Purification of Aluminium Cast Alloy Melts through Precipitation of Fe-Containing Intermetallic Compounds
Abstract
:1. Introduction
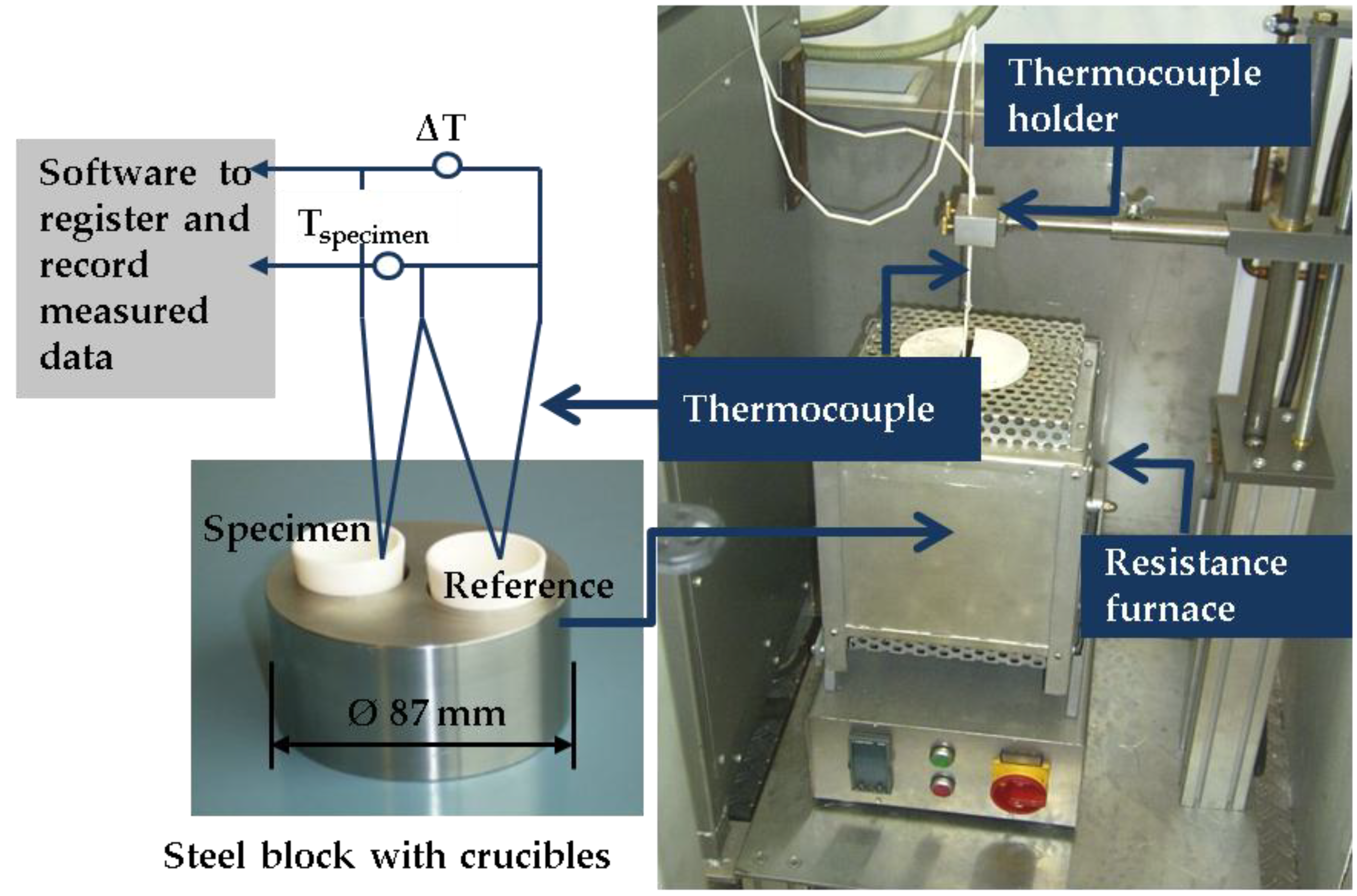
2. Materials and Methods
