Microstructural and Mechanical Characterization of the Transition Zone of 9%Ni Steel Cladded with Ni-Based Superalloy 625 by GTAW-HW
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Welding
2.2. Microstructural Characterization
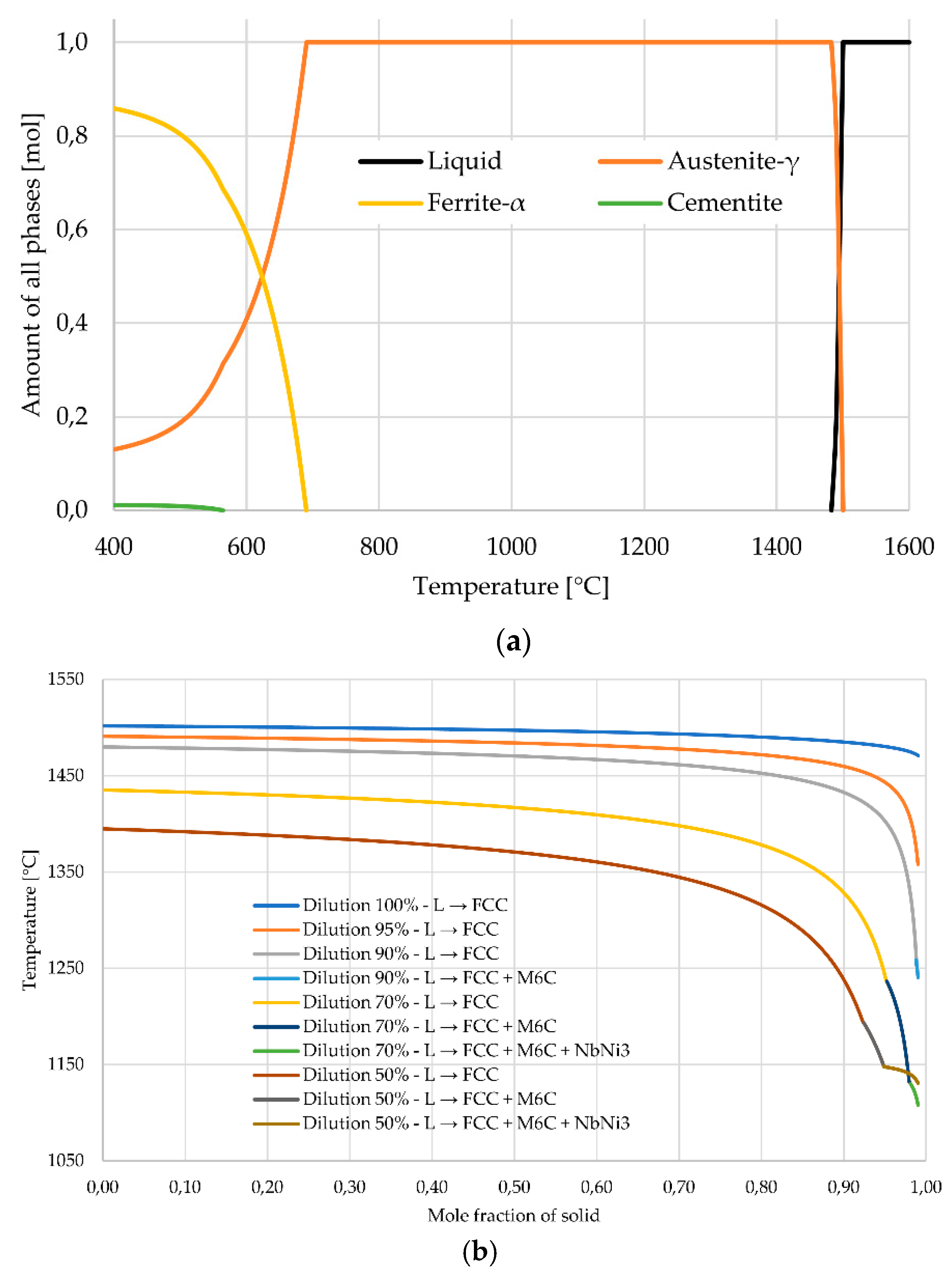
2.3. Thermodynamic Simulation
2.4. Mechanical Characterization
3. Results
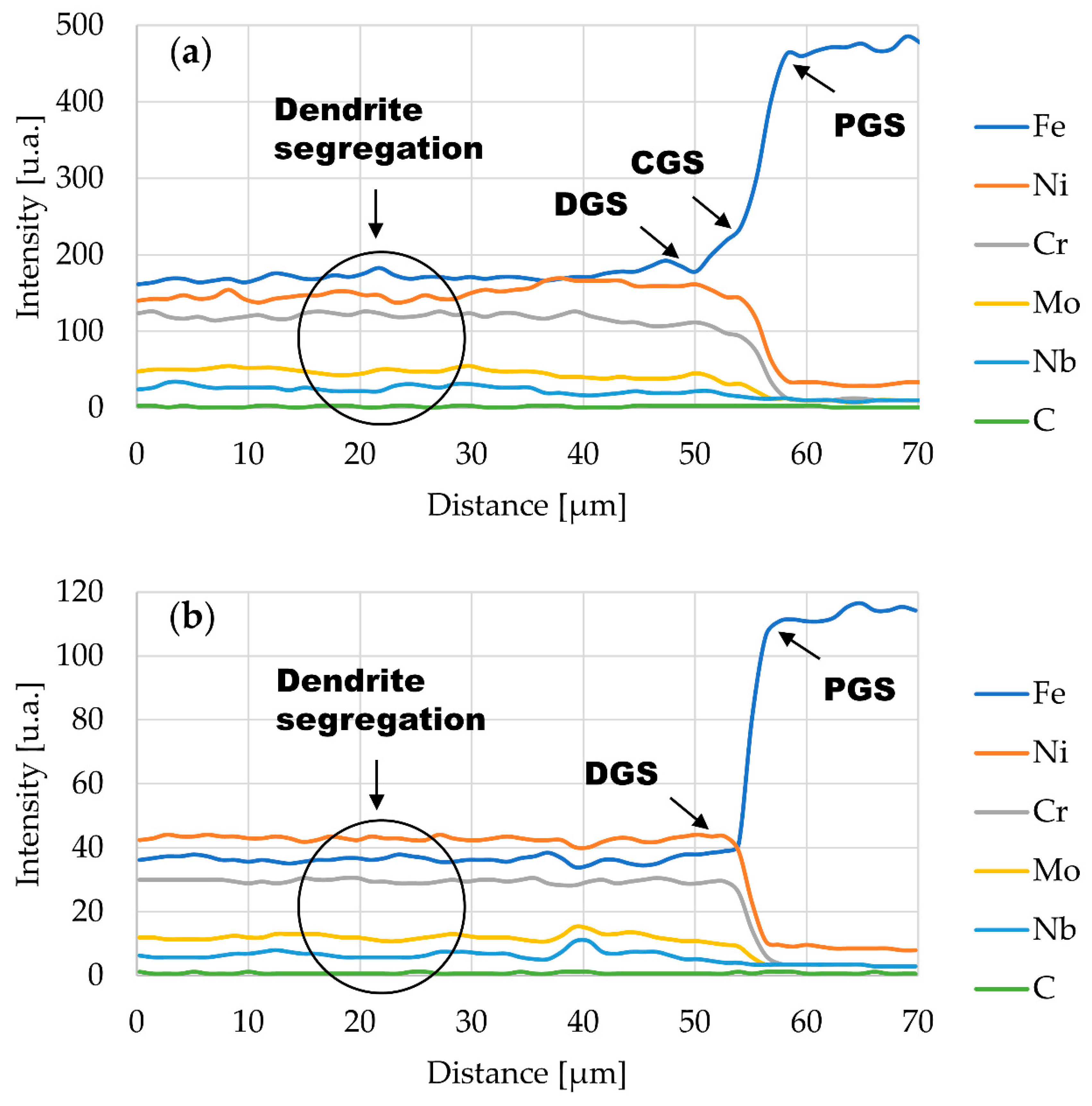
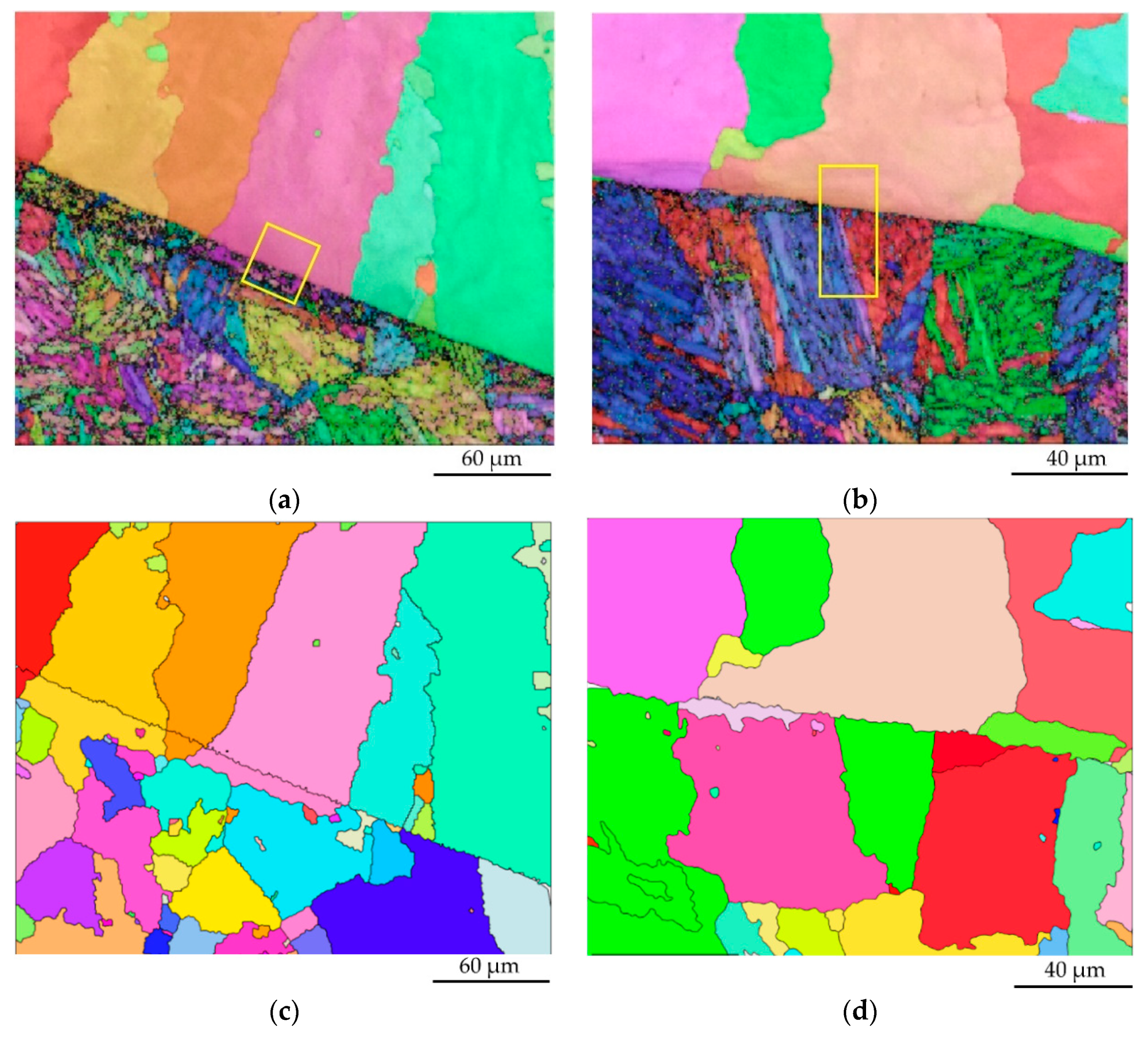
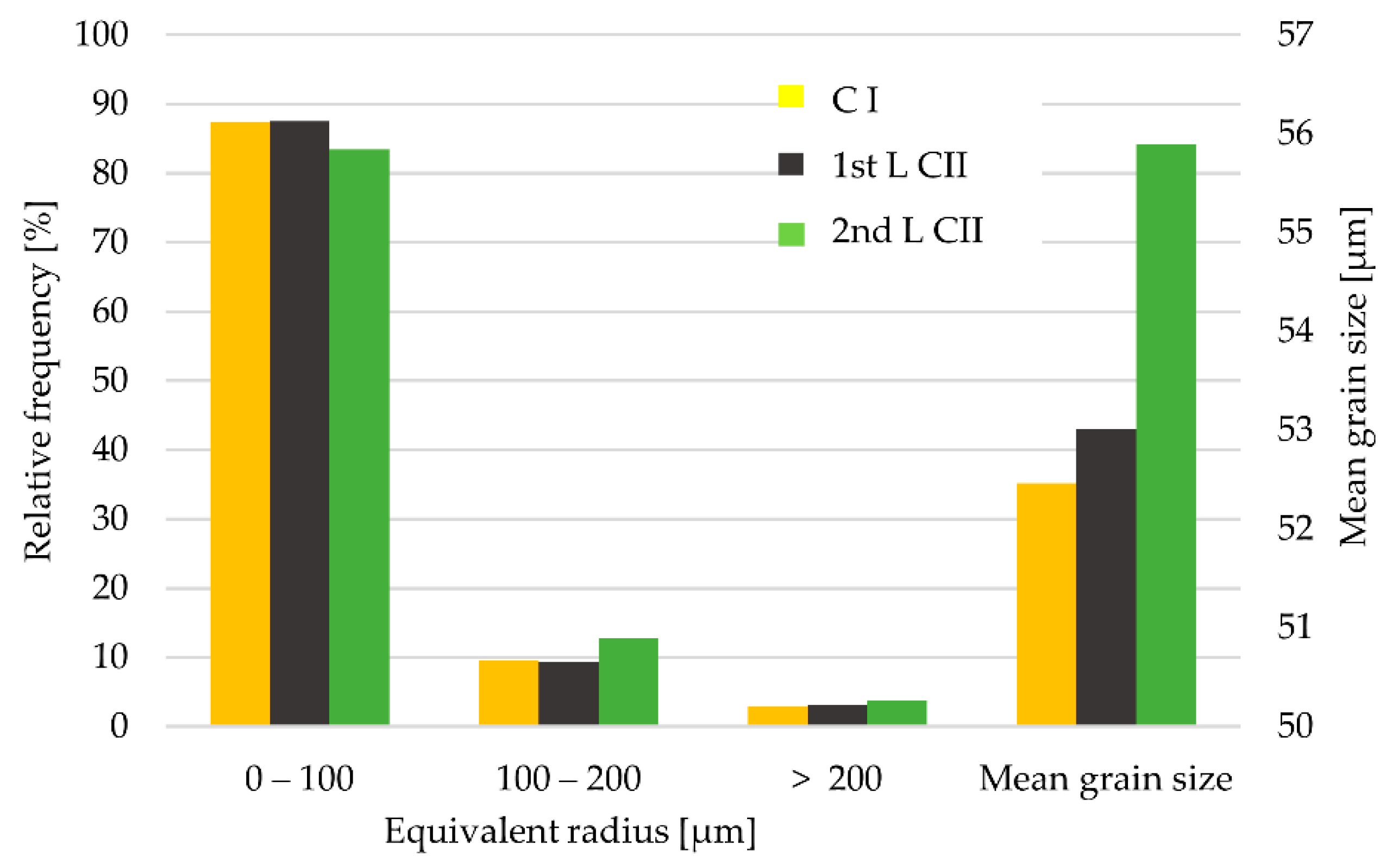
3.1. Microstructural Characterization
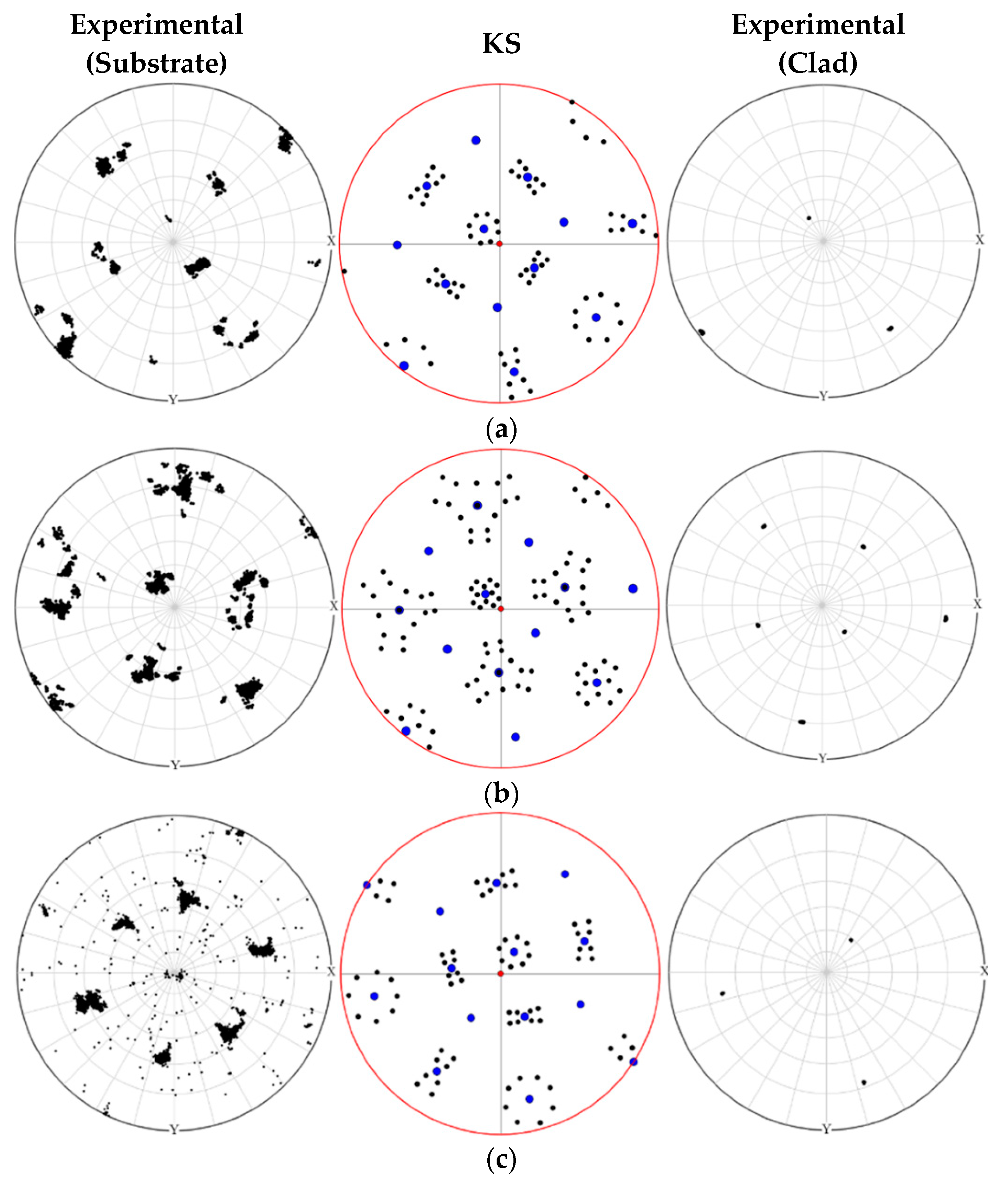
3.2. Crystallographic Relationship between the Substrate and the Clad
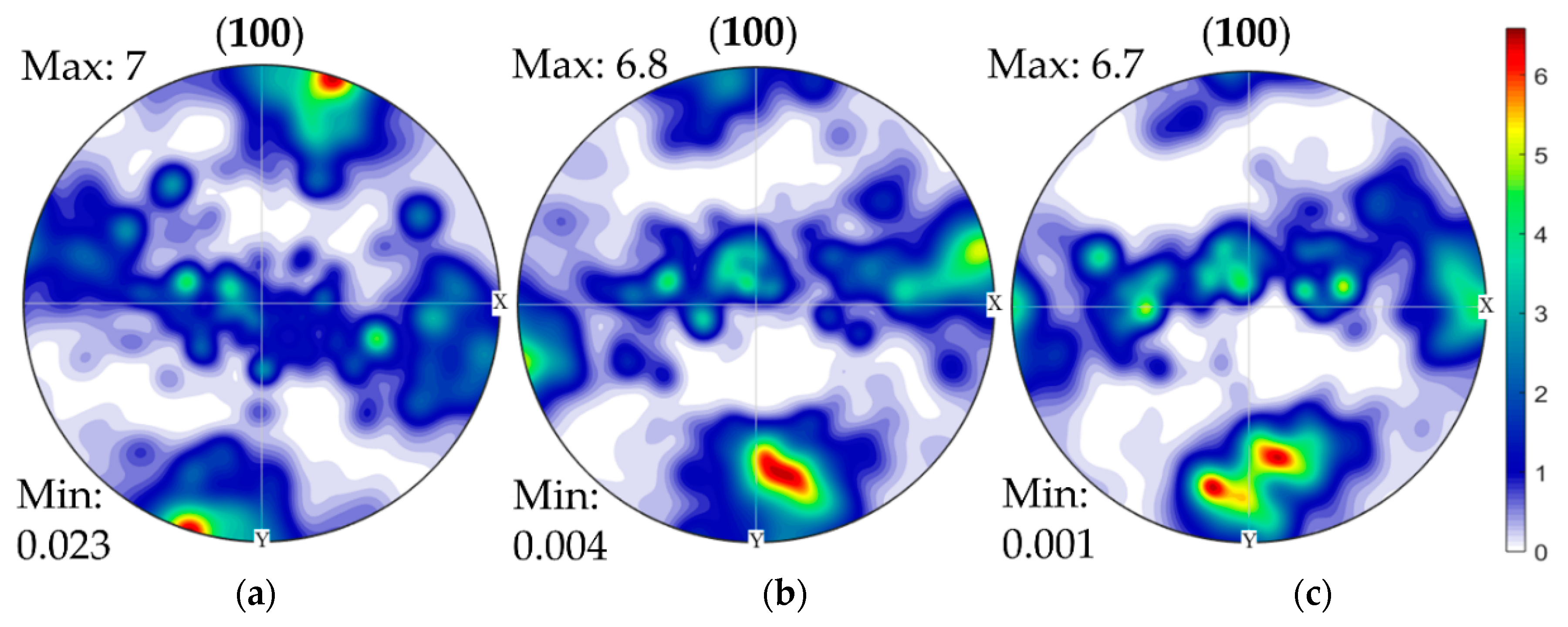
3.3. Crystallographic Texture of the Clad Layers
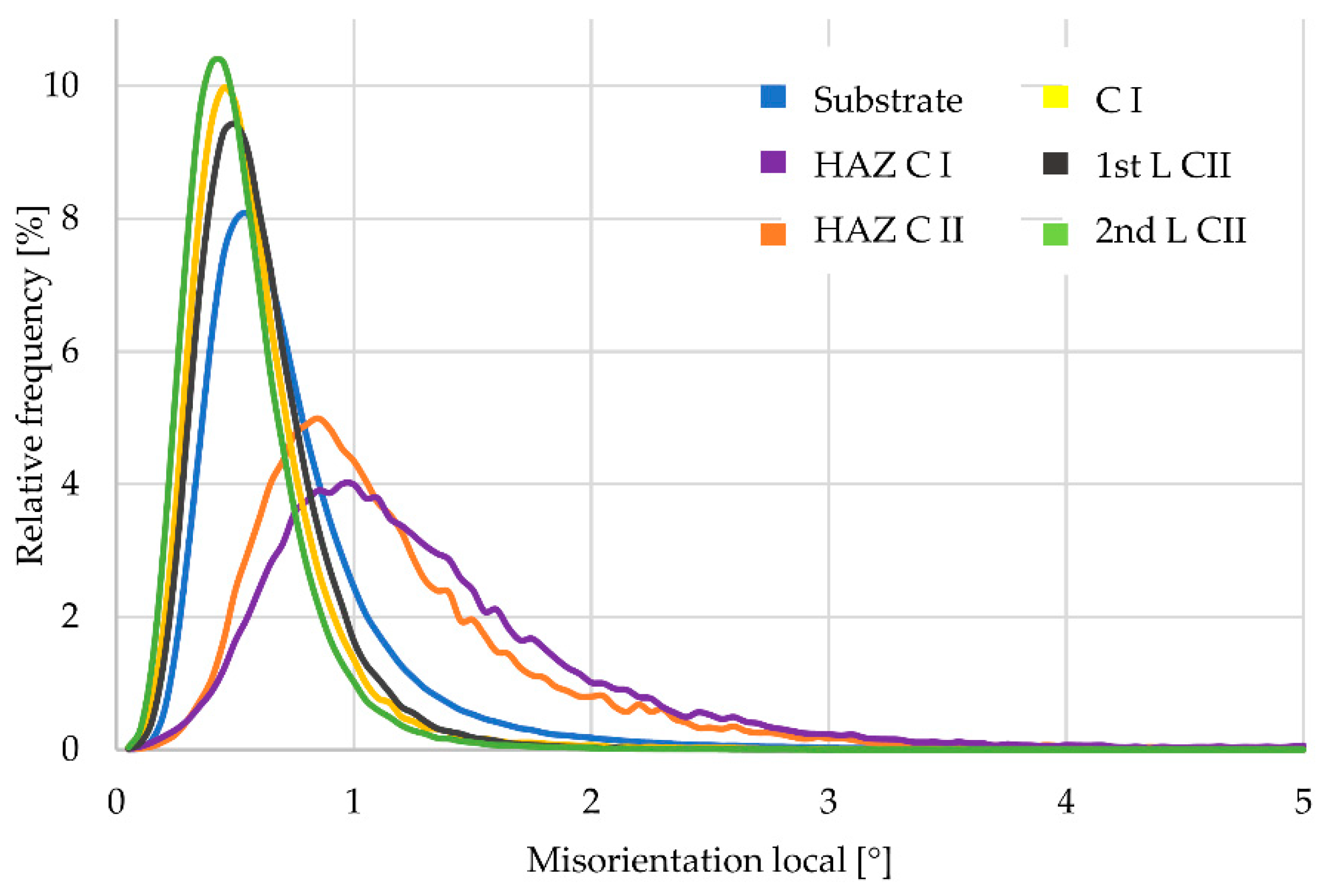
3.4. Kernel Average Neighbor Misorientation (KANM)
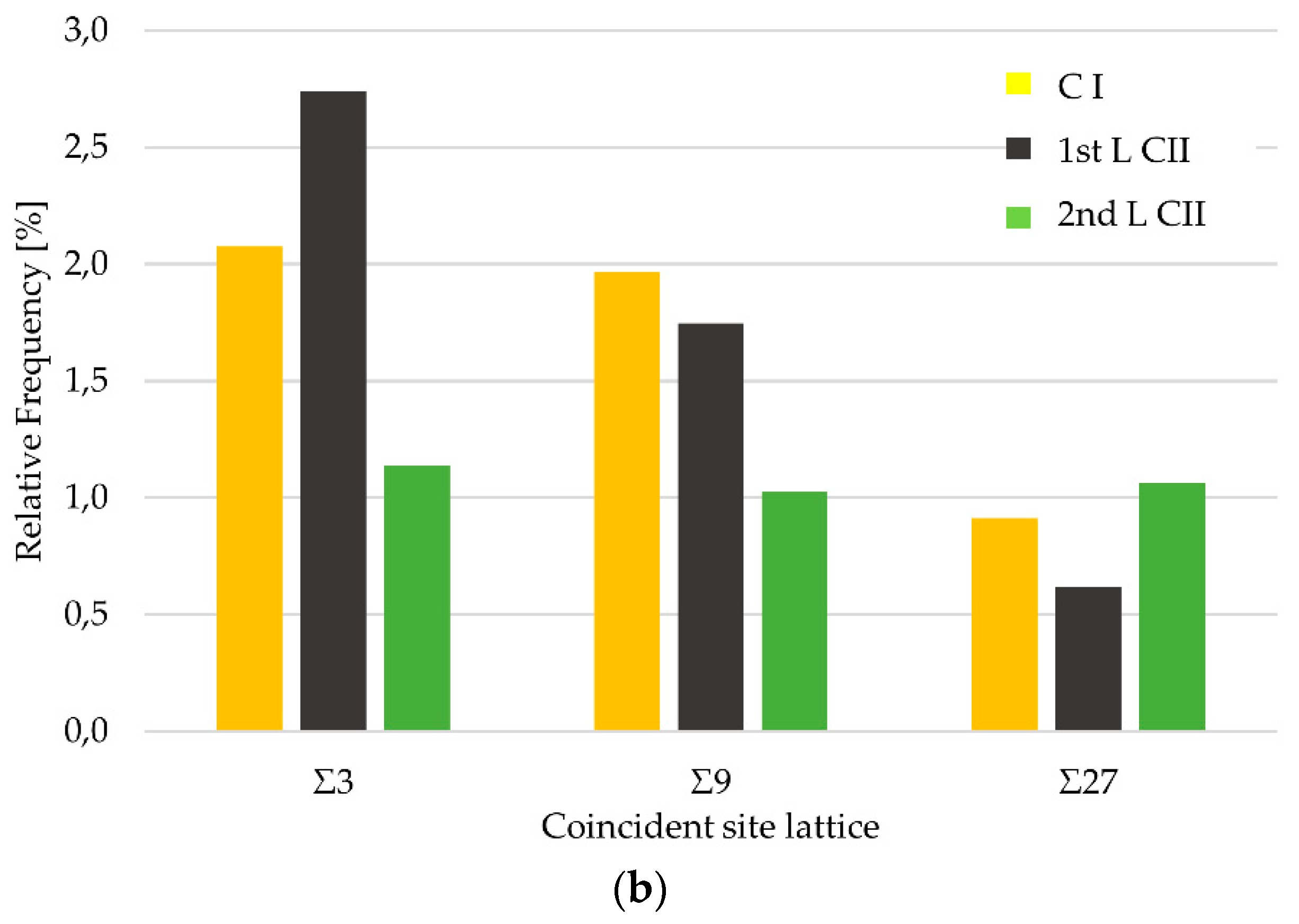
3.5. Grain Boundary Characteristic Distribution (GBCD)
3.6. Mechanical Properties
4. Discussion
5. Conclusions
- Although there is martensite in the transition zone of the substrate/clad, this zone does not present a high density of finely dispersed carbides (MC, M7C3, and M23C6 type), this being very beneficial to its ductility and fracture toughness;
- The unexpected presence of type II boundaries in the dissimilar weld with a base metal that does not have allotropic transformations (δ → α) during the welding thermal cycle requires further analysis of the stress corrosion cracking behavior of the 9%Ni steel cladded pipe in environments with a high H2S concentration, such as the one the in the CO2 injection unit;
- Although the clads have residual strain and stress, MC-type carbides, low melting point eutectic γ/Laves, and a partially oriented microstructure in the easy growth direction, the weldability of the 9%Ni steel cladded with the Ni-based superalloy 625 is good as there were no discontinuities and mainly no solidification nor liquation hot cracks;
