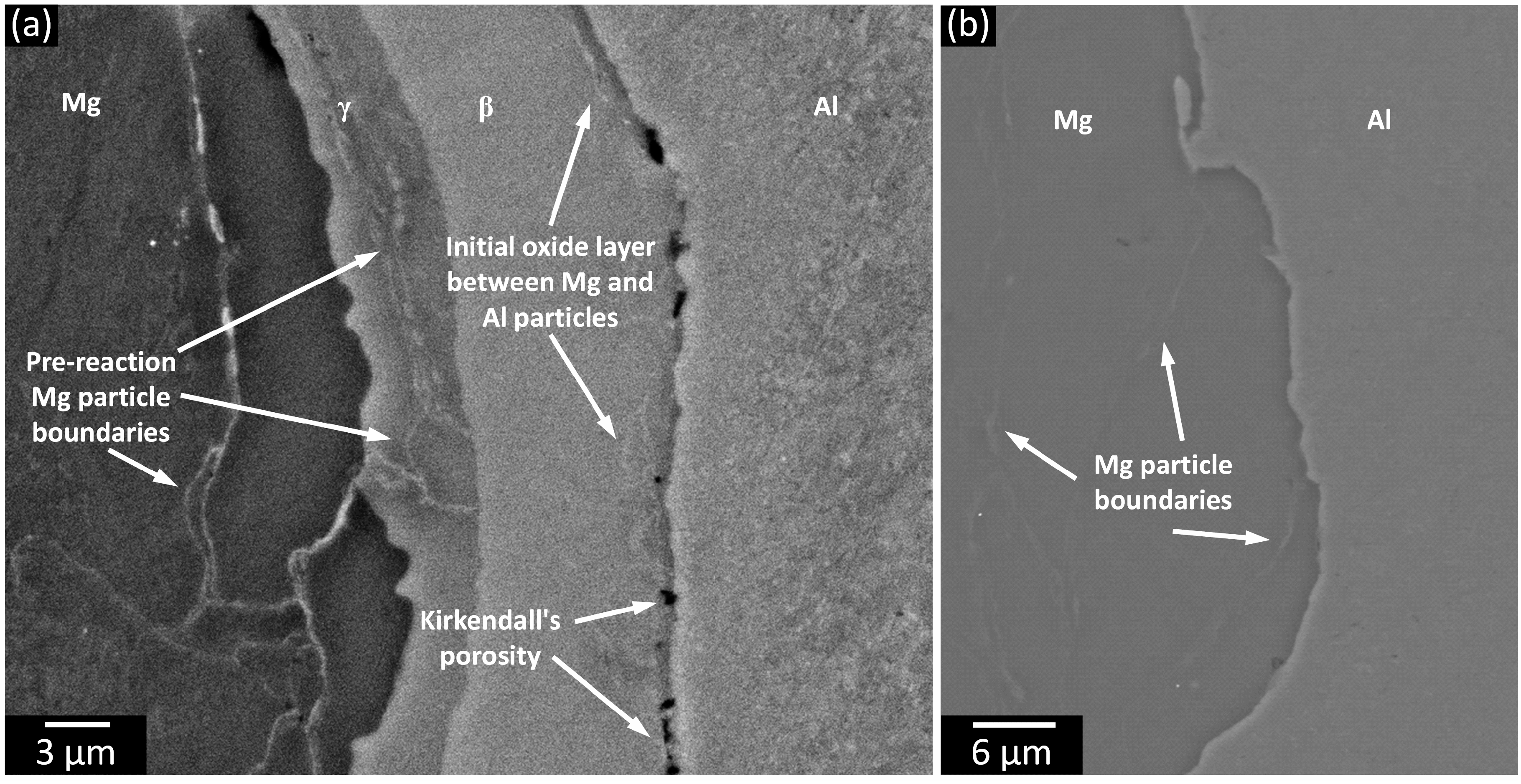
3.1. Phase Analysis and Microstructural Characterization
3.2. Phase Formation During the Reaction of Al and Mg
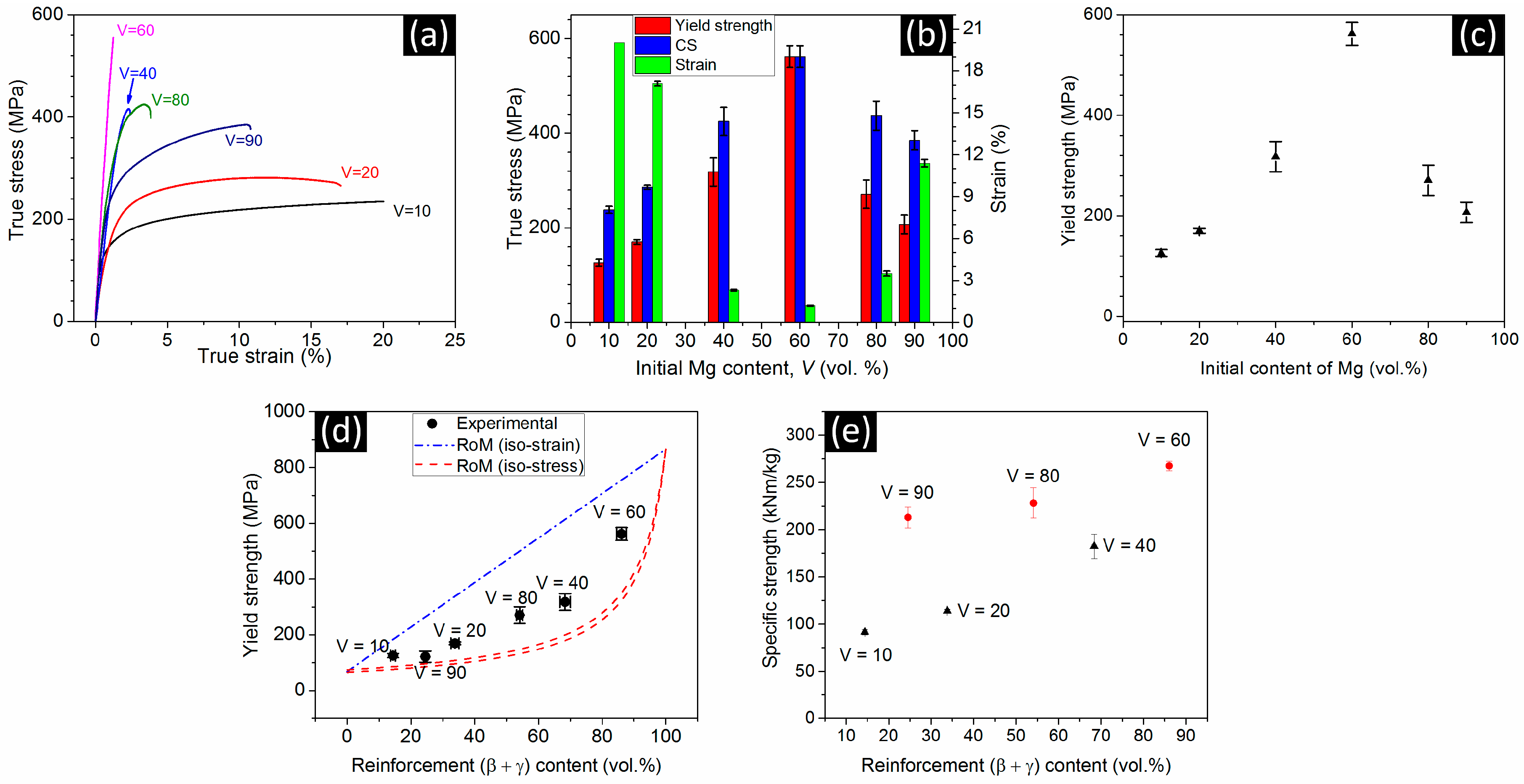
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allison, J.E.; Cole, G.S. Metal-matrix composites in the automotive industry: Opportunities and challenges. JOM 1993, 45, 19–24. [Google Scholar] [CrossRef]
- Immarigeon, J.P.; Holt, R.T.; Koul, A.K.; Zhao, L.; Wallace, W.; Beddoes, J.C. Lightweight materials for aircraft applications. Mater. Charact. 1995, 35, 41–67. [Google Scholar] [CrossRef]
- Jambor, A.; Beyer, M. New cars—New materials. Mater. Des. 1997, 18, 203–209. [Google Scholar] [CrossRef]
- Cole, G.S.; Sherman, A.M. Light weight materials for automotive applications. Mater. Charact. 1995, 35, 3–9. [Google Scholar] [CrossRef]
- González Palencia, J.C.; Furubayashi, T.; Nakata, T. Energy use and CO2 emissions reduction potential in passenger car fleet using zero emission vehicles and lightweight materials. Energy 2012, 48, 548–565. [Google Scholar] [CrossRef]
- Evans, A.G. Lightweight materials and structures. MRS Bull. 2013, 26, 790–797. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Alaneme, K.K.; Chown, L.H. Aluminium matrix hybrid composites: A review of reinforcement philosophies; mechanical, corrosion and tribological characteristics. J. Mater. Res. Technol. 2015, 4, 434–445. [Google Scholar] [CrossRef]
- Kainer, K.U. Metal Matrix Composites Custom-Made Materials for Automotive and Aerospace Engineering; Wiley: Weinheim, Germany, 2006. [Google Scholar]
- Lesuer, D.R.; Kipouros, G.J. Lightweight materials for transportation applications. JOM 1995, 47, 17. [Google Scholar] [CrossRef]
- Kaufman, J.G.; Rooy, E.L. Aluminum Alloy Castings: Properties, Processes, and Applications; ASM International: Materials Park, Novelty, OH, USA, 2004. [Google Scholar]
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondoh, K.; Umeda, J.; Fugetsu, B. Fabrication of magnesium based composites reinforced with carbon nanotubes having superior mechanical properties. Mater. Chem. Phys. 2011, 127, 451–458. [Google Scholar] [CrossRef]