3. Results and Discussions
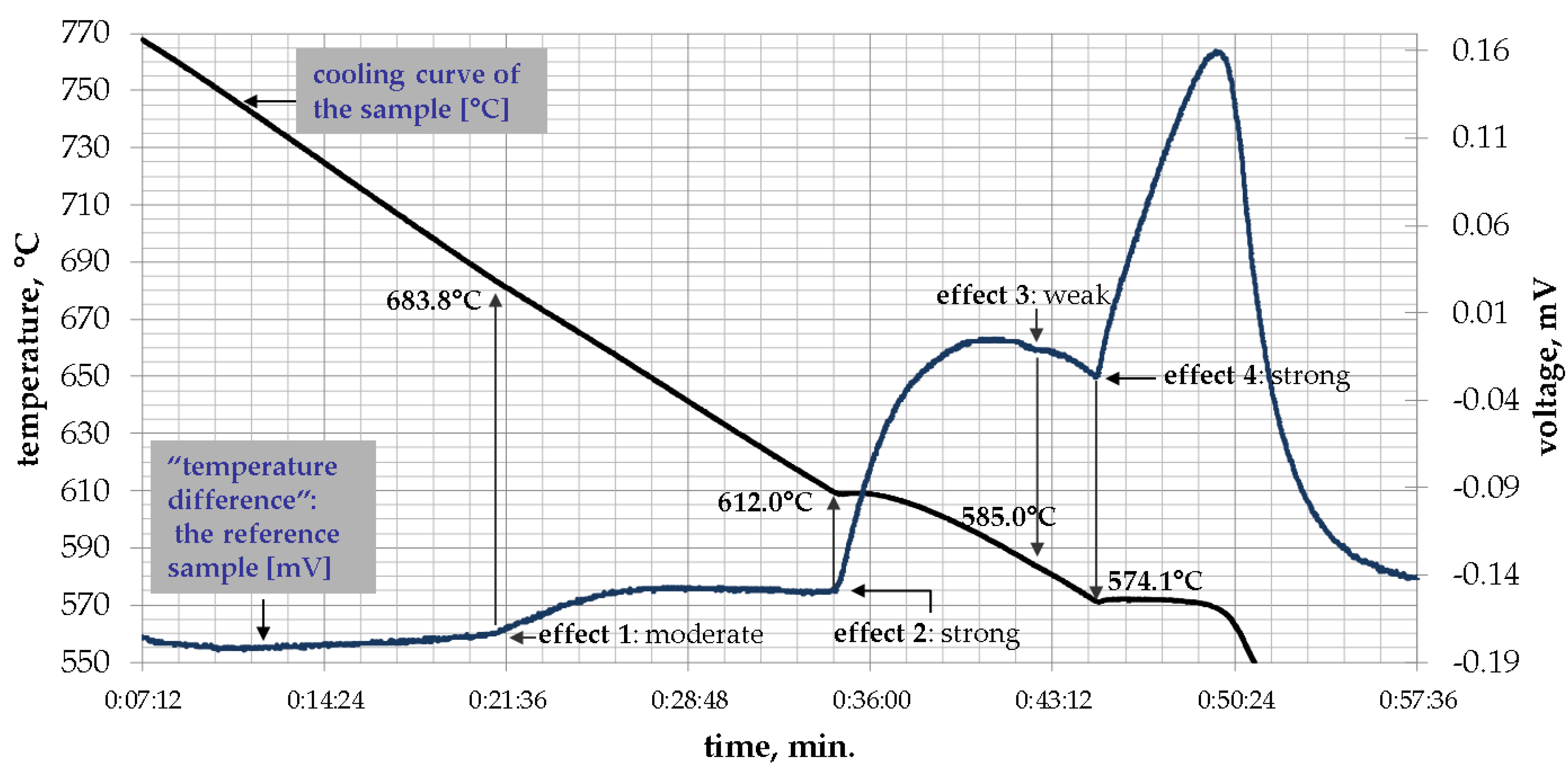
3.1. DTA Experimental Results
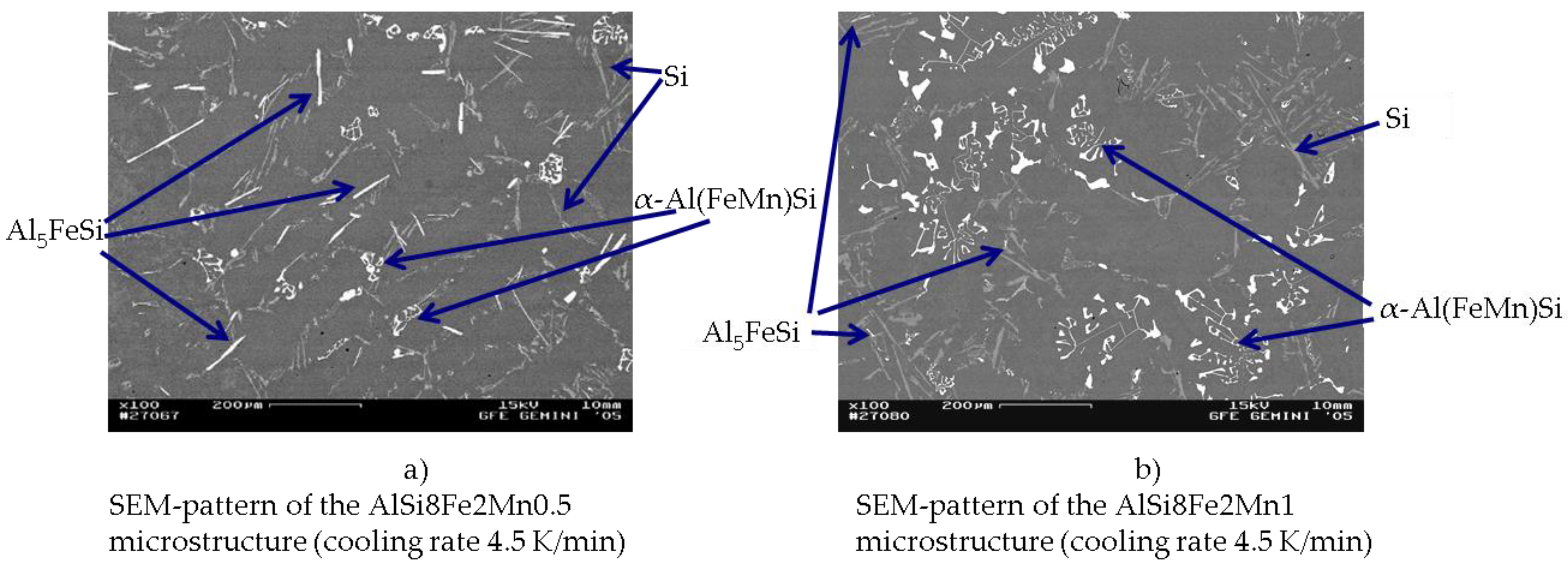
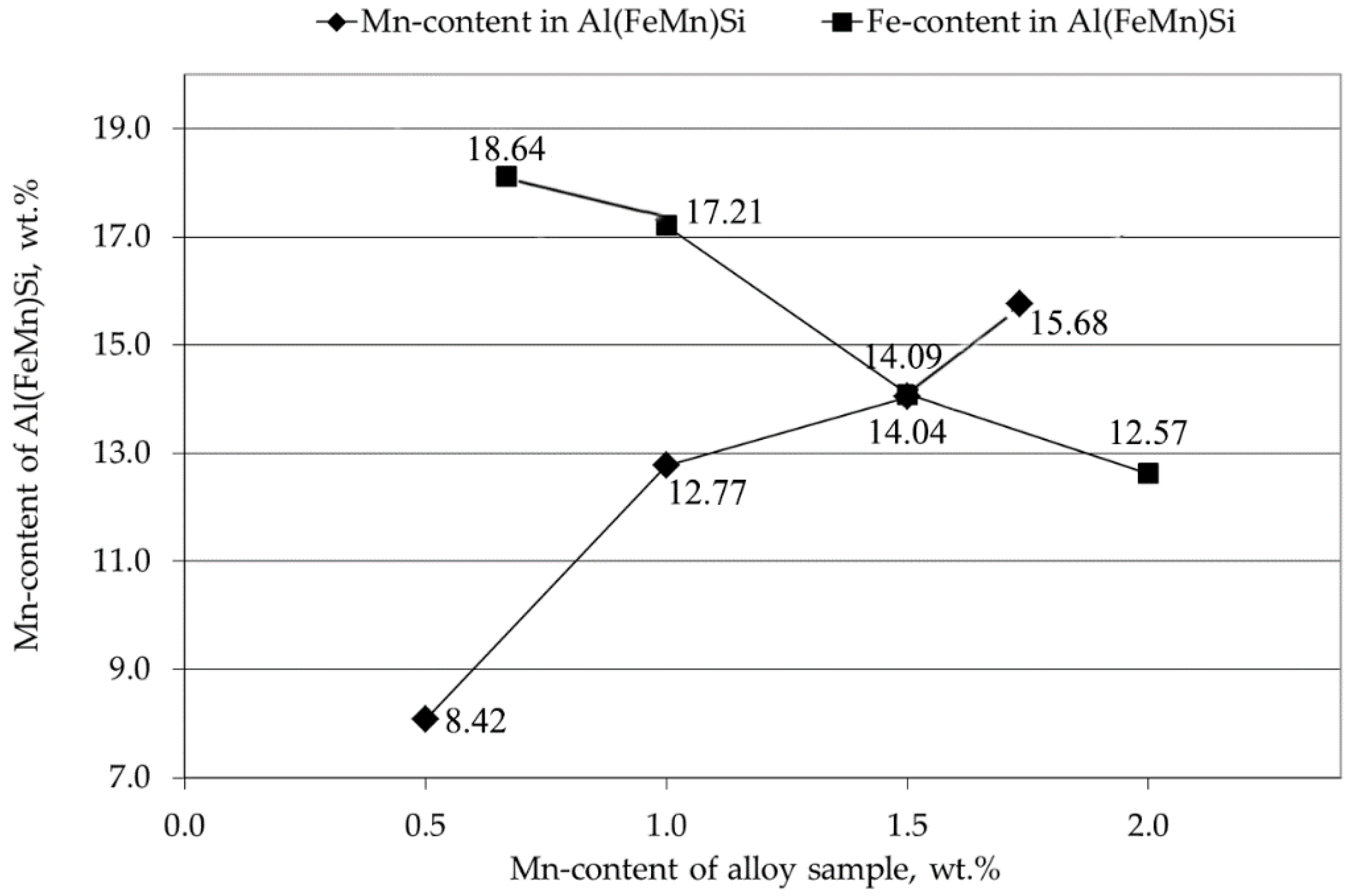
3.2. Precipitated Phases