- The transition zone of substrate/clad has good metallurgical characteristics and mechanical properties, which allows the oil and gas industry to use the 9%Ni steel pipe cladded with Ni-based superalloy 625 in CO2 injection units.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beltrão, R.L.C.; Sombra, C.L.; Lage, C.V.M.; Netto, J.R.F.; Henriques, C.C.D. Challenges and New Technologies for the Development of the Pre-Salt Cluster, Santos Basin, Brazil. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 4–7 May 2009. [Google Scholar] [CrossRef]
- Ding, M.; Yuan, F.; Wang, Y.; Xia, X.; Chen, W.; Liu, D. Oil Recovery from a CO2 Injection in Heterogeneous Reservoirs: The Influence of Permeability Heterogeneity, CO2-Oil Miscibility and Injection Pattern. J. Nat. Gas Sci. Eng. 2017, 44, 140–149. [Google Scholar] [CrossRef]
- Bowman, J. The Greenhouse Effect. Land Use Policy 1982, 16, 101–108. [Google Scholar] [CrossRef]
- Vollmer, L.W. Hydrogen Sulphide corrosion Cracking of Steel. Corrosion 1952, 8, 326–332. [Google Scholar] [CrossRef]
- NACE International Standard TM00177-1996. Laboratory Testing of Metals for Resistance to Sulfide Stress Cracking and Stress Corrosion Cracking in H2S Environments; NACE: Houston, TX, USA, 1996; pp. 1–32. [Google Scholar]
- Zhao, X.Q.; Pan, T.; Wang, Q.F.; Su, H.; Yang, C.F.; Yang, Q.X. Effect of Tempering Temperature on Microstructure and Mechanical Properties of Steel Containing Ni of 9%. J. Iron Steel Res. Int. 2011, 18, 47–51. [Google Scholar] [CrossRef]
- Nippes, E.; Balaguer, J. A Study of the Weld Heat-Affected Zone Toughness of 9% Nickel Steel. Weld. Res. Suppl. 1986, 57, 237–243. [Google Scholar]
- Zeemann, A.; Emygdio, G. 9%Ni Steel Alloy for H2S Service. In Proceedings of the Corrosion, Santo Antonio, TX, USA, 9–13 Mach 2014; NACE: Houston, TX, USA, 2014. [Google Scholar]
- Gooch, T.G. Effect of Iron Dilution on Corrosion Resistance of Ni-Cr-Mo Alloy Cladding. Br. Corros. J. 1996, 31, 4–9. [Google Scholar] [CrossRef]
- Rao, N.V.; Reddy, M.G.; Nagarjuna, S. Weld Overlay Cladding of High Strength Low Alloy Steel with Austenitic Stainless Steel—Structure and Properties. Mater. Des. 2011, 32, 2496–2506. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.; Ding, X.; Shoji, T. Materials Characterization Microstructures and Microhardness at Fusion Boundary of 316 Stainless Steel/Inconel 182 Dissimilar Welding. Mater. Charact. 2015, 107, 255–261. [Google Scholar] [CrossRef]