- Chawla, N.; Chawla, K.K. Metal Matrix Composites; Springer: New York, NY, USA, 2006. [Google Scholar]
- Miracle, D. Metal matrix composites—From science to technological significance. Comp. Sci. Technol. 2005, 65, 2526–2540. [Google Scholar] [CrossRef]
- Chua, B.W.; Lu, L.; Lai, M.O. Deformation behaviour of ultrafine and nanosize-grained Mg alloy synthesized via mechanical alloying. Philos. Mag. 2006, 86, 2919–2939. [Google Scholar] [CrossRef]
- Han, B.Q.; Lavernia, E.J.; Mohamed, F.A.; Bampton, C.C. Improvement of toughness and ductility of a cryomilled Al-Mg alloy via microstructural modification. Metall. Mater. Trans. A 2005, 36, 2081–2091. [Google Scholar] [CrossRef]
- Cao, G.; Konishi, H.; Li, X. Mechanical properties and microstructure of Mg/SiC nanocomposites fabricated by ultrasonic cavitation based nanomanufacturing. J. Manuf. Sci. Eng. 2008, 130, 031105. [Google Scholar] [CrossRef]
- Xiao, J.; Shu, D.W.; Wang, X.J. Effect of strain rate and temperature on the mechanical behavior of magnesium nanocomposites. Int. J. Mech. Sci. 2014, 89, 381–390. [Google Scholar] [CrossRef]
- Clyne, T.W.; Withers, P.J. An Introduction to Metal Matrix Composites (Cambridge Solid State Science Series); Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Tan, M.J.; Zhang, X. Powder metal matrix composites: Selection and processing. Mater. Sci. Eng. A 1998, 244, 80–85. [Google Scholar] [CrossRef]
- Jiang, Q.C.; Wang, H.Y.; Ma, B.X.; Wang, Y.; Zhao, F. Fabrication of B4C particulate reinforced magnesium matrix composite by powder metallurgy. J. Alloys Compd. 2005, 386, 177–181. [Google Scholar] [CrossRef]
- Scudino, S.; Liu, G.; Prashanth, K.G.; Bartusch, B.; Surreddi, K.B.; Murty, B.S.; Eckert, J. Mechanical properties of Al-based metal matrix composites reinforced with Zr-based glassy particles produced by powder metallurgy. Acta Mater. 2009, 57, 2029–2039. [Google Scholar] [CrossRef]
- Ling, Y.; Hua, H.; Yuhong, Z.; Xiaomin, Y. Microstructure and mechanical properties of squeeze casting quasicrystal reinforced AZ91D magnesium matrix composites. Rare Metal Mater. Eng. 2016, 45, 1978–1982. [Google Scholar] [CrossRef]
- Wan, D.; Yang, G.; Zhu, M.; Xu, Q.; Zhou, Y. Solidification of Mg-28%Zn-2%Y alloy involving icosahedral quasicrystal phase. Trans. Nonferrous Met. Soc. China 2007, 17, 586–589. [Google Scholar] [CrossRef]
