Recovery of Cerium from Glass Polishing Waste: A Critical Review
Abstract
:1. Introduction
1.1. Rare-Earth Elements
Cerium
1.2. Glass Polishing Powder and Its Applications
1.3. Production of Glass Polishing Powder
1.4. Polishing Process
1.5. Glass Polishing Powder Waste
2. Characterization of Glass Polishing Waste
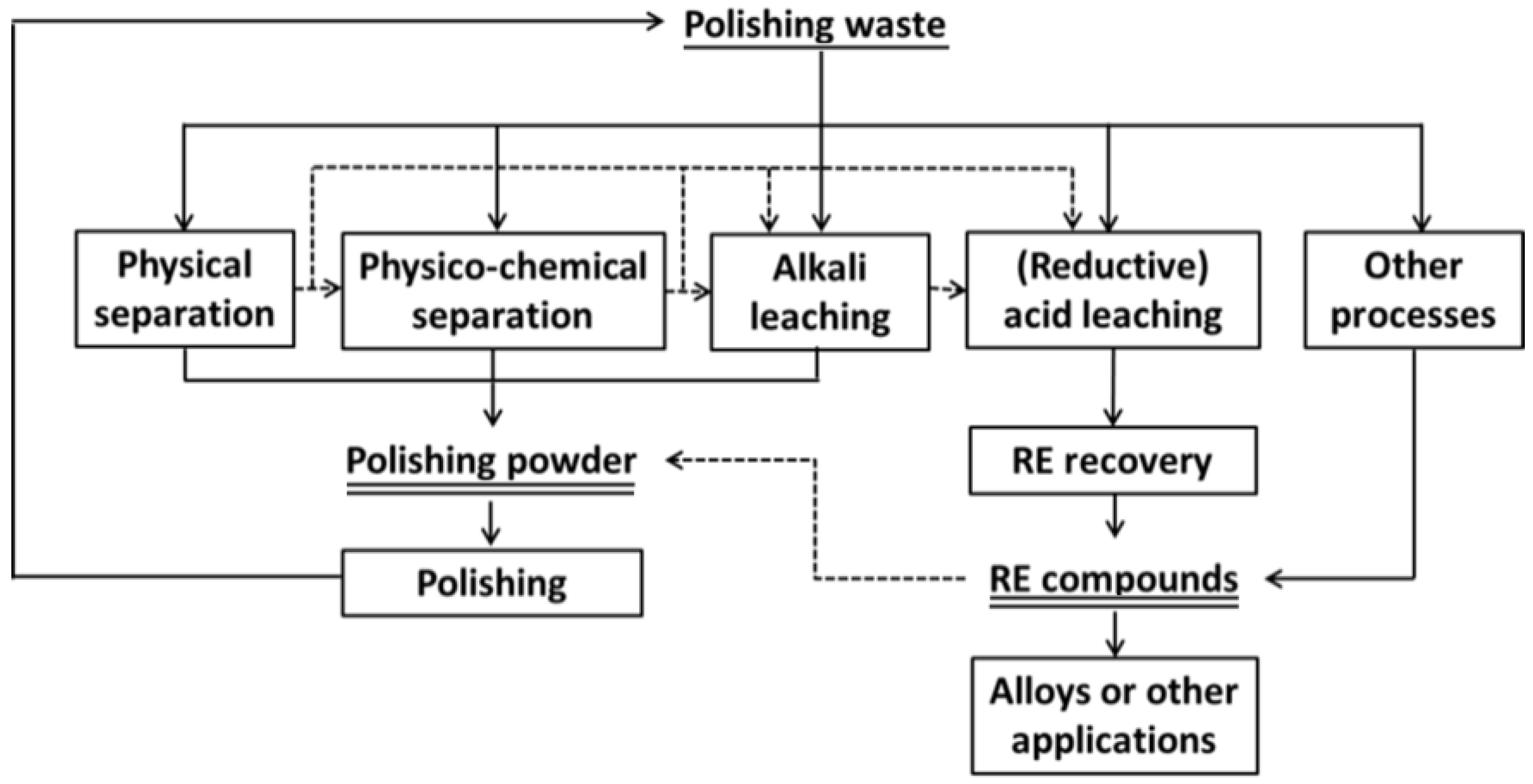
3. Recovery of Polishing Powder (Cerium Compounds)
3.1. Physical Separation
3.2. Chemical Separation
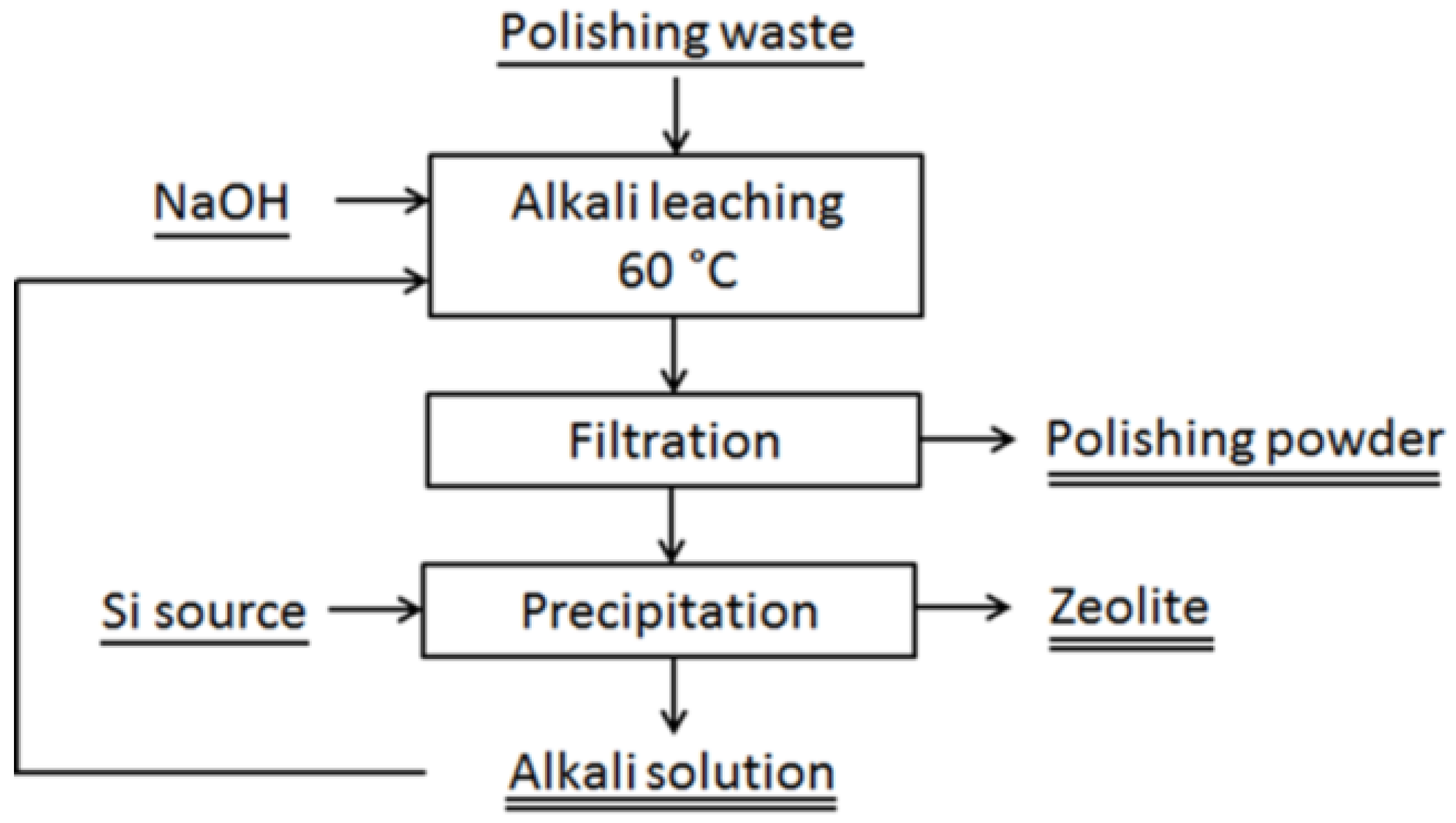
3.2.1. Alkali Leaching—for Reuse of Polishing Powder
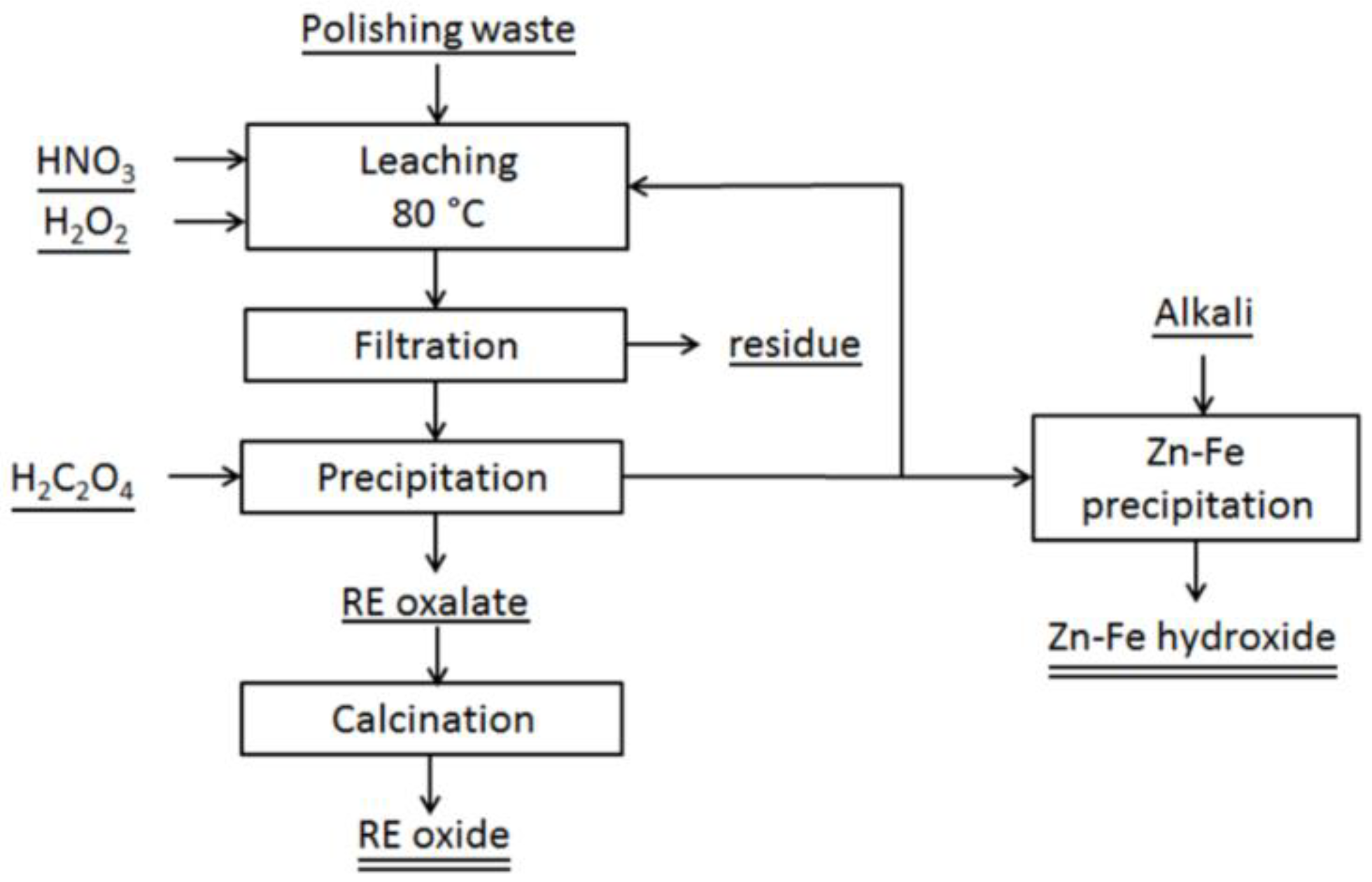
3.2.2. Acid Leaching—for REE Recovery
Direct Leaching
- Concentrated H2SO4 leaching: After removal of silica from glass polishing waste by flotation and after alkali leaching, Kim et al. performed oxidative roasting on dried samples at 600 °C to convert cerium compounds to CeO2 [29]. Cerium was leached from the roasted mass by concentrated sulfuric acid solution at 60 °C. These authors claimed that high amount of oxidation of cerium oxide dissolves more cerium in to the leach solution. However, for a small increase in cerium recovery (about 10%) the roasting of the powder is difficult to be justified.
- Concentrated H2SO4 digestion—water leaching: Poscher et al. [25] digested polishing powder with conc. sulfuric acid (>96 wt%) at temperatures above 100 °C. During digestion the slurry was solidified. The digested material was subsequently leached with water to dissolve rare earth sulfates.
- Two stage H2SO4 leaching: Um et al. leached a synthetic mixture with sulfuric acid in two stages for selective recovery of cerium [37]. In the first stage, the mixture was treated with 2 mol/dm3 sulfuric acid at 90 °C for leaching La, Nd, Pr and Ca. In the second stage, the leach residue from first stage was treated with 12 mol/dm3 and 120 °C. After leaching, the leach solution was diluted with water to dissolve Ce(SO4)2.
