Interaction between Ni/Ti Nanomultilayers and Bulk Ti-6Al-4V during Heat Treatment
Abstract
:1. Introduction
2. Experimental Details
2.1. Deposition Technique
2.2. Characterization Technique
3. Results
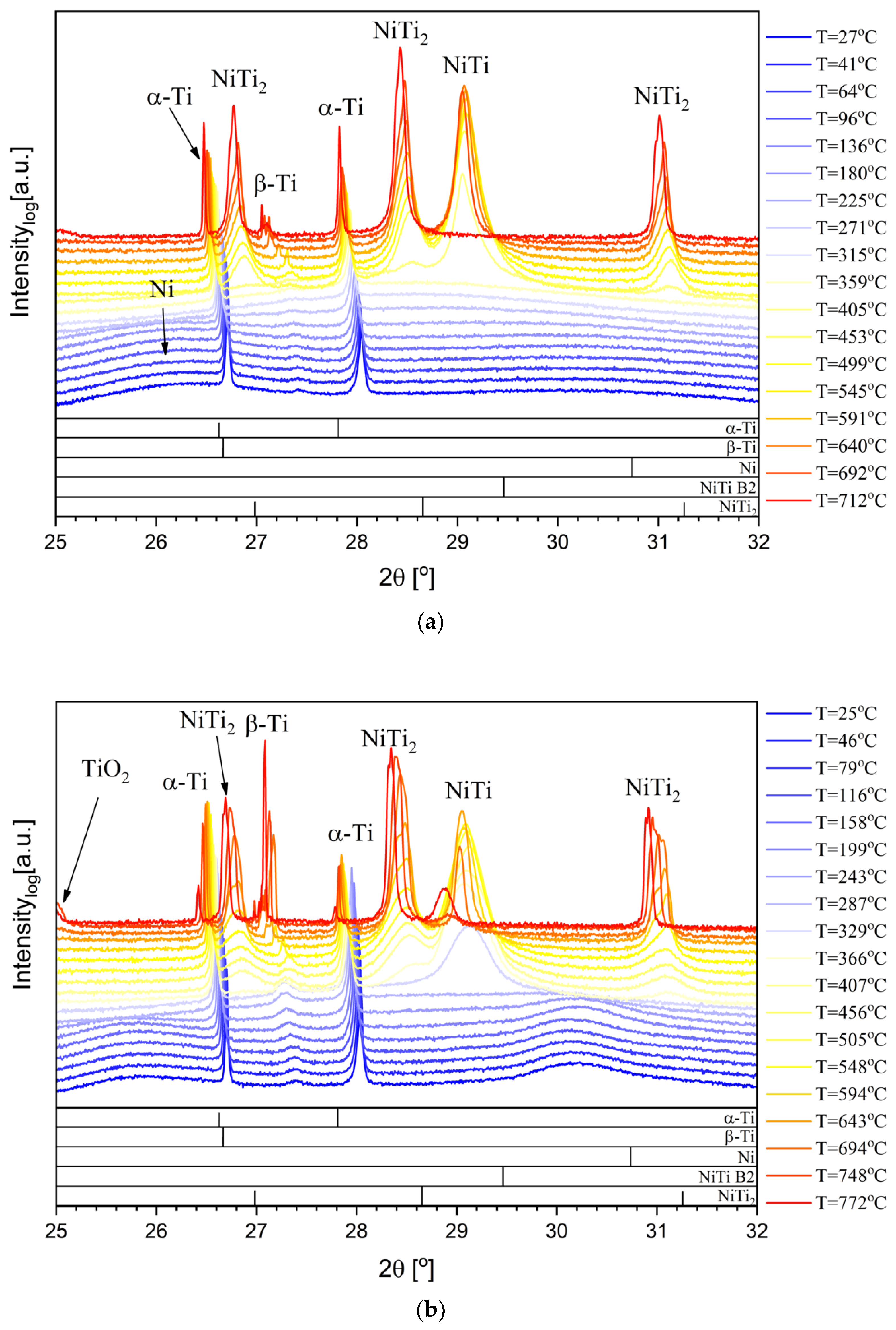
3.1. In Situ Characterization during Heat Treatment
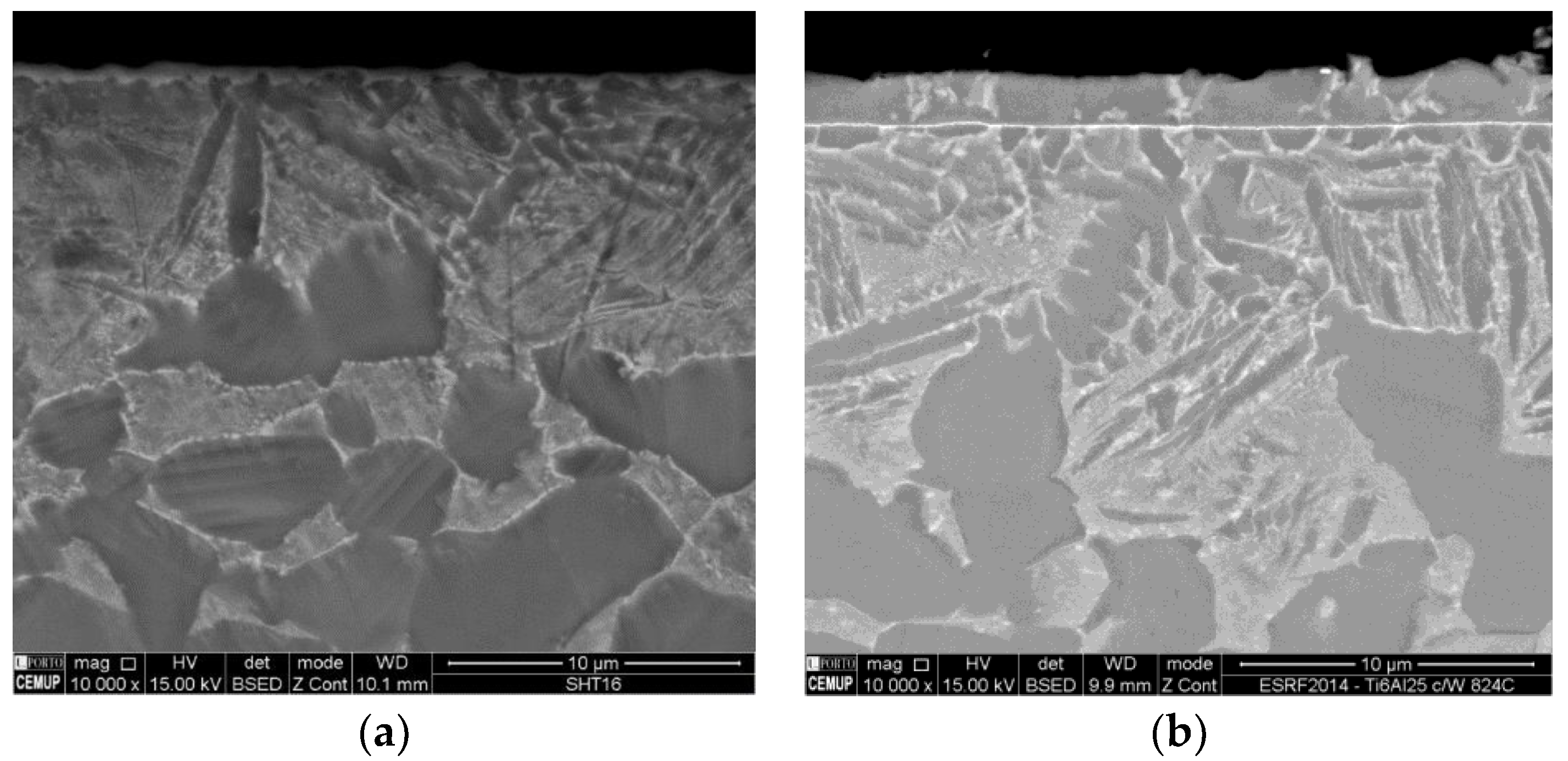
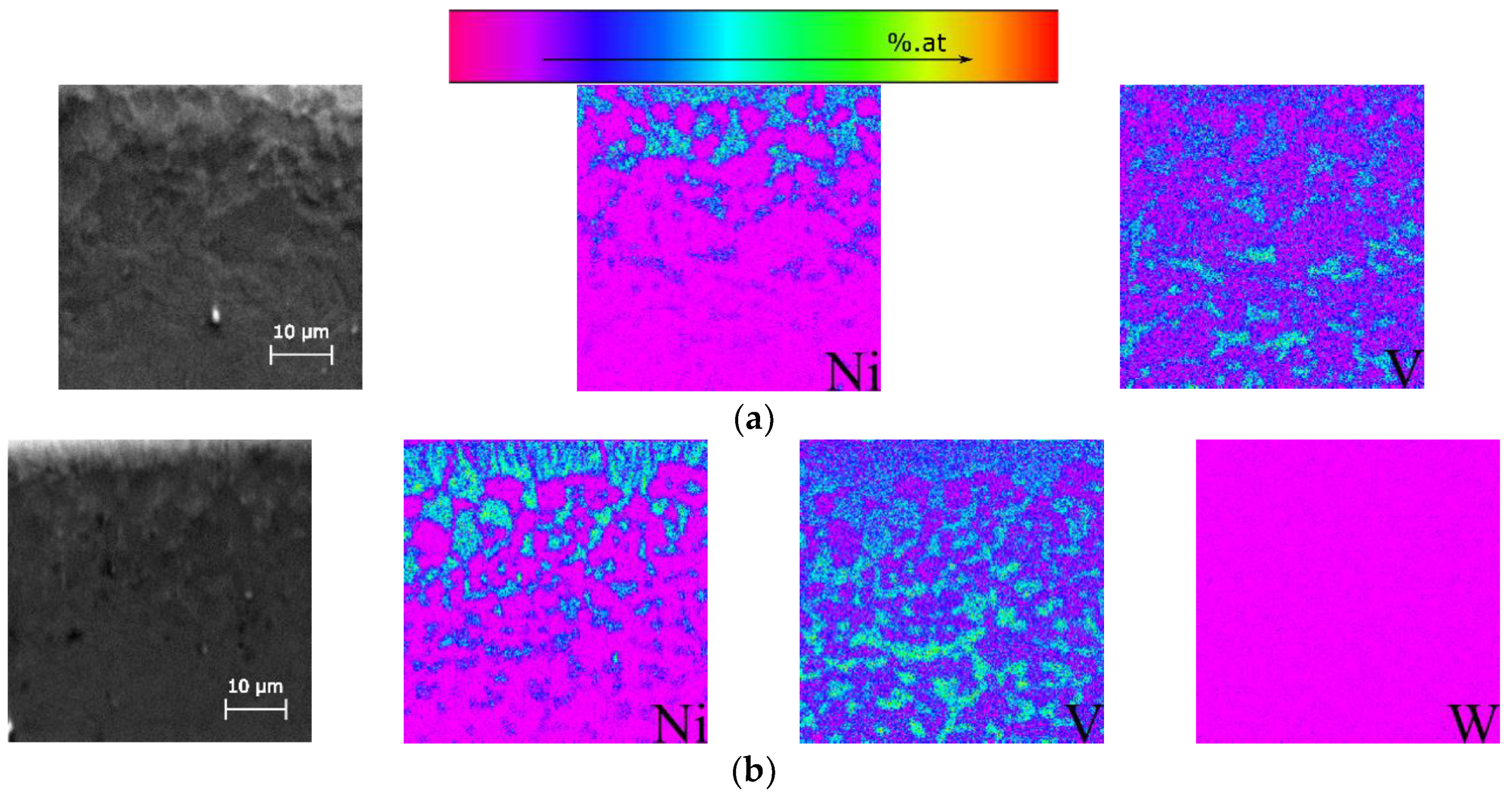
3.2. Ex Situ Characterization after Heat Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. 1996, A213, 103–114. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Zoeram, A.S.; Mousavi, S.A.A.A. Laser welding of Ti-6Al-4V to nitinol. Mater. Des. 2014, 61, 185–190. [Google Scholar] [CrossRef]
- Miranda, R.M.; Assunção, E.; Silva, R.J.C.; Oliveira, J.P.; Quintino, L. Fiber laser Welding of NiTi to Ti-6Al-4V. Int. J. Adv. Manuf. Technol. 2015, 81, 1533–1538. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Panton, B.; Zeng, Z.; Andrei, C.M.; Zhou, N.; Miranda, R.M.; Fernandes, F.M.B. Laser Joining of NiTi to Ti6Al4V using a Niobium interlayer. Acta Mater. 2016, 105, 9–15. [Google Scholar] [CrossRef]
- Shiue, R.K.; Wu, S.K.; Chen, Y.T.; Shiue, C.Y. Infrared brazing of Ti50Al50 and Ti-6Al-4V using two Ti-based filler metals. Intermetallics 2008, 16, 1083–1089. [Google Scholar] [CrossRef]