- Dubois, J.-M.; Belin-Ferre, E.; Urban, K. Complex Metallic Alloys: Fundamentals and Applications, 1st ed.; Wiley: Weinheim, Germany, 2011; p. 434. [Google Scholar]
- Scudino, S.; Sakaliyska, M.; Surreddi, K.B.; Ali, F.; Eckert, J. Structure and mechanical properties of Al–Mg alloys produced by copper mold casting. J. Alloys Compd. 2010, 504, S483–S486. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, B.; Zhao, D.; Li, X.; Zhai, T.; Han, Q.; Liu, F. In-situ synthesis of AlN/Mg-Al composites with high strength and high plasticity. J. Alloys Compd. 2017, 699, 627–632. [Google Scholar] [CrossRef]
- Contreras, A.; Angeles-Chávez, C.; Flores, O.; Perez, R. Structural, morphological and interfacial characterization of Al-Mg/TiC composites. Mater. Charact. 2007, 58, 685–693. [Google Scholar] [CrossRef]
- Sharifi, H.; Ostovan, K.; Tayebi, M.; Rajaee, A. Dry sliding wear behavior of open-cell Al-Mg/Al2O3 and Al-Mg/SiC-Al2O3 composite preforms produced by a pressureless infiltration technique. Tribol. Int. 2017, 116, 244–255. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, Z.; Chen, Y. Crystallographic orientations of intermetallic compounds of a multi-pass friction stir processed Al/Mg composite materials. Mater. Charact. 2017, 128, 156–164. [Google Scholar] [CrossRef]
- Hou, L.; Li, B.; Wu, R.; Cui, L.; Ji, P.; Long, R.; Zhang, J.; Li, X.; Dong, A.; Sun, B. Microstructure and mechanical properties at elevated temperature of Mg-Al-Ni alloys prepared through powder metallurgy. J. Mater. Sci. Tech. 2017, 33, 947–953. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Y.F. Influence of process control agent on interdiffusion between Al and Mg during mechanical alloying. J. Alloys Compd. 1999, 290, 279–283. [Google Scholar] [CrossRef]
- Chen, J.; Bao, C.; Chen, W.; Zhang, L.; Liu, J. Mechanical properties and fracture behavior of Mg-Al/AlN composites with different particle contents. J. Mater. Sci. Tech. 2017, 33, 668–674. [Google Scholar] [CrossRef]
- Chen, J.; Bao, C.; Chen, F. Evolutions of microstructure and mechanical properties for Mg-Al/AlN composites under hot extrusion. Mater. Sci. Eng. A 2016, 667, 426–434. [Google Scholar] [CrossRef]