- Silva, C.C.; Miranda, H.C.; Farias, J.P.; De Abreu, H.F.G. Chemistry and Crystallographic Evaluation of Ni-based Alloy and Steel Dissimilar Interface. In Proceedings of the 9th International Conference in Welding Research, Chicago, IL, USA, 4–8 June 2013. [Google Scholar]
- Beaugrand, V.C.M.; Smith, L.S.; Gittos, M.F. Subsea dissimilar joints: Failure mechanisms and opportunities for mitigation. In Proceedings of the Corrosion, Atlanta, GA, USA, 22–26 March 2009; NACE International: Houston, TX, USA, 2009. [Google Scholar]
- Mikami, Y.; Sogabe, K.; Hashimoto, T.; Nishikawa, S.; Mochizuki, M. Evaluation of Residual Stress Distribution in Ni Base Alloy Clad Welds by Numerical Simulation and X-Ray Stress Measurement. Sci. Technol. Weld. Join. 2013, 18, 114–120. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 199–214. ISBN 0-471-43491-4. [Google Scholar]
- Ocelík, V.; Furár, I.; De Hosson, J.T.M. Microstructure and Properties of Laser Clad Coatings Studied by Orientation Imaging Microscopy. Acta Mater. 2010, 58, 6763–6772. [Google Scholar] [CrossRef]
- Rozmus-Górnikowska, M.; Cieniek, L.; Blicharski, M.; Kusiński, J. Microstructure and Microsegregation of an Inconel 625 Weld Overlay Produced on Steel Pipes by the Cold Metal Transfer Technique. Arch. Metall. Mater. 2014, 59, 1081–1084. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, B.T.; Lippold, J.C.; Sowards, J.W.; Hope, A.T.; Saltzmann, D.R. Fusion Boundary Microstructure Evolution Associated with Embrittlement of Ni-Base Alloy Overlays Applied to Carbon Steel. Weld. World 2013, 57, 39–53. [Google Scholar] [CrossRef]
- Soysal, T.; Kou, S.; Tat, D.; Pasang, T. Macrosegregation in Dissimilar-Metal Fusion Welding. Acta Mater. 2016, 110, 149–160. [Google Scholar] [CrossRef]
- Rowe, M.D.; Nelson, T.W.; Lippold, J.C. Hydrogen-Induced Cracking along the Fusion Boundary of Dissimilar Metal Welds. Weld. Res. Suppl. 1999, 1, 31–37. [Google Scholar]