- HCl leaching: Yamada et al. [40] were able to recover cerium selectively by using 1.4 kmol/m3 HCl at 55 °C. The recovery of cerium is about 65% and that of lanthanum is very low. From this study it looks that cerium can be selectively dissolved. However, the recovery of cerium or lanthanum mainly depends on the presence of different compounds [25]. For example, if the lanthanum is present in a fluoride phase then it is difficult to dissolve. However, part of lanthanum in oxyfluoride may dissolve during leaching according to the following reaction.LaO0.65F1.7 + 1.3HCl ⇌ 0.433LaCl3 + 0.566LaF3 + 0.65 H2O
Acid Leaching Together with a Reductant
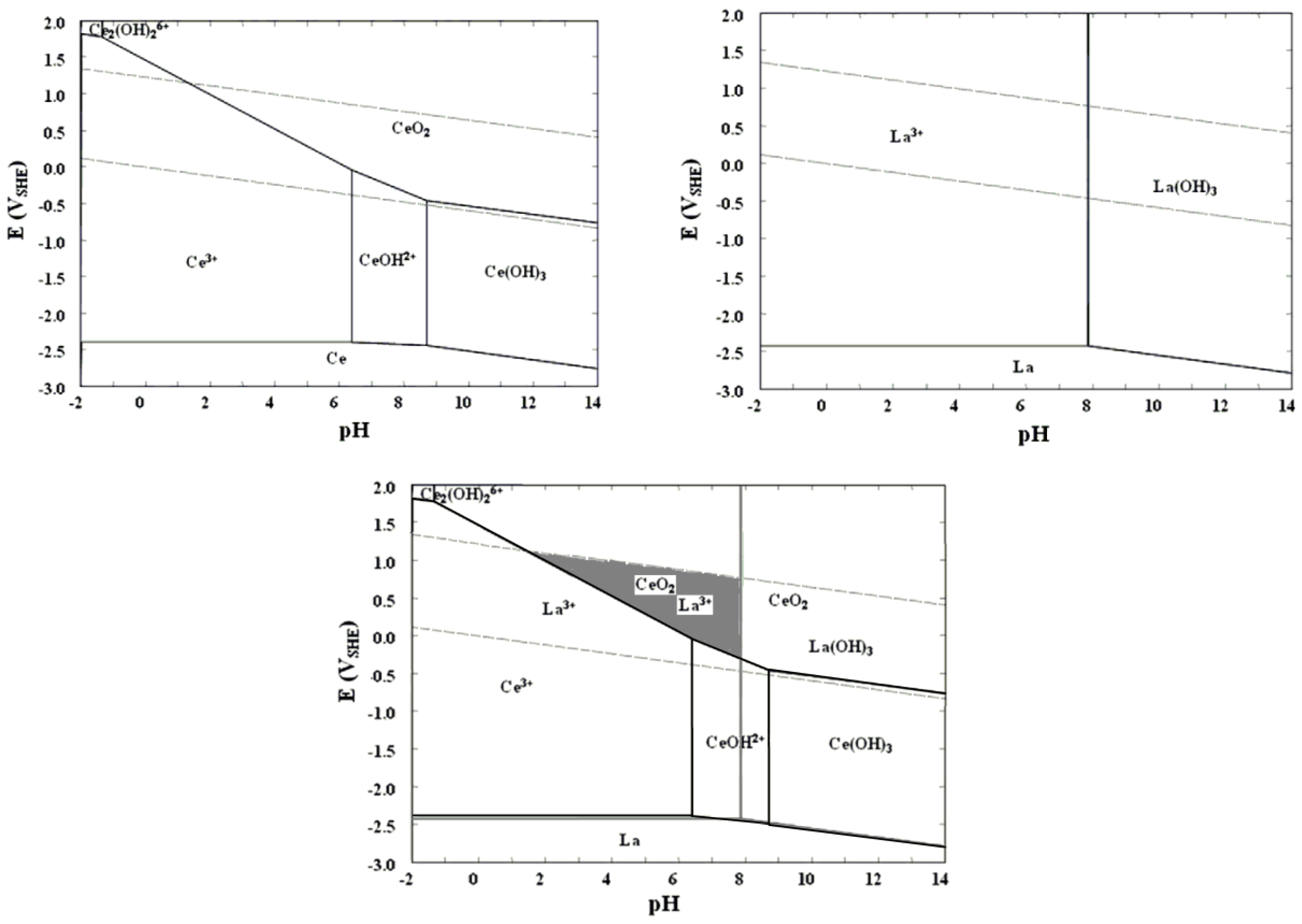
3.2.3. Recovery of REEs from Polishing Waste Leach Solutions
3.2.4. Other Processes for REE Recovery
4. Applications of Recovered Cerium
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2015; ISBN 1466576383. [Google Scholar]
- Gambogi, J. Rare earths. In Mineral Commodity Summeries; USGS: Reston, VA, USA, 2018; pp. 132–133. [Google Scholar]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Guyonnet, D.; Planchon, M.; Rollat, A.; Escalon, V.; Tuduri, J.; Charles, N.; Vaxelaire, S.; Dubois, D.; Fargier, H. Material flow analysis applied to rare earth elements in Europe. J. Clean. Prod. 2015, 107, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Gambogi, J. USGS 2014 Minerals Yearbook: Rare Earths; USGS: Reston, VA, USA, 2016. [Google Scholar]
- Argus Media Analysing the Changing Global Rare Earths Supply and Demand Outlook. Available online: http://www.argusmedia.jp/~/media/files/pdfs/regional-specific/jp/downloads/argus-metal-pages-forum082016-rareearths.pdf/?la=en (accessed on 16 February 2017).