- Bohn, R.; Haubold, T.; Birringer, R.; Gleiter, H. Nanocrystalline intermetallic compounds—An approach to ductility? Scr. Metall. Mater. 1991, 25, 811–816. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K. Nickel and iron aluminides: An overview on properties, processing, and applications. Intermetallics 1996, 4, 357–375. [Google Scholar] [CrossRef]
- Davis, J.R. Aluminum and aluminum alloys. In ASM Specialty Handbook; ASM International: Materials Park, Novelty, OH, USA, 1993. [Google Scholar]
- Tjong, S.C.; Ma, Z.Y. Microstructural and mechanical characteristics of in situ metal matrix composites. Mater. Sci. Eng. R 2000, 29, 49–113. [Google Scholar] [CrossRef]
- Tang, F.; Anderson, I.E.; Gnaupel-Herold, T.; Prask, H. Pure Al matrix composites produced by vacuum hot pressing: Tensile properties and strengthening mechanisms. Mater. Sci. Eng. A 2004, 383, 362–373. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.H.; Park, J.S.; Kim, J.C.; Kim, W.T.; Kim, D.H. Fabrication of Ni–Nb–Ta metallic glass reinforced al-based alloy matrix composites by infiltration casting process. Scr. Mater. 2004, 50, 1367–1371. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Simchi, A.; Kokabi, A.H.; Gerlich, A.P. Friction stir processing of an aluminum-magnesium alloy with pre-placing elemental titanium powder: In-situ formation of an Al3Ti-reinforced nanocomposite and materials characterization. Mater. Charact. 2015, 108, 102–114. [Google Scholar] [CrossRef]
- Hashim, J.; Looney, L.; Hashmi, M.S.J. Particle distribution in cast metal matrix composites—Part I. J. Mater. Process. Technol. 2002, 123, 251–257. [Google Scholar] [CrossRef]
- Ali, F.; Scudino, S.; Anwar, M.S.; Shahid, R.N.; Srivastava, V.C.; Uhlenwinkel, V.; Stoica, M.; Vaughan, G.; Eckert, J. Al-based metal matrix composites reinforced with Al-Cu-Fe quasicrystalline particles: Strengthening by interfacial reaction. J. Alloys Compd. 2014, 607, 274–279. [Google Scholar] [CrossRef]
- Shahid, R.N.; Scudino, S. Microstructural strengthening by phase transformation in Al-Fe3Al composites. J. Alloys Compd. 2017, 705, 590–597. [Google Scholar] [CrossRef]