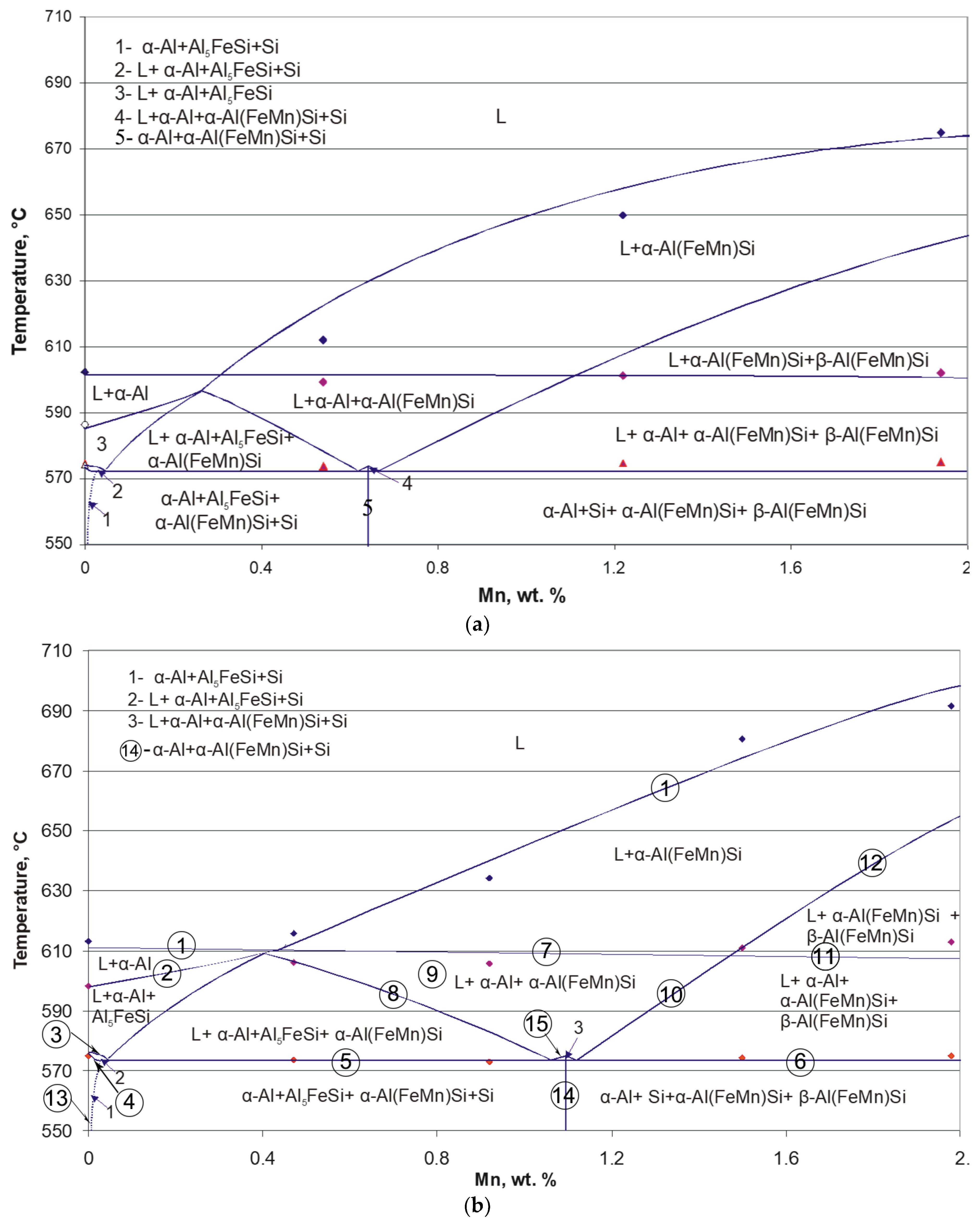
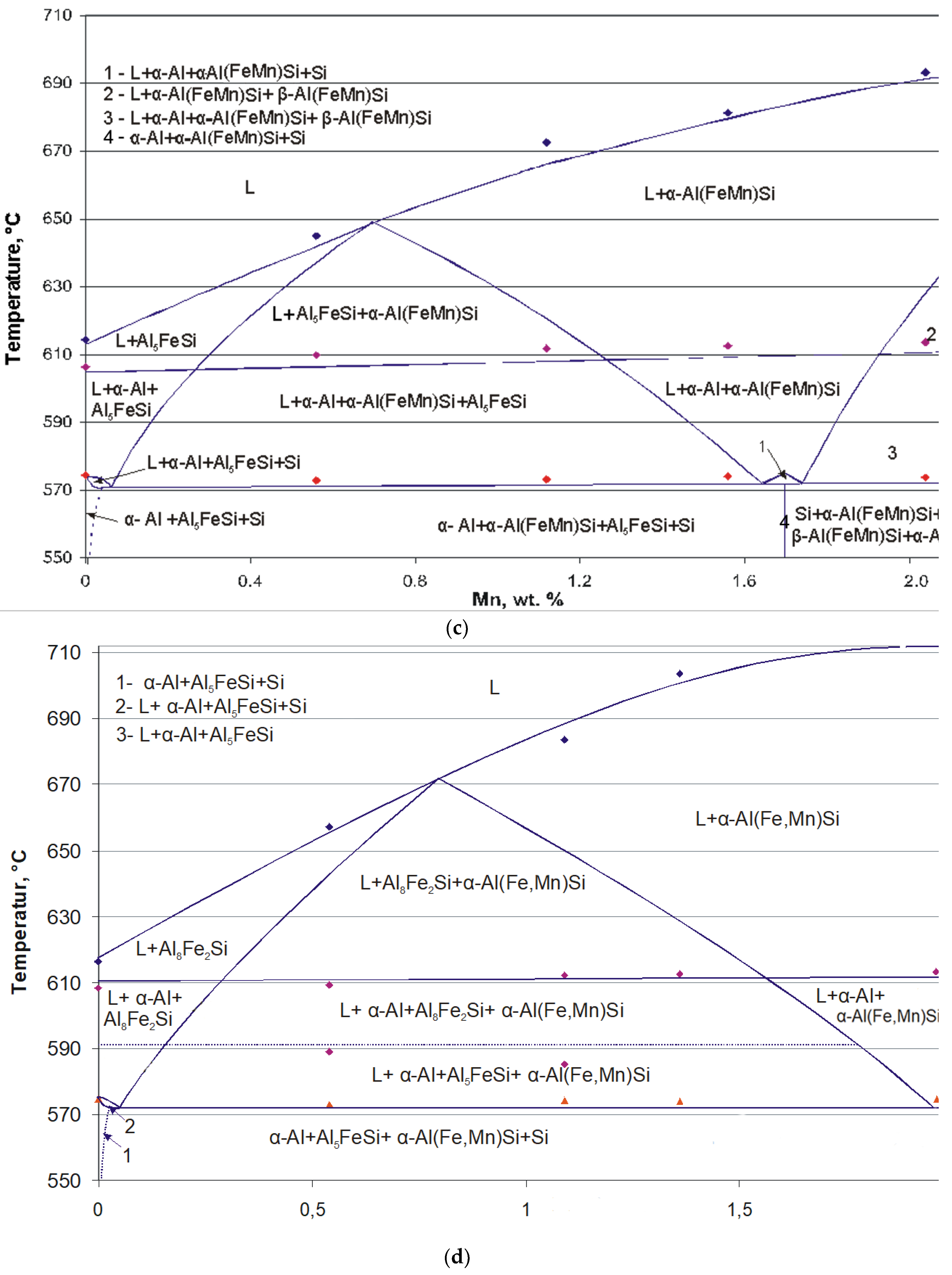
3.3. Developing Isopleths from DTA and Phase Analysis Results
- (1)
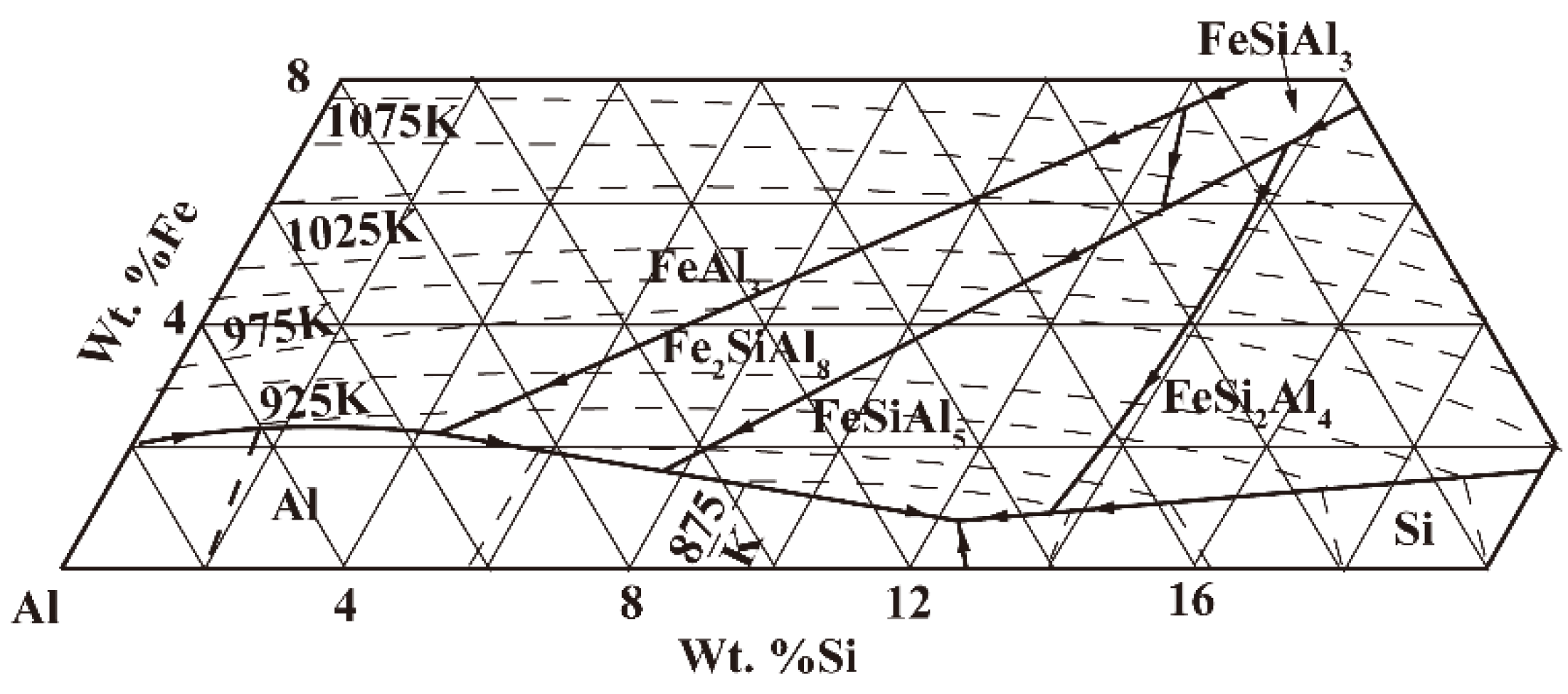
- The quaternary Al(FeMn)Si are differentiated by the Mn/Fe ratio into α-Al(FeMn)Si if Mn/Fe ≤ 1.1 and β-Al(FeMn)Si if Mn/Fe > 1.1. These three systems are formed depending on the Mn/Fe ratio of the alloy: if Mn/Fe < 1.1, after crystallization, the alloys consist of Al–α-Al(FeMn)Si–Si–Al5FeSi; if Mn/Fe > 1.1, the alloys consist of Al–α-Al(FeMn)Si–Si–β-Al(FeMn)Si; if Mn/Fe = 1.1, only Al–α-Al(FeMn)Si–Si coexist [12].