- Cao, J.; Song, X.G.; Li, C.; Zhao, L.Y.; Feng, J.C. Brazing ZrO2 ceramic to Ti–6Al–4V alloy using NiCrSiB amorphous filler foil: Interfacial microstructure and joint properties. Mater. Charact. 2013, 81, 85–91. [Google Scholar] [CrossRef]
- Ferrante, M.; Pigoretti, E.V. Diffusion bonding of Ti6Al4V to AISI 316L stainless steel: Mechanical resistance and interface microstructure. J. Mater. Sci. 2002, 37, 2825–2833. [Google Scholar] [CrossRef]
- Kundu, S.; Sam, S.; Chatterjee, S. Interface microstructure and strength properties of Ti6Al4V and microduplex stainless steel diffusion bonded joints. Mater. Des. 2011, 32, 2997–3003. [Google Scholar] [CrossRef]
- Kundu, S.; Sam, S.; Mishra, B.; Chatterjee, S. Diffusion bonding of microduplex stainless steel and Ti alloy with and without interlayer: Interface microstructure and strength properties. Metall. Mater. Trans. 2014, 45A, 371–383. [Google Scholar] [CrossRef]
- Balasubramanian, M. Characterization of diffusion-bonded titanium alloy and 304 stainless steel with Ag as an interlayer. Int. J. Adv. Manuf. Technol. 2016, 82, 153–162. [Google Scholar] [CrossRef]