- Fenske, J.A.; Robertson, I.M.; Ayer, R.; Hukle, M.; Lillig, D.A.N.; Newbury, B. Microstructure and Hydrogen-Induced Failure Mechanisms in Fe and Ni Alloy Weldments. Metall. Mater. Trans. A 2012, 43, 3011–3022. [Google Scholar] [CrossRef]
- Wu, Y. Hydrogen Related Disbonding of Weld Overlay of Corrosion Resistant Alloys. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 1993. [Google Scholar] [CrossRef]
- Nelson, T.W.; Lippold, J.C.; Mills, M.J. Investigation of Boundaries and Structures in Dissimilar Metal Welds. Sci. Technol. Weld. Join. 1998, 3, 249–255. [Google Scholar] [CrossRef]
- Nelson, B.Y.T.W.; Lippold, J.C.; Mills, M.J. Nature and Evolution of the Fusion Boundary in Ferritic-Austenitic Dissimilar Weld Metals, Part 1—Nucleation and Growth. Weld. J. 1999, 2, 237–243. [Google Scholar]
- Nelson, B.Y.T.W.; Lippold, J.C.; Mills, M.J. Nature and Evolution of the Fusion Boundary in Ferritic-Austenitic Dissimilar Metal Welds—Part 2: On-Cooling Trans. Weld. J. 2000, 5, 267–277. [Google Scholar]
- Dong, L.; Peng, Q.; Xue, H.; Han, E.-H.; Ke, W.; Wang, L. Correlation of microstructure and stress corrosion cracking initiation behaviour of the fusion boundary region in a SA508 Cl. 3-Alloy 52M dissimilar weld joint in primary pressurized water reactor environment. Corros. Sci. 2018, 132, 9–20. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, Y.; Sun, D.; Zhu, J.; Wu, Y. Microstructure and Properties of High-Power Laser Welding of SUS304 to SA553 for Cryogenic Applications. J. Mater. Process. Technol. 2015, 225, 56–66. [Google Scholar] [CrossRef]
- Hielscher, R.; Schaeben, H. A novel pole figure inversion method: Specification of the MTEX algorithm. J. Appl. Cryst. 2008, 41, 1024–1037. [Google Scholar] [CrossRef]
- Nyyssönen, T. Quenching and Partitioning of High-Aluminum Steels. Ph.D. Thesis, Tampere University of Technology, Tampere, Finland, 2017. [Google Scholar]
- Gu, X.F.; Furuhara, T.; Zhang, W.Z. PTCLab: Free and Open-Source Software for Calculating Phase Transformation Crystallography. J. Appl. Crystallogr. 2016, 49, 1099–1106. [Google Scholar] [CrossRef]