- (2)
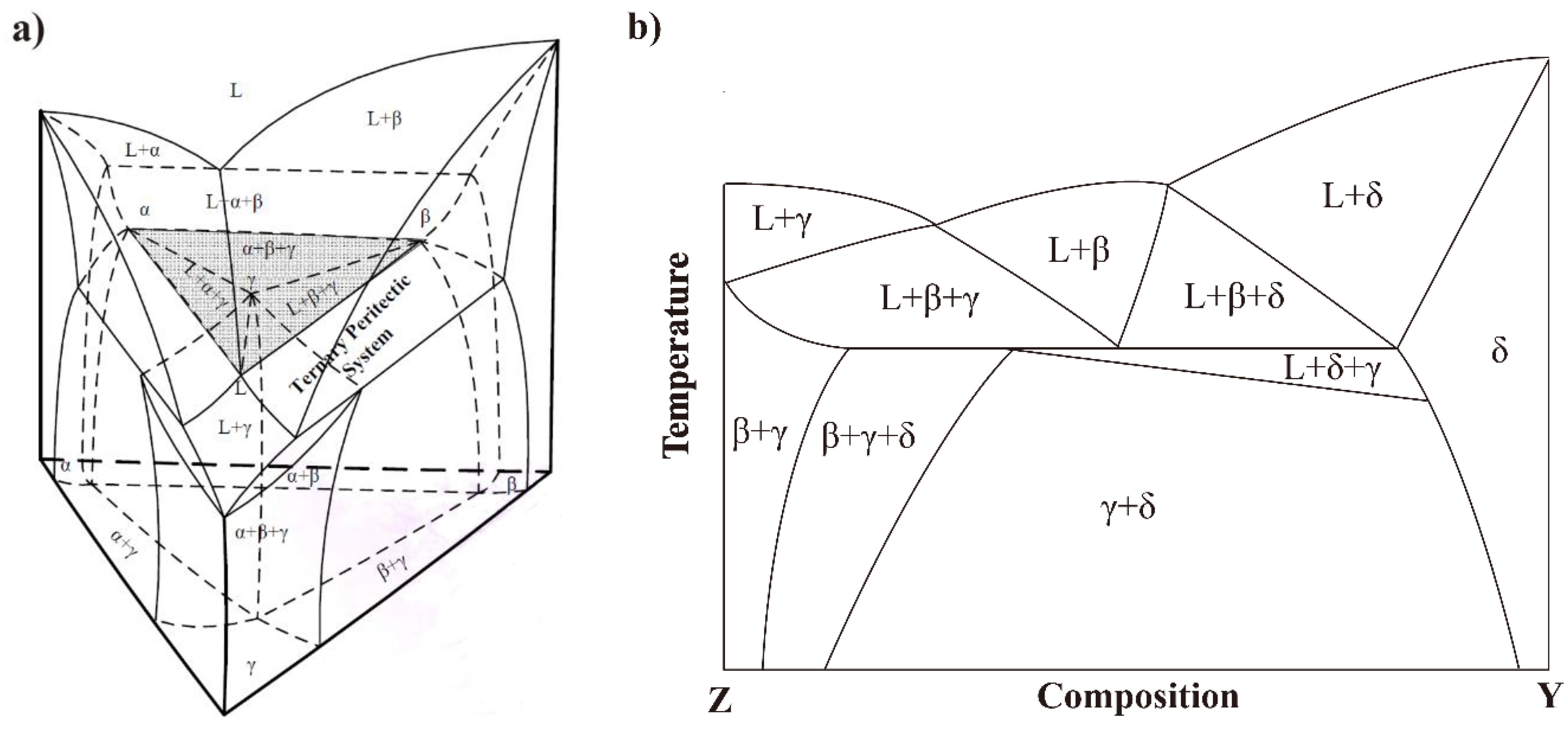
- Crossing the tilted phase boundary line leads to exhaust or precipitation of one phase, whereas passing through the horizontal phase boundary line, where eutectic or peritetic reactions occur, causes exhaust of one phase and precipitation of one phase, respectively. Crossing a point-phase boundary results in either exhaust (precipitation) of two phases or exhaust of one phase and precipitation of the other [20].
- (1)
- L + α-Al + α-Al(FeMn)Si + Al5FeSi = α-Al + α-Al(FeMn)Si + Si + Al5FeSi and
- (2)
- L + α-Al + α-Al(FeMn)Si + β-Al(FeMn)Si = α-Al + α-Al(FeMn)Si + Si + β-Al(FeMn)Si
4. Conclusions
- (1)
- L + α-Al + α-Al(FeMn)Si + Al5FeSi = α-Al + α-Al(FeMn)Si + Si + Al5FeSi;
- (2)
- L + α-Al + α-Al(FeMn)Si + β-Al(FeMn)Si = α-Al + α-Al(FeMn)Si + Si + β-Al(FeMn)Si.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ostermann, F. Anwendungstechnologie Aluminium, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany; New York, NY, USA, 1998; p. 18. ISBN 978-3-662-05788-9. [Google Scholar]
- Liste der Aluminiumgusslegierungen; Angaben von VAR Verband der Aluminiumrecyclingindustrie e.V.: Düsseldorf, Germany, 2004.