- Simões, S.; Viana, F.; Ramos, A.S.; Vieira, M.T.; Vieira, M.F. Reaction zone formed during diffusion bonding of TiNi to Ti6Al4V using Ni/Ti nanolayers. J. Mater. Sci. 2013, 48, 7718–7727. [Google Scholar] [CrossRef]
- Cavaleiro, A.J.; Ramos, A.S.; Fernandes, F.M.B.; Schell, N.; Vieira, M.T. In Situ Characterization of NiTi/Ti6Al4V Joints During Reaction-Assisted Diffusion Bonding Using Ni/Ti Multilayers. J. Mater. Eng. Perform. 2014, 23, 1625–1629. [Google Scholar] [CrossRef]
- Cavaleiro, A.J.; Ramos, A.S.; Vieira, M.T.; Martins, R.M.S.; Fernandes, F.M.B.; Morgiel, J.; Baehtz, C. Phase transformations in Ni/Ti multilayers investigated by synchrotron radiation-based x-ray diffraction. J. Alloys Compd. 2015, 646, 1165–1171. [Google Scholar] [CrossRef]
- Cavaleiro, A.J.; Santos, R.J.; Ramos, A.S.; Vieira, M.T. In-situ thermal evolution of Ni/Ti multilayer thin films. Intermetallics 2014, 51, 11–17. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Zhao, Y.; Liu, Q.; Zhu, L.; Song, X.; Zhang, Y.; Hao, J. Diffusion behavior and mechanical properties of Cu-Ni coating on TC4 alloy. Vacuum 2017, 143, 150–157. [Google Scholar] [CrossRef]
- Bitzer, M.; Bram, M.; Buchkremer, H.P.; Stöver, D. Phase transformation behavior of hot isostatically pressed NiTi-X (X = Ag, Nb, W) alloys for functional engineering applications. J. Mater. Eng. Perform. 