- Banovic, S.W.; Dupont, J.N.; Marder, A.R. Dilution and Microsegregation in Dissimilar Metal Welds between Super Austenitic Stainless Steel and Nickel Base Alloys. Sci. Technol. Weld. Join. 2002, 7, 374–383. [Google Scholar] [CrossRef]
- Yang, Y.K.; Kou, S. Macrosegregation Mechanisms in Arc Welds Made with Dissimilar Filler Metals. Sci. Technol. Weld. Join. 2010, 15, 15–31. [Google Scholar] [CrossRef]
- Perricone, M.J.; Dupont, J.N. Effect of Composition on the Solidification Behavior of Several Ni-Cr-Mo and Fe-Ni-Cr-Mo Alloys. Metall. Mater. Trans. A 2006, 37, 1267–1280. [Google Scholar] [CrossRef]
- Dupont, J.N. Solidification of an Alloy 625 Weld Overlay. Metall. Mater. Trans. A 1996, 27, 3612–3620. [Google Scholar] [CrossRef]
- Frei, J.; Alexandrov, B.T.; Rethmeier, M. Low Heat Input Gas Metal Arc Welding for Dissimilar Metal Weld Overlays Part II: The Transition Zone. Weld. World 2018, 62, 317–324. [Google Scholar] [CrossRef]
- Chen, Z.R.; Lu, Y.H. TEM Observation of Martensite Layer at the Weld Interface of an A508III to Inconel 82 Dissimilar Metal Weld Joint. Metall. Mater. Trans. A 2015, 46, 5494–5498. [Google Scholar] [CrossRef]
- Barrick, E.J.; Jain, D.; Dupont, J.N.; Seidman, D.N. Effects of Heating and Cooling Rates on Phase Transformations in 10 Wt Pct Ni Steel and Their Application to Gas Tungsten Arc Welding. Metall. Mater. Trans. A 2017, 48, 5890–5910. [Google Scholar] [CrossRef]
- Filho, J.d.C.P. Improvement of Welding Techniques and Heat Treatments of 9%Ni Steel; Technical Report for Shell Brasil Ltda.: Rio de Janeiro, RJ, Brazil, September 2018. [Google Scholar]
- Silva, C.C.; de Albuquerque, V.H.C.; Miná, E.M.; Moura, E.P.; Tavares, J.M.R.S. Mechanical Properties and Microstructural Characterization of Aged Nickel-Based Alloy 625 Weld Metal. Metall. Mater. Trans. A 2018, 49, 1653–1673. [Google Scholar] [CrossRef]
- Solecka, M.; Kopia, A.; Radziszewska, A.; Rutkowski, B. Microstructure, Microsegregation and Nanohardness of CMT Clad Layers of Ni-Base Alloy on 16Mo3 Steel. J. Alloys Compd. 2018, 751, 86–95. [Google Scholar] [CrossRef]