- Gao, J.; Shu, D.; Wang, J.; Sun, B. Effects of Na2B4O7 on the elimination of iron from aluminum melt. Scr. Mater. 2007, 57, 197–200. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Subramnian, S.; Hajra, J.P. Electroslag refining of commercial aluminium. (Retroactive coverage). Trans. Indian Inst. Met. 1996, 39, 646–651. [Google Scholar]
- Zhang, J.; Sun, B.; He, B.; Mao, H.; Chen, G.; Ge, A. Principle and control of new-style purification equipment of 5 N high purity aluminum. Chin. J. Mech. Eng. 2006, 42, 64–68. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, H. The development of 85kA three-layer electrolysis cell for refining of aluminium. TMS Light Metals. 2008, 533–535. [Google Scholar]
- Van Der Donk, H.M.; Nijhof, G.H.; Castelijns, C.A.M. The removal of iron from molten aluminum. In Proceedings of the Third International Symposium: Recycling of Metals, and Engineered Materials, Point Clear, AL, USA, 12–15 November 1995; pp. 651–661. [Google Scholar]
- De Moraes, H.L.; De Oliveira, J.R.; Espinosa, D.C.R.; Tenorio, J.A.S. Removal of iron from molten recycled aluminum through intermediate phase filtration. Mater. Trans. 2006, 47, 1731–1736. [Google Scholar] [CrossRef]
- Matsubara, H.; Izawa, N.; Nakanishi, M. Macroscopic segregation in Al-11 mass% Si alloy containing 2 mass% Fe solidified under centrifugal force. J. Jpn. Inst. Light Met. 1998, 48, 93–97. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Aluminium Alloys: Structure and Properties, 1st ed.; Butterworths: London, UK; Boston, MA, USA, 1976; pp. 282–289, 534–536, 529–530, 661–663. ISBN 0408706805. [Google Scholar]
- Petzow, G.; Effenberg, G. Ternary Alloys, 1st ed.; VCH Publishers: New York, NY, USA, 1992; Volume 5, pp. 250–264, 394–438. ISBN 0895738953. [Google Scholar]
- Zakharov, A.M.; Gul’din, I.T.; Arnol’d, A.A.; Matsenko, Y.A. Phase Equlibria in the Al-Si-Fe-Mn System in the 10–14%Si, 0–3%Fe and 0–4%Mn Concentration Ranges. Izvest Vyssh Uchebn Zaved Tsvetn Metall. 1988, 4, 89–94. [Google Scholar]
- DIN EN 1706:1998-06. Available online: https://www.beuth.de/en/standard/din-en-1706/3359595 (accessed on 19 August 2018).
- Villars, P.; Prince, A.; Okamoto, H. Handbook of Ternary Alloy Phase Diagramms, 1st ed.; ASM International: Geauga Country, OH, USA, 1995; Volume 3, pp. 3501–3514, 3597–3621. ISBN 0871705257. [Google Scholar]
- Belov, N.A.; Eskin, D.G.; Aksenov, A.A. Multicomponent Phase Diagrams: Applications for Commercial Aluminium Alloys, 1st ed.; ELSEVIER Ltd.: Oxford, UK, 2005; pp. 1–19. ISBN 0080445373. [Google Scholar]
- Rhines, F.N. Phase Diagrams in Metallurgy, Their Development and Application, 1st ed.; McGraw-Hill Book Company Inc.: New York, NY, USA, 1956; pp. 186, 197, 220–229, 290–302. ISBN 0070520704. [Google Scholar]
- Gnatko, M. Untersuchungen zur Entfernung von Eisen aus Verunreinigten Aluminiumgusslegierungen Durch Intermetallische Fällung. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 19 May 2008. [Google Scholar]
- Hanemann, H.; Schrader, A. Ternäre Legierungen des Aluminiums, Atlas Metallographicus, 1st ed.; Band III, Teil 2; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1952; pp. 35–38. [Google Scholar]
- Atlas of Microstructures of Industrial Alloys. In Metals Handbook, 8th ed.; ASM: Metals park, OH, USA, 1961–1972; Volume 7, pp. 256–263.