- Tercero Espinoza, L.; Hummen, T.; Brunot, A.; Hovestad, A.; Peña Garay, I.; Velte, D.; Smuk, L.; Todorovic, J.; Van Der Eijk, C.; Joce, C. Critical Raw Materials Substitution Profiles; CRM InnoNet: Karlsruhe, Germany, 2015. [Google Scholar]
- Lucas, J.; Lucas, P.; Le Mercier, T.; Rollat, A.; Davenport, W.; Le Mercier, T.; Rollat, A.; Davenport, W. Chapter 12—Polishing with Rare Earth Oxides Mainly Cerium Oxide CeO2. In Rare Earths; Elsevier: Amsterdam, The Netherlands, 2015; pp. 191–212. ISBN 978-0-444-62735-3. [Google Scholar]
- Cook, L.M. Chemical processes in glass polishing. J. Non-Cryst. Solids 1990, 120, 152–171. [Google Scholar] [CrossRef]
- Hedrick, J.B.; Sinha, S.P. Cerium-based polishing compounds: Discovery to manufacture. J. Alloys Compd. 1994, 207–208, 377–382. [Google Scholar] [CrossRef]
- Kato, K.; Yoshioka, T.; Okuwaki, A. Study for recycling of ceria-based glass polishing powder. Ind. Eng. Chem. Res. 2000, 39, 943–947. [Google Scholar] [CrossRef]
- Matsui, H.; Harada, D.; Takeuchi, M. Method for Recovery of Cerium Oxide. U.S. Patent 20130152483A1, 20 June 2013. [Google Scholar]
- Xu, T.; Peng, H. Formation cause, composition analysis and comprehensive utilization of rare earth solid wastes. J. Rare Earths 2009, 27, 1096–1102. [Google Scholar] [CrossRef]
- Janoš, P.; Ederer, J.; Pilařová, V.; Henych, J.; Tolasz, J.; Milde, D.; Opletal, T. Chemical mechanical glass polishing with cerium oxide: Effect of selected physico-chemical characteristics on polishing efficiency. Wear 2016, 362–363, 114–120. [Google Scholar] [CrossRef]
- Komiya, H.; Yamaguchi, S.; Hisatsune, T.; Takenaka, A.; Yonemori, S. Method for Evaluating the Quality of Abrasive Grains, Polishing Method and Abrasive for Polishing Glass. U.S. Patent 7025796B2, 11 April 2006. [Google Scholar]
- Ikeda, H.; Akagami, Y. Highly efficient polishing technology for glass substrates using tribo-chemical polishing with electrically controlled slurry. J. Manuf. Process. 2013, 15, 102–107. [Google Scholar] [CrossRef]
- Kaller, A. The basic mechanism of glass polishing. Naturwissenschaften 2000, 87, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Bhushan, B. Physics and tribology of chemical mechanical planarization. J. Phys. Condens. Matter 2008, 20, 225011–225023. [Google Scholar] [CrossRef]
- Moon, W.-J.; Na, S.-O.; Oh, H.-Y. Method for Recycling Cerium Oxide Abrasive. U.S. Patent 20110219704A1, 15 September 2011. [Google Scholar]
- Ozaki, T.; Machida, K.; Adachi, G. Recovery of rare earths from used polishes by chemical vapor transport process. Mater. Sci. Forum 1999, 315–317, 297–305. [Google Scholar] [CrossRef]
- Ozaki, T.; Machida, K.; Adachi, G. Extraction and mutual separation of rare earths from used polishes by chemical vapor transport. Metall. Mater. Trans. B 1999, 30, 45–51. [Google Scholar] [CrossRef]
- Janoš, P.; Kuráň, P.; Ederer, J.; Š͗astný, M.; Vrtoch, L.; Pšenička, M.; Henych, J.; Mazanec, K.; Skoumal, M. Recovery of Cerium Dioxide from Spent Glass-Polishing Slurry and Its Utilization as a Reactive Sorbent for Fast Degradation of Toxic Organophosphates. Adv. Mater. Sci. Eng. 2015, 2015, 241421. [Google Scholar] [CrossRef]
- Poscher, A.; Luidold, S.; Antrekowitsch, H. Extraction of cerium and lanthanum from spent glass polishing agent. In Materials Science & Technology 2013; London, I.M., Goode, J.R., Moldoveanu, G., Rayat, M.S., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montréal, QC, Canada, 2013; pp. 543–552. [Google Scholar]
- Poscher, A.; Luidold, S.; Schnideritsch, H.; Antrekowitsch, H. Extraction of Lanthanides from Spent Polishing Agent. In Proceedings of the ERES2014-1st European Rare Earth Resources Conference, Milos, Greece, 4–7 September 2014; pp. 209–222. [Google Scholar]