- Böllinghaus, T.; Herold, H. Hot Cracking Phenomena in Welds, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 141–164. ISBN 3-540-22332-0. [Google Scholar]
- Wu, Y.; Cai, Y.; Wang, H.; Shi, S.; Hua, X.; Wu, Y. Investigation on Microstructure and Properties of Dissimilar Joint between SA553 and SUS304 Made by Laser Welding with Filler Wire. Mater. Des. 2015, 87, 567–578. [Google Scholar] [CrossRef]
- Crocker, A.G. The Phenomenological Theories of Martensite Crystallography. J. Phys. Colloq. 1982, 43, 209–214. [Google Scholar] [CrossRef]
- Wayman, C.M. The Phenomenological Theory of Martensite Crystallography: Interrelationships. Metall. Mater. Trans. A 1994, 25, 1787–1795. [Google Scholar] [CrossRef]
- Kinney, C.C.; Pytlewski, K.R.; Khachaturyan, A.G.; Junior, J.W.M. The Microstructure of Lath Martensite in Quenched 9Ni Steel. Acta Mater. 2014, 69, 372–385. [Google Scholar] [CrossRef]
- Kitahara, H.; Ueji, R.; Tsuji, N.; Minamino, Y. Crystallographic Features of Lath Martensite in Low-Carbon Steel. Acta Mater. 2006, 54, 1279–1288. [Google Scholar] [CrossRef]
- Mas, F.; Tassin, C.; Roch, F.; Yescas, M.; Todeschini, P.; Bréchet, Y. Growth Morphologies and Primary Solidification Modes in a Dissimilar Weld between a Low-Alloy. Metals 2018, 8, 284. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H. Effect of Welding Heat Input on Microstructure and Texture of Inconel 625 Weld Overlay Studied Using the Electron Backscatter Diffraction Method. Metall. Mater. Trans. A 2016, 47, 6109–6120. [Google Scholar] [CrossRef]
- Moat, R.J.; Pinkerton, A.J.; Li, L.; Withers, P.J.; Preuss, M. Crystallographic Texture and Microstructure of Pulsed Diode Laser-Deposited Waspaloy. Acta Mater. 2009, 57, 1220–1229. [Google Scholar] [CrossRef]
- He, Q.; Yang, Y. On Lattice Distortion in High Entropy Alloys. Front. Mater. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Eghlimi, A.; Shamanian, M.; Eskandarian, M.; Zabolian, A.; Nezakat, M.; Szpunar, J.A. Evaluation of Microstructure and Texture across the Welded Interface of Super Duplex Stainless Steel and High Strength Low Alloy Steel. Surf. Coat. Technol. 2015, 264, 150–162. [Google Scholar] [CrossRef]