- Zakharov, A.M. Phase Diagrams of Binary and Ternary Systems, 3rd ed.; Metallurgia: Moskwa, Russia, 1990; pp. 230–239. ISBN 5229005173. [Google Scholar]

| Alloy Identification | Alloy Composition Limits, wt. % | ||||||
|---|---|---|---|---|---|---|---|
| Numerical | Chemical | Si | Fe | Cu | Mn | Mg | Other |
| cast alloys for pressure casting | |||||||
| EN AC-44300 | EN AC-AlSi12(Fe) | 10.5–13.5 | 1.0 | 0.10 | 0.55 | - | 0.55 |
| EN AC-46000 | EN AC-AlSi9Cu3(Fe) | 8.0–11.0 | 1.30 | 2.0–4.0 | 0.55 | 0.05–0.55 | 2.75 |
| cast alloys for common application | |||||||
| EN AC-44200 | EN AC-AlSi12(a) | 10.5–13.5 | 0.55 | 0.05 | 0.35 | - | 0.40 |
| EN AC-46200 | EN AC-AlSi8Cu3 | 7.5–9.5 | 0.8 | 2.0–3.5 | 0.15–0.65 | 0.05–0.55 | 2.45 |
| Phases | Components, wt. % | |||
|---|---|---|---|---|
| Al | Mn | Fe | Si | |
| Al8Fe2Si | 56.0–62.6 | – | 30.0 | 7.4–11.0 |
| Al5FeSi | 59.4–60.9 | <0,8 | 25.5–26.5 | 12.8–13.3 |
| Al16(FeMn)4Si3 | 53.0–64.6 | 14.6–19.7 | 10.4–15.3 | 10.4–12.0 |
| Al15Mn3Si2 | 58.0–60.3 | 27.7–29.5 | <1.8 | 10.2–10.7 |
| Al4FeSi2 | 46.9–48.0 | <0.8 | 25.9 | 25.3–26.4 |
| Group | Iron/Manganese Content, wt. % | |||
|---|---|---|---|---|
| Fe/Mn Step 0.5 | Fe/Mn Step 0.5 | Fe/Mn Step 0.5 | Fe/Mn Step 0.5 | |
| AlSi6FeMn | 0.5/0–2 | 1.0/0–2 | 1.5/0–2 | 2.0/0–2 |
| AlSi8FeMn | 0.5/0–2 | 1.0/0–2 | 1.5/0–2 | 2.0/0–2 |
| AlSi10FeMn | 0.5/0–2 | 1.0/0–2 | 1.5/0–2 | 2.0/0–2 |
| Alloy | Mn, | Effect 1 | Effect 2 | Effect 3 | Effect 4 |
|---|---|---|---|---|---|
| (Target) | wt. % | T, °C | T, °C | T, °C | T, °C |
| AlSi8Fe0.5 | 0.00 | 602.3 | 586.4 | - | 574.4 |
| AlSi8Fe0.5Mn0.5 | 0.54 | 612.0 | 599.2 | 585.0 | 573.7 |
| AlSi8Fe0.5Mn1.0 | 1.22 | 649.9 | 601.2 | 597.0 | 574.7 |
| AlSi8Fe0.5Mn2.0 | 1.94 | 675.0 | 633.0 | 602.1 | 574.9 |
| AlSi8Fe1.0 | 0.00 | 613.0 | 598.1 | - | 574.9 |
| AlSi8Fe1.0Mn0.5 | 0.47 | 620.7 | 606.0 | 600.0 | 573.5 |
| AlSi8Fe1.0Mn1.0 | 0.92 | 634.2 | 605.7 | 580.0 | 573.0 |
| Al Si8 Fe1.0Mn1.5 | 1.50 | 680.5 | 611.0 | 607.0 | 574.3 |
| Al Si8 Fe1.0Mn2.0 | 1.98 | 691.4 | 640.0 | 612.9 | 574.8 |
| AlSi8Fe1.5 | 0.00 | 614.3 | 606.4 | - | 574.4 |
| AlSi8Fe1.5Mn0.5 | 0.56 | 645.0 | 638.0 | 609.9 | 572.8 |
| AlSi8 Fe1.5Mn1.0 | 1.12 | 672.4 | 614.0 | 611.7 | 573.2 |
| AlSi8Fe1.5Mn1.5 | 1.56 | 681.3 | 612.6 | 576.0 | 574.0 |
| AlSi8Fe1.5Mn2.0 | 2.04 | 693.1 | 630.0 | 613.6 | 573.8 |
| AlSi8Fe2.0 | 0.00 | 616.3 | 608.4 | - | 574.4 |
| AlSi8Fe2.0Mn0.5 | 0.54 | 657.1 | 609.2 | 589.1 | 573.0 |
| AlSi8Fe2.0Mn1.0 | 1.09 | 683.8 | 612.0 | 585.0 | 574.1 |
| AlSi8Fe2.0Mn1.5 | 1.36 | 703.6 | 638.0 | 612.5 | 573.8 |
| AlSi8Fe2.0Mn2.0 | 1.99 | 710.5 | 613.2 | 575.0 | 573.6 |
| Phase | Components, wt. % | |||
|---|---|---|---|---|
| Al | Mn | Fe | Si | |
| Al matrix | 98.37–99.66 | 0.0–0.45 | 0.0–0.50 | 0.73–2.55 |
| Al5FeSi | 55.02–56.03 | 1.92–2.59 | 23.73–26.21 | 16.86–17.65 |
| α-Al(FeMn)Si | 57.77–61.46 | 8.07–17.39 | 12.62–19.85 | 10.06–13.70 |
| β-Al(FeMn)Si | 56.47–62.58 | 12.63–17.93 | 11.25–13.44 | 10.84–11.34 |
| Si | 0.30–3.50 | - | - | 96.50–99.77 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnatko, M.; Li, C.; Arnold, A.; Friedrich, B. Purification of Aluminium Cast Alloy Melts through Precipitation of Fe-Containing Intermetallic Compounds. Metals 2018, 8, 796. https://doi.org/10.3390/met8100796
Gnatko M, Li C, Arnold A, Friedrich B. Purification of Aluminium Cast Alloy Melts through Precipitation of Fe-Containing Intermetallic Compounds. Metals. 2018; 8(10):796. https://doi.org/10.3390/met8100796
Chicago/Turabian StyleGnatko, Marina, Cong Li, Alexander Arnold, and Bernd Friedrich. 2018. "Purification of Aluminium Cast Alloy Melts through Precipitation of Fe-Containing Intermetallic Compounds" Metals 8, no. 10: 796. https://doi.org/10.3390/met8100796