- Poscher, A.; Luidold, S.; Schnideritsch, H.; Antrekowitsch, H. Extraction of Lanthanides from Spent Polishing Agent; Elsevier Inc.: San Diego, CA, USA, 2015; ISBN 9780128023280. [Google Scholar]
- Henry, P.; Lamotte, S.; Bier, J. Recycling of rare earth materials at Hydrometal (Belgium). In 52nd Conference of Metallurgists (COM), Hosting by Materials Science Technology Conference (MS&T); London, I.M., Goode, J.R., Moldoveanu, G., Rayat, M.S., Eds.; Canadian Institute of Mining, Metallurgy and Petroleum: Montréal, QC, Canada, 2013; pp. 537–542. [Google Scholar]
- Wang, X.; Liu, J.; Yang, Q.; Du, J.; Wang, F.; Tao, W. Decomposition process and kinetics of waste rare earth polishing powder TG-DTA-FTIR studies. J. Therm. Anal. Calorim. 2012, 109, 419–424. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, U.S.; Byeon, M.S.; Kang, W.K.; Hwang, K.T.; Cho, W.S. Recovery of cerium from glass polishing slurry. J. Rare Earths 2011, 29, 1075–1078. [Google Scholar] [CrossRef]
- Byeon, M.S.; Kim, J.Y.; Hwang, K.T.; Kim, U.; Cho, W.S.; Kang, W.K. Recovery and purification of cerium from glass polishing slurry. In Proceedings of the 18th International Conference on Composite Materials, Jeju, Korea, 21–26 August 2011. [Google Scholar]
- Kumar, V.; Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Lee, J.Y. Recovery of Rare Earth Metals (REMs) from Primary and Secondary Resources: A Review. In Rare Metal Technology 2014; Neale, R., Neelameggham, N.R., Alam, S., Oosterhof, H., Jha, A., Wang, S., Eds.; Wiley: San Diego, CA, USA, 2014; pp. 81–88. [Google Scholar]
- Sims, Z.C.; Weiss, D.; McCall, S.K.; McGuire, M.A.; Ott, R.T.; Geer, T.; Rios, O.; Turchi, P.A.E. Cerium-Based, Intermetallic-Strengthened Aluminum Casting Alloy: High-Volume Co-product Development. JOM 2016, 68, 1940–1947. [Google Scholar] [CrossRef]
- Willbold, E.; Gu, X.; Albert, D.; Kalla, K.; Bobe, K.; Brauneis, M.; Janning, C.; Nellesen, J.; Czayka, W.; Tillmann, W. Effect of the addition of low rare earth elements (lanthanum, neodymium, cerium) on the biodegradation and biocompatibility of magnesium. Acta Biomater. 2015, 11, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Zhang, J.; Chen, H.-L.; Su, Y.-H.; Kuo, C.-L.; Su, Y.-H.; Chen, S.-H.; Lin, K.-J.; Hsieh, P.-H.; Hwang, W.-S. Effects of rare earth metals on steel microstructures. Materials 2016, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.; Lucas, P.; Le Mercier, T.; Rollat, A.; Davenport, W. Epilogue. In Rare Earths; Elsevier: Amsterdam, The Netherlands, 2015; pp. 351–362. ISBN 9780444627353. [Google Scholar]
- Lucas, J.; Lucas, P.; Le Mercier, T.; Rollat, A.; Davenport, W. Chapter 18—Rare Earth Recycle; Elsevier: Amsterdam, The Netherlands, 2015; pp. 333–350. [Google Scholar] [CrossRef]
- Um, N.; Hirato, T. Dissolution Behavior of La2O3, Pr2O3, Nd2O3, CaO and Al2O3 in Sulfuric Acid Solutions and Study of Cerium Recovery from Rare Earth Polishing Powder Waste via Two-Stage Sulfuric Acid Leaching. Mater. Trans. 2013, 54, 713–719. [Google Scholar] [CrossRef]
- Lebedeva, M.I.; Dzidziguri, E.L.; Argatkina, L.A. Research of structure and polishing properties of nanopowders based on cerium dioxide. In Nanostructures, Nanomaterials, and Nanotechnologies to Nanoindustry; Apple Academic Press: Waretown, NJ, USA, 2014; pp. 215–225. [Google Scholar]
- Borra, C.R.; Vlugt, T.J.; Yang, Y.; Offerman, S.E. Characterisation of glass polishing waste samples. In Proceedings of the ERES 2017: The Second Conference on European Rare Earth Resources, Santorini, Greece, 28–31 May 2017; pp. 215–216. [Google Scholar]
- Tanaka, M.; Oki, T.; Koyama, K.; Narita, H.; Oishi, T. Recycling of Rare Earths from Scrap, 1st ed.; Elsevie: Amsterdam, The Netherlands, 2013; Volume 43, ISBN 9780444595362. [Google Scholar]