- Shamanian, M.; Eghlimi, A.; Eskandarian, M.; Szpunar, J.A. Interface Microstructure across Cladding of Super Duplex Stainless Steel with Austenitic Stainless Steel Buffer Layer. Surf. Coat. Technol. 2014, 259, 532–542. [Google Scholar] [CrossRef]
- Örnek, C.; Engelberg, D.L. Towards understanding the effect of deformation mode on stress corrosion cracking susceptibility of grade 2205 duplex stainless steel. Mater. Sci. Eng. A 2016, 666, 269–279. [Google Scholar] [CrossRef]
- Brandon, D.G. The structure of high-angle grain boundaries. Acta Mater. 1966, 14, 1479–1484. [Google Scholar] [CrossRef]
- Guo, Y.; Han, E.H.; Wang, J. Effects of Forging and Heat Treatments on the Microstructure and Oxidation Behavior of 316LN Stainless Steel in High Temperature Water. J. Mater. Sci. Technol. 2015, 31, 403–412. [Google Scholar] [CrossRef]
- Randle, V. Mechanism of twinning-induced grain boundary engineering in low stacking-fault energy materials. Acta Mater. 1999, 47, 4187–4196. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nakamura, M.; Tsurekawa, S.; Watanabe, T. Effect of Grain Boundary Microstructure on Fatigue Crack Propagation in Austenitic Stainless Steel. J. Mater. Sci. 2011, 46, 4254–4260. [Google Scholar] [CrossRef]
- Zhou, S.; Ma, G.; Wu, D.; Chai, D.; Lei, M. Ultrasonic Vibration Assisted Laser Welding of Nickel-Based Alloy and Austenite Stainless Steel. J. Manuf. Process. 2018, 31, 759–767. [Google Scholar] [CrossRef]
- Durst, K.; Go, M. Finite Element Study for Nanoindentation Measurements on Two-Phase Materials. J. Mater. Res. 2004, 19, 85–93. [Google Scholar] [CrossRef]
- Mukherjee, P.; Sarkar, A.; Barat, P.; Jayakumar, T.; Mahadevan, S.; Rai, S.K. Lattice Misfit Measurement in Inconel 625 by X-Ray Diffraction Technique. Int. J. Mod. Phys. B 2008, 22, 3977–3985. [Google Scholar] [CrossRef]
- Jang, J.-I.; Ju, J.-B.; Lee, B.-W.; Kwon, D.; Kim, W.-S. Micromechanism of local brittle zone phenomenon in weld heat-affected zones of advanced 9% Ni steel. J. Mater. Sci. Lett. 2001, 20, 2149–2152. [Google Scholar] [CrossRef]