- Mallick, A. Improvement of mechanical properties in light weight Mg-based materials. Procedia Eng. 2016, 149, 283–287. [Google Scholar] [CrossRef]
- Fang, C.; Wang, L.; Hao, H.; Zhang, X. Distribution of TiB2 reinforcements in magnesium matrix composites by a multi-physical coupling field. J. Mater. Process. Technol. 2014, 214, 551–555. [Google Scholar] [CrossRef]
- Liu, Z.; Han, Q.; Li, J.; Huang, W. Effect of ultrasonic vibration on microstructural evolution of the reinforcements and degassing of in situ TiB2P/Al–12Si–4Cu composites. J. Mater. Process. Technol. 2012, 212, 365–371. [Google Scholar] [CrossRef]
- Pitkethly, M.J. Nanomaterials—The driving force. Mater. Today 2004, 7, 20–29. [Google Scholar] [CrossRef]
- Garcés, G.; Domínguez, F.; Pérez, P.; Caruana, G.; Adeva, P. Effect of extrusion temperature on the microstructure and plastic deformation of PM-AZ92. J. Alloys Compd. 2006, 422, 293–298. [Google Scholar] [CrossRef]
- Nakashima, K.; Iwasaki, H.; Mori, T.; Mabuchi, M.; Nakamura, M.; Asahina, T. Mechanical properties of a powder metallurgically processed Mg-5Y-6Re alloy. Mater. Sci. Eng. A 2000, 293, 15–18. [Google Scholar] [CrossRef]
- Lü, L.; Lai, M.O.; Liang, W. Magnesium nanocomposite via mechanochemical milling. Comp. Sci. Technol. 2004, 64, 2009–2014. [Google Scholar] [CrossRef]
- Torralba, J.M.; da Costa, C.E.; Velasco, F. P/M aluminum matrix composites: An overview. J. Mater. Process. Technol. 2003, 133, 203–206. [Google Scholar] [CrossRef]
- Harrigan, W.C. Commercial processing of metal matrix composites. Mater. Sci. Eng. A 1998, 244, 75–79. [Google Scholar] [CrossRef]
- Das, D.K.; Mishra, P.C.; Singh, S.; Pattanaik, S. Fabrication and heat treatment of ceramic-reinforced aluminium matrix composites—A review. Int. J. Mech. Mater. Eng. 2014, 9, 6. [Google Scholar] [CrossRef]
- Das, D.K.; Mishra, P.C.; Singh, S.; Thakur, R.K. Properties of ceramic-reinforced aluminium matrix composites—A review. Int. J. Mech. Mater. Eng. 2014, 9, 12. [Google Scholar] [CrossRef]
- Long, R.A. Fabrication and Properties of Hot-Pressed Molybdenum Disilicide; Lewis Flight Propulsion Lab, National Advisory Committee for Aeronautics (NACA): Washington, DC, USA, 1950.