2012, 21, 2535–2545. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, M.; Kulkarni, S.K.; Kharrazi, S.; Gupta, A.; Chaudhari, S.M. Thermal stability of nanometer range Ti/Ni multilayers. Thin Solid Films 2006, 515, 2213–2219. [Google Scholar] [CrossRef]
- Hubbel, J.H.; Seltzer, S.M. Tables of X-ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients from 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; NIST Interagency/Internal Report (NISTIR): Gaithersburg, MD, USA, 1995. [Google Scholar]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaleiro, A.J.; Ramos, A.S.; Fernandes, F.B.; Baehtz, C.; Vieira, M.T. Interaction between Ni/Ti Nanomultilayers and Bulk Ti-6Al-4V during Heat Treatment. Metals 2018, 8, 878. https://doi.org/10.3390/met8110878
Cavaleiro AJ, Ramos AS, Fernandes FB, Baehtz C, Vieira MT. Interaction between Ni/Ti Nanomultilayers and Bulk Ti-6Al-4V during Heat Treatment. Metals. 2018; 8(11):878. https://doi.org/10.3390/met8110878
Chicago/Turabian StyleCavaleiro, André João, Ana Sofia Ramos, Francisco Braz Fernandes, Carsten Baehtz, and Maria Teresa Vieira. 2018. "Interaction between Ni/Ti Nanomultilayers and Bulk Ti-6Al-4V during Heat Treatment" Metals 8, no. 11: 878. https://doi.org/10.3390/met8110878