- Kato, K.; Yoshioka, T.; Okuwaki, A. Recyle of Ceria-Based Glass Polishing Powder Using NaOH Solution. Nippon Kagaku Kaishi 2000, 10, 725–732. [Google Scholar] [CrossRef]
- Janoš, P.; Novak, J.; Broul, M. A Procedure for Obtaining Salts of Rare Earth Elements. U.S. Patent 21039151, 31 October 1988. [Google Scholar]
- Terziev, A.L.; Minkova, N.L.; Todorovsky, D.S. Regeneration of waste rare earth oxides based polishing materials. Bulg. Chem. Commun. 1996, 29, 274–284. [Google Scholar]
- Um, N.; Hirato, T. A hydrometallurgical method of energy saving type for separation of rare earth elements from rare earth polishing powder wastes with middle fraction of ceria. J. Rare Earths 2016, 34, 536–542. [Google Scholar] [CrossRef]
- Um, N.; Hirato, T. Conversion kinetics of cerium oxide into sodium cerium sulfate in Na2SO4-H2SO4-H2O solutions. Mater. Trans. 2012, 53, 1992–1996. [Google Scholar] [CrossRef]
- Um, N.; Hirato, T. Synthesis of Sodium Cerium Sulfate (NaCe(SO4)2 ·H2O) from Cerium Oxide in Sulfuric Acid Solutions. In Zero-Carbon Energy Kyoto 2011: Special Edition of Jointed Symposium of Kyoto University Global COE “Energy Science in the Age of Global Warming”; Yao, T., Ed.; Springer: Tokyo, Japan, 2012; pp. 171–176. ISBN 978-4-431-54067-0. [Google Scholar]
- Yoon, H.; Kim, C.; Kim, S.; Lee, J.; Cho, S.; Kim, J. Separation of Rare Earth and Aluminium from the Dried Powder of Waste Cerium Polishing Slurry. J. Korean Inst. Resour. Recycl. 2003, 12, 10–15. [Google Scholar]
- Um, N.; Hirato, T. Precipitation of cerium sulfate converted from cerium oxide in sulfuric acid solutions and the conversion kinetics. Mater. Trans. 2012, 53, 1986–1991. [Google Scholar] [CrossRef]
- Janoš, P.; Novák, J.; Broul, M.; Loučka, T. Regeneration of polishing powders based on REE oxides from glass polishing sludges. Chem. Prum. 1987, 37–62, 189–194. [Google Scholar]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Byrne, R.H.; Li, B. Comparative complexation behavior of the rare earths. Geochim. Cosmochim. Acta 1995, 59, 4575–4589. [Google Scholar] [CrossRef]
- Kim, E.; Osseo-Asare, K. Aqueous stability of thorium and rare earth metals in monazite hydrometallurgy: Eh–pH diagrams for the systems Th–, Ce–, La–, Nd–(PO4)–(SO4)–H2O at 25 °C. Hydrometallurgy 2012, 113–114, 67–78. [Google Scholar] [CrossRef]
- Marsac, R.; Réal, F.; Banik, N.L.; Pédrot, M.; Pourret, O.; Vallet, V. Aqueous chemistry of Ce(iv): Estimations using actinide analogues. Dalt. Trans. 2017, 46, 13553–13561. [Google Scholar] [CrossRef] [PubMed]
- Janoš, P.; Kuran, P.; Kormunda, M.; Stengl, V.; Grygar, T.M.; Dosek, M.; Stastny, M.; Ederer, J.; Pilarova, V.; Vrtoch, L. Cerium dioxide as a new reactive sorbent for fast degradation of parathion methyl and some other organophosphates. J. Rare Earths 2014, 32, 360–370. [Google Scholar] [CrossRef]




| Polishing Powder | Hardness (Mohs Scale) | Type of Glass | Hardness (Mohs Scale) |
|---|---|---|---|
| Diamond | 10 | Silica | 7 |
| Alumina | 9 | Soda lime | 5.3 |
| Zirconia | 8 | Borosilicate | 5.8 |
| Ceria | 7–8 | Lead | 4.8 |
| Oxide | La2O3 | CeO2 | Pr6O11 | Nd2O3 | Reference |
|---|---|---|---|---|---|
| wt% | 31.5 | 65 | 3.5 | - | [5] |
| 34.2 | 43.8 | 3.4 | 10.9 | [12] | |
| - | 62.1 | - | - | [13] | |
| 0–35 | 50–99 | 0–5 | 0–15 | [14] | |
| - | 30–99.9 | - | - | [9] |
| Compound, wt% | Reference | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CeO2 | La2O3 | Pr6O11 | Nd2O | F (elem.) | SiO2 | Al2O3 | Fe2O3 | Na2O | P2O5 | ZnO | K2O | CaO | PbO | ||
| 1 | 8.8 | 1.5 | - | - | - | 57.6 | 1 | - | 13.3 | - | 2.0 | 6.9 | 2.9 | - | [23] |
| 2 | 69 | 8 | - | 1 | - | 11 | 1.3 | - | 1 | 2 | 2 | - | 1 | - | [24,25,26] |
| 3 | 49–80 | 1–10 | - | 0.1–0.8 | - | 5-10 | - | 1.4–2.9 | - | - | 1.2–2.5 | - | - | 1–2 | [27] |