- Huffadine, J.B. The fabrication and properties of molybdenum disilicide and molybdenum disilicide–alumina. In Special Ceramics; Proper, P., Ed.; Academic Press: New York, USA, 1960; pp. 220–236. [Google Scholar]
- Smith, E. Macro process for direct production of tungsten monocarbide. Met. Powder Rep. Met. Powder Rep. 1979, 35, 53. [Google Scholar]
- Coble, R.L. Reactive sintering. In Sintering-Theory and Practice; Kolar, D., Pejovnik, S., Ristic, M., Eds.; Elsevier Scientific Publishing Co.: Amsterdam, The Netherlands, 1982; p. 145. [Google Scholar]
- Eisen, W.; Ferguson, B.; German, R.; Iacocca, R.; Lee, P.; Madan, D.; Moyer, K.; Sanderow, H.; Trudel, Y. Powder Metal Technologies and Applications; OSTI. GOV: Oak Ridge, TN, USA, 1998.
- Dunand, D.C. Reactive synthesis of aluminide intermetallics. Mater. Manuf. Proc. 1995, 10, 373–403. [Google Scholar] [CrossRef]
- Alman, D. Powder fabrication of monolithic and composite NiAl. IJPM 1991, 27, 29–41. [Google Scholar]
- Fahrenholtz, W.; Ewsuk, K.; Loehmann, R.; Tomasia, A. In-situ reactions for synthesis of composites, ceramics and intermetallics. Miner. Met. Mater. Soc. Warrenda 1995, 99, 327–342. [Google Scholar]
- Horton, J.; Baker, I.; Hanada, S.; Noebe, R.D.; Schwartz, D.S. High-Temperature Ordered Intermetallic Alloys VI; Materials Research Scociety: Pittsburgh, PA, USA, 1994. [Google Scholar]
- Zhang, H.; Zhao, Y.; Yan, Y.; Fan, J.; Wang, L.; Dong, H.; Xu, B. Microstructure evolution and mechanical properties of mg matrix composites reinforced with al and nano SiC particles using spark plasma sintering followed by hot extrusion. J. Alloys Compd. 2017, 725, 652–664. [Google Scholar] [CrossRef]
- Förster, W.; Binotsch, C.; Awiszus, B.; Dietrich, D.; Nickel, D. Interface engineering of aluminum-magnesium compounds. Mater. Today Proc. 2015, 2, 3–8. [Google Scholar] [CrossRef]
- Kittner, K.; Feuerhack, A.; Förster, W.; Binotsch, C.; Graf, M. Recent developments for the production of Al-Mg compounds. Mater. Today Proc. 2015, 2, S225–S232. [Google Scholar] [CrossRef]
- Li, C.; Chi, C.; Lin, P.; Zhang, H.; Liang, W. Deformation behavior and interface microstructure evolution of Al/Mg/Al multilayer composite sheets during deep drawing. Mater. Des. 2015, 77, 15–24. [Google Scholar] [CrossRef]
- Skorpen, K.G.; Mauland, E.; Reiso, O.; Roven, H.J. Novel method of screw extrusion for fabricating Al/Mg (macro-) composites from aluminum alloy 6063 and magnesium granules. Trans. Nonferrous Met. Soc. China 2014, 24, 3886–3893. [Google Scholar] [CrossRef]
- Guo, B.; Yi, J.; Ni, S.; Shen, R.; Song, M. Factors affecting the microstructure and mechanical properties of Ti-Al3Ti core-shell-structured particle-reinforced Al matrix composites. Philos. Mag. 2016, 96, 1197–1211. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kang, S.B.; Sato, T.; Tezuka, H.; Kamio, A. Fabrication of Al/Al3Fe composites by plasma synthesis method. Mater. Sci. Eng. 2003, A343, 199–209. [Google Scholar] [CrossRef]
- Laplanche, G.; Joulain, A.; Bonneville, J.; Schaller, R.; El Kabir, T. Microstructures and mechanical properties of Al-base composite materials reinforced by Al-Cu-Fe particles. J. Alloys Compd. 2010, 493, 453–460. [Google Scholar] [CrossRef]
- Qi, N.; Hu, M.; Wang, Z.; Lu, Z.; Xie, C. Synthesis of Al/Fe3Al core-shell intermetallic nanoparticles by chemical liquid deposition method. Adv. Powder Technol. 2013, 24, 926–931. [Google Scholar] [CrossRef]