| 4 | 54 | - | - | - | - | 12 | - | - | - | - | - | - | - | - | [13] |
| 5 | 22.3 | 17.7 | 1.41 | 0.21 | 1.8 | 0.1 | 11.5 | 0.2 | - | - | - | - | 9.3 | - | [28] |
| 6 | 48.4 | 23.5 | - | - | 3.7 | 0.6 | 8.1 | 0.9 | 0.4 | 0.7 | 0.1 | - | 0.3 | - | [29,30] |
| 7 | 60 | 25.8 | 13.6 (elem.) | 15.3 (elem.) | [20] | ||||||||||
| 8 | 22.1 | 17.8 | 2.30 | 5.1 | - | 12.6 | 24.8 | - | - | - | - | - | - | - | [12] |
| 9 | 50 | 28 | 7.4 | 4 | 9.5 | 0.6 | - | 0.3 | - | 0.1 | - | - | 0.7 | - | [21] |
| 10 | 38 | 28 | 3.8 | 10 | 12 | 1.7 | - | 0.2 | - | 1.7 | - | - | 1.3 | - | [22] |
| Physical or Physio-Chemical Separation | Chemical Separation | Recovery of REEs/Reuse of Polishing Powder | Reference |
|---|---|---|---|
| Coarse glass particles: sieving Filtration with vibrating screen | Product: for reuse in polishing | [40] | |
| Elutriation Rapid settling: glass Slow settling (3 days): rare earths | Product: for reuse in polishing | [40] | |
| Flotation: dodecylesulfate (collector) | 40% with a purity of 44% (35% in feed sample) | [40] | |
| Flotation | HCl (1.4 kmol/m3) 55 °C, 60 min Selective Ce leaching | D2EHPA and PC-88ACe recovered preferentially over La | [40] |
| Flocculent removal: oxalic or citric acid leaching (pH–1.5) Silica removal: flotation aided by sonication | Silica: alkali leaching (pH–11.5) Roasting: 600 °C, 2 h Rare earths: Sulfuric acid (3 mol/L), S/L—1:10, 60 °C, 3 h | Other rare earths: Double sulfate precipitation, Na2SO4/REO—0.5 to 1 Ce Yield: 60% | [29,30] |
| NaOH: 4 mol/kg, 1 h 50–60 °C | Residue: for reuse in polishing Aluminum and silicon in the leach solution were recovered as zeolites | [12,41] | |
| Alkali Leaching NaOH, NaF, Na2CO3 90 °C, 2 h | Residue: for reuse in polishing | [20] | |
| NaOH (2 mol/L) KOH (3.5 mol/L) | Residue: for reuse in polishing | [13] | |
| HCl: 15–35 wt% (10–20% excess). Iodide: 10–20% excess 15–60 min | [42] | ||
| HNO3 and H2O2 H2O2/HNO3 = 0.15 80 °C, S/L: 1:5 | RE recovery: 80% Precipitation with ammonium carbonate | [43] | |
| Pure oxides Step 1: 0.4 mol/L Na2SO4 and 8 mol/L H2SO4 for double salt precipitation Step 2: RE hydroxide precipitation from double salt with NaOH Step 3: Oxidation of cerium Step 4: HCl (selective) leaching of rare earths except cerium Step 5: H2SO4 (selective) leaching of cerium | [44,45,46] | ||
| Pure oxides H2SO4 (1–8 mol/dm3), 30–90 °C | [37] | ||
| HCl: 32 wt%, H2O2: 30 wt% 80 °C, 4 h Ce recovery (>90%), La recovery (>60%) | Oxalic acid (27% excess) precipitation | [24,25,26] | |
| HNO3: 47%, H2O2: 30 wt% 70 °C, 1 h Ce recovery (>90%), La recovery (>60%) | |||
| H2SO4: 98%, 100–200 °C followed by water leaching Ce, La recovery (>90%) | |||
| HNO3 and H2O2: 20% excess (with respect to the total REE content) 70 °C, 3 h | Carbonate precipitation by CO2 and NH3 followed by annealing in rotary kiln | [23] | |
| HNO3 and H2O2 (stoichiometric) 80 °C | Oxalate precipitation | [27] | |
| Carbochlorination: Active carbon, N2-Cl2 gas, 1000 °C, | Rare earth chloride deposition: <1000 °C to 450 °C. | [21,22] | |
| Sulfation: H2SO4, 250 °C, 2 h Water leaching 50 °C, 2 h, L/S: 10 | Double sulfate precipitation | [47] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borra, C.R.; Vlugt, T.J.H.; Yang, Y.; Offerman, S.E. Recovery of Cerium from Glass Polishing Waste: A Critical Review. Metals 2018, 8, 801. https://doi.org/10.3390/met8100801
Borra CR, Vlugt TJH, Yang Y, Offerman SE. Recovery of Cerium from Glass Polishing Waste: A Critical Review. Metals. 2018; 8(10):801. https://doi.org/10.3390/met8100801
Chicago/Turabian StyleBorra, Chenna Rao, Thijs J. H. Vlugt, Yongxiang Yang, and S. Erik Offerman. 2018. "Recovery of Cerium from Glass Polishing Waste: A Critical Review" Metals 8, no. 10: 801. https://doi.org/10.3390/met8100801