- Wang, Y.; Song, M.; Ni, S.; Xue, Y. In situ formed core-shell structured particle reinforced aluminum matrix composites. Mater. Des. 2014, 56, 405–408. [Google Scholar] [CrossRef]
- Xue, Y.; Shen, R.; Ni, S.; Xiao, D.; Song, M. Effects of sintering atmosphere on the mechanical properties of Al-Fe particle-reinforced Al-based composites. J. Mater. Eng. Perform. 2015, 24, 1890–1896. [Google Scholar] [CrossRef]
- Azizieh, M.; Mazaheri, M.; Balak, Z.; Kafashan, H.; Kim, H.S. Fabrication of Mg/Al12Mg17 in-situ surface nanocomposite via friction stir processing. Mater. Sci. Eng. A 2018, 712, 655–662. [Google Scholar] [CrossRef]
- Brennan, S.; Bermudez, K.; Kulkarni, N.S.; Sohn, Y. Interdiffusion in the Mg-Al system and intrinsic diffusion in β-Mg2Al3. Metall. Mater. Trans. A 2012, 43, 4043–4052. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Huang, W. Interface microstructure and diffusion kinetics in diffusion bonded Mg/Al joint. React. Kinet. Catal. Lett. 2008, 95, 71–79. [Google Scholar] [CrossRef]
- Seyyed Afghahi, S.S.; Jafarian, M.; Paidar, M.; Jafarian, M. Diffusion bonding of Al 7075 and Mg AZ31 alloys: Process parameters, microstructural analysis and mechanical properties. Trans. Nonferrous Met. Soc. China 2016, 26, 1843–1851. [Google Scholar] [CrossRef]
- Liu, W.; Long, L.; Ma, Y.; Wu, L. Microstructure evolution and mechanical properties of Mg/Al diffusion bonded joints. J. Alloys Compd. 2015, 643, 34–39. [Google Scholar] [CrossRef]
- Dietrich, D.; Nickel, D.; Krause, M.; Lampke, T.; Coleman, M.P.; Randle, V. Formation of intermetallic phases in diffusion-welded joints of aluminium and magnesium alloys. J. Mater. Sci. 2011, 46, 357–364. [Google Scholar] [CrossRef]
- Su, H.L.; Harmelin, M.; Donnadieu, P.; Baetzner, C.; Seifert, H.J.; Lukas, H.L.; Effenberg, G.; Aldinger, F. Experimental investigation of the Mg-Al phase diagram from 47 to 63 at % Al. J. Alloys Compd. 1997, 247, 57–65. [Google Scholar] [CrossRef]
- Panteli, A.; Robson, J.D.; Brough, I.; Prangnell, P.B. The effect of high strain rate deformation on intermetallic reaction during ultrasonic welding aluminium to magnesium. Mater. Sci. Eng. A 2012, 556, 31–42. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, J.S.; Jo, Y.M.; Lee, S.E.; Heo, J.; Chang, Y.W.; Lee, Y.S. Interface-correlated deformation behavior of a stainless steel-Al-Mg 3-ply composite. Mater. Charact. 2013, 75, 138–149. [Google Scholar] [CrossRef]
- Negendank, M.; Mueller, S.; Reimers, W. Coextrusion of Mg-Al macro composites. J. Mater. Process. Technol. 2012, 212, 1954–1962. [Google Scholar] [CrossRef]
- Njiokep, T.; Eugene, M.; Salamon, M.; Mehrer, H. Growth of Intermetallic Phases in the Al-Mg System. In Defect and Diffusion Forum; Trans. Tech. Publ.: Zürich, Switzerland, 2001; pp. 1581–1586. [Google Scholar]
- Funamizu, Y.; Watanabe, K. Interdiffusion in the Al–Mg system. Trans. Jpn. Inst. Met. 1972, 13, 278–283. [Google Scholar] [CrossRef]
- Laidler, K.J. Chemical Kinetics, 3rd ed.; Harper Collins: NewYork, NY, USA, 1987. [Google Scholar]
- Svoboda, J.; Fischer, F.D. Incorporation of vacancy generation/annihilation into reactive diffusion concept—Prediction of possible kirkendall porosity. Comp. Mater. Sci. 2017, 127, 136–140. [Google Scholar] [CrossRef]
- Bhadeshia, H. The Kirkendall Effect. Available online: http://www.msm.cam.ac.uk/phase-trans/kirkendall.html (accessed on 13 April 2018).
- ASTM E6-03. Standard Terminology Relating to Methods of Mechanical Testing, Annual Book of ASTM Standards; ASTM international: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Scudino, S.; Surreddi, K.B.; Sager, S.; Sakaliyska, M.; Kim, J.S.; Löser, W.; Eckert, J. Production and mechanical properties of metallic glass-reinforced Al-based metal matrix composites. J. Mater. Sci. 2008, 43, 4518–4526. [Google Scholar] [CrossRef]
- Chawla, K.K. Composite Materials: Science and Engineering; Springer: New York, NY, USA, 1987. [Google Scholar]
- Kim, H.S. On the rule of mixtures for the hardness of particle reinforced composites. Mater. Sci. Eng. A 2000, 289, 30–33. [Google Scholar] [CrossRef]
- Kim, H.S.; Hong, S.I.; Kim, S.J. On the rule of mixtures for predicting the mechanical properties of composites with homogeneously distributed soft and hard particles. J. Mater. Process. Technol. 2001, 112, 109–113. [Google Scholar] [CrossRef]
- Kim, J.Y.; Scudino, S.; Kühn, U.; Kim, B.S.; Lee, M.H.; Eckert, J. Production and characterization of brass-matrix composites reinforced with Ni59Zr20Ti16Si2Sn3 glassy particles. Metals 2012, 2, 79–94. [Google Scholar] [CrossRef]
- Li, Z.; Schmauder, S.; Dong, M. A simple mechanical model to predict fracture and yield strengths of particulate two-phase materials. Comp. Mater. Sci. 1999, 15, 11–21. [Google Scholar] [CrossRef]
- Wang, Z.; Scudino, S.; Stoica, M.; Zhang, W.; Eckert, J. Al-based matrix composites reinforced with short Fe-based metallic glassy fiber. J. Alloys Compd. 2015, 651, 170–175. [Google Scholar] [CrossRef]
- Singh, A.; Solanki, K.; Manuel, M.; Neelameggham, N. Magnesium Technology; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Zolriasatein, A.; Khosroshahi, R.A.; Emamy, M.; Nemati, N. Mechanical and wear properties of Al-Al3Mg2 nanocomposites prepared by mechanical milling and hot pressing. Int. J. Min. Met. Mater. 2013, 20, 290–297. [Google Scholar] [CrossRef]
- Scudino, S.; Liu, G.; Sakaliyska, M.; Surreddi, K.B.; Eckert, J. Powder metallurgy of Al-based metal matrix composites reinforced with β-Al3Mg2 intermetallic particles: Analysis and modeling of mechanical properties. Acta Mater. 2009, 57, 4529–4538. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, R.N.; Scudino, S. Microstructure and Mechanical Behavior of Al-Mg Composites Synthesized by Reactive Sintering. Metals 2018, 8, 762. https://doi.org/10.3390/met8100762
Shahid RN, Scudino S. Microstructure and Mechanical Behavior of Al-Mg Composites Synthesized by Reactive Sintering. Metals. 2018; 8(10):762. https://doi.org/10.3390/met8100762
Chicago/Turabian StyleShahid, Rub Nawaz, and Sergio Scudino. 2018. "Microstructure and Mechanical Behavior of Al-Mg Composites Synthesized by Reactive Sintering" Metals 8, no. 10: 762. https://doi.org/10.3390/met8100762
APA StyleShahid, R. N., & Scudino, S. (2018). Microstructure and Mechanical Behavior of Al-Mg Composites Synthesized by Reactive Sintering. Metals, 8(10), 762. https://doi.org/10.3390/met8100762