- Cao, Y.; Di, H.; Zhang, J.; Zhang, J.; Ma, T.; Misra, R.D.K. An Electron Backscattered Diffraction Study on the Dynamic Recrystallization Behavior of a Nickel—Chromium Alloy (800H) during Hot Deformation. Mater. Sci. Eng. A 2013, 585, 71–85. [Google Scholar] [CrossRef]
- Kianezhad, M.; Sajjadi, S.A.; Vafaeenezhad, H. A Numerical Approach to the Prediction of Hardness at Different Points of a Heat-Treated Steel. J. Mater. Eng. Perform. 2015, 24, 1516–1521. [Google Scholar] [CrossRef]
- Nambu, S.; Michiuchi, M.; Inoue, J.; Koseki, T. Effect of Interfacial Bonding Strength on Tensile Ductility of Multilayered Steel Composites. Compos. Sci. Technol. 2009, 69, 1936–1941. [Google Scholar] [CrossRef]
- De Araújo, A.A.; Bastian, F.L.; Castrodeza, E.M. CTOD-R Curves of the Metal-Clad Interface of API X52 Pipes Cladded with an Inconel 625 Alloy by Welding Overlay. Fatigue Fract. Eng. Mater. Struct. 2016, 39, 1477–1487. [Google Scholar] [CrossRef]
- Xie, G.; Luo, Z.; Wang, G.; Li, L.; Wang, G. Interface Characteristic and Properties of Stainless Steel/HSLA Steel Clad Plate by Vacuum Rolling Cladding. Mater. Trans. 2011, 52, 1709–1712. [Google Scholar] [CrossRef] [Green Version]

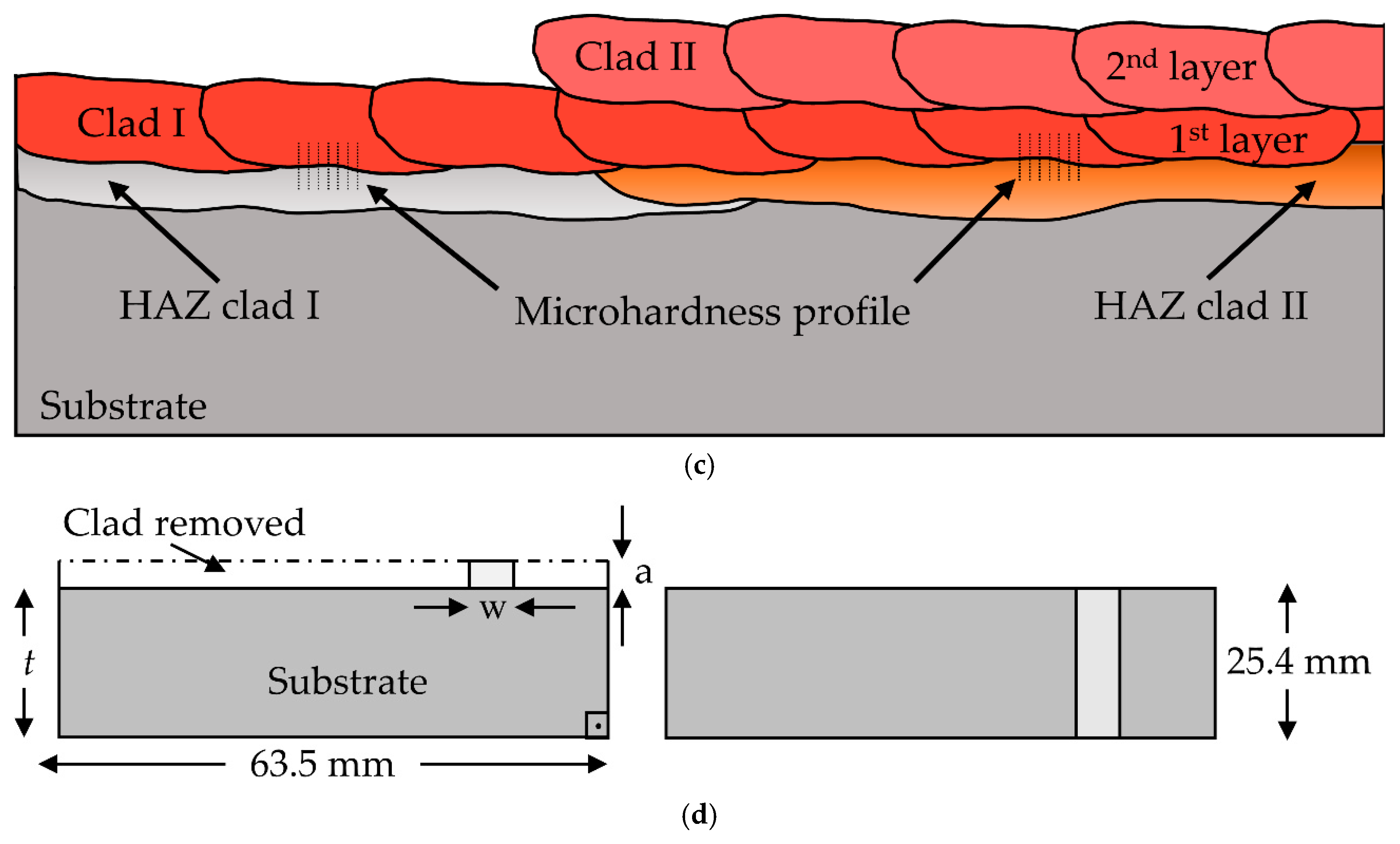

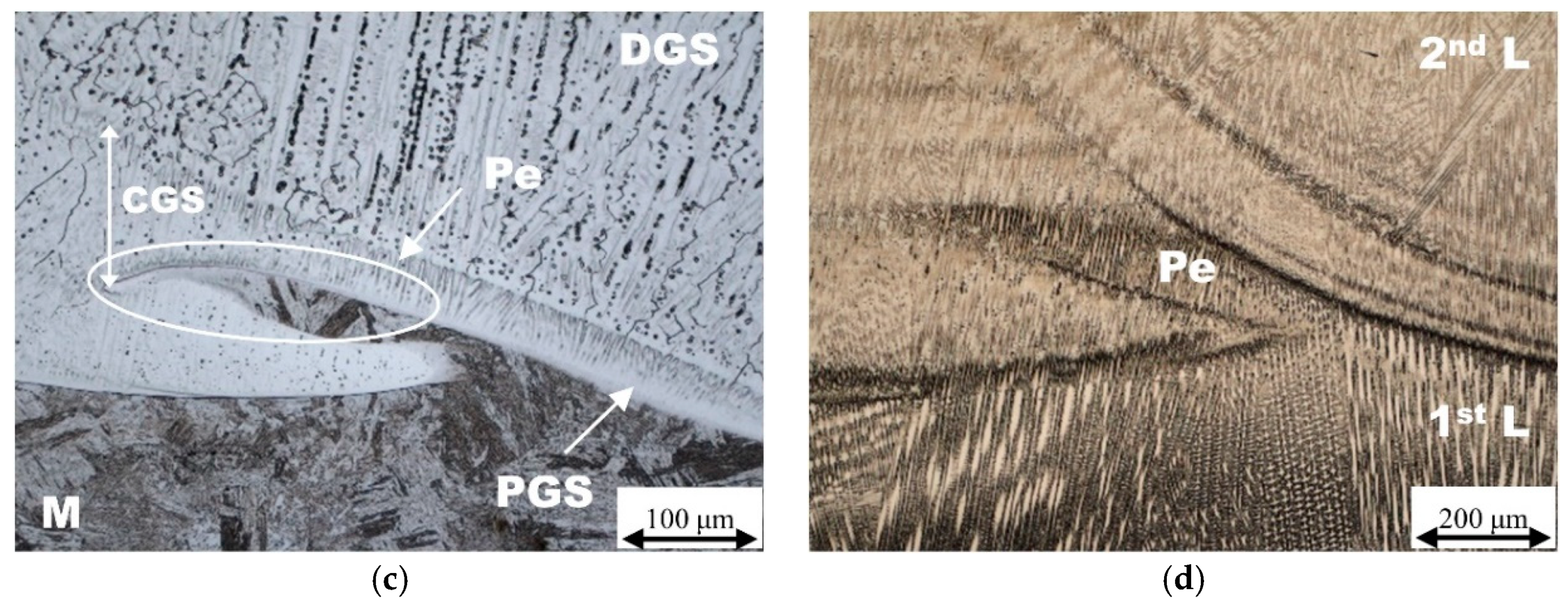


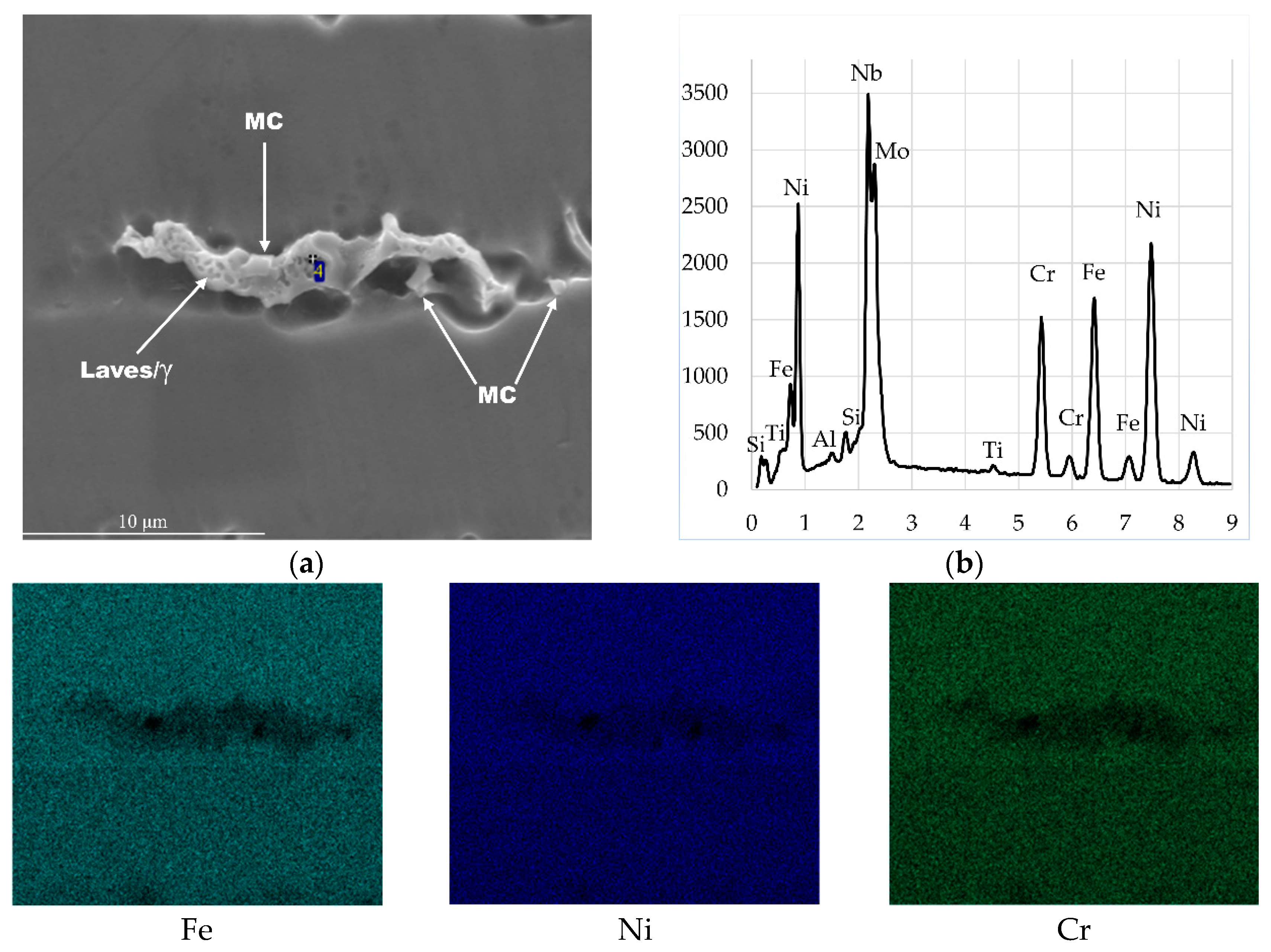
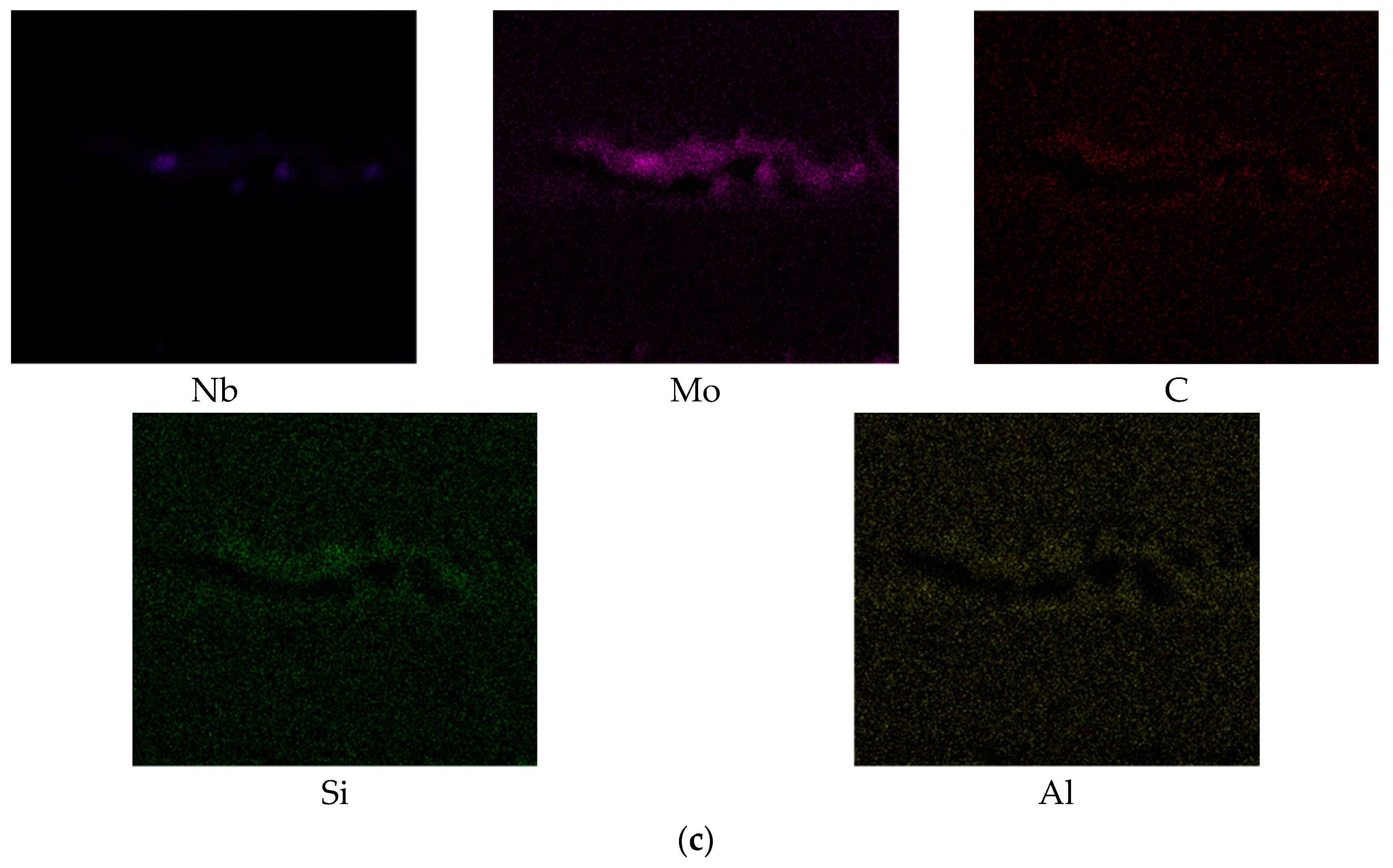




| Chemical Composition (wt. %) | ||||||
|---|---|---|---|---|---|---|
| Element | Substrate | Filler Metal | Clad * | |||
| ASTM A333 Gr. 8 | AWS ER NiCrMo-3 | |||||
| 9%Ni Steel | Ni-based Superalloy 625 | |||||
| Specified (max.) | Analyzed | Specified (max.) | Analyzed | Analyzed | ||
| 1st Layer | 2nd Layer | |||||
| C | 0.13 | 0.067 | 0.1 | 0.028 | 0.038 | 0.031 |
| Mn | 0.90 | 0.614 | 0.5 | 0.234 | 0.326 | 0.26 |
| Si | 0.13–0.32 | 0.271 | 0.5 | 0.242 | 0.249 | 0.244 |
| P | 0.025 | 0.007 | 0.02 | 0.001 | 0.002 | 0.001 |
| S | 0.025 | 0.004 | 0.015 | 0.003 | 0.003 | 0.003 |
| Ni | 8.4–9.6 | 9.231 | >58 | 63.414 | 51.9 | 61.6 |
| Cu | N. A. | 0.004 | 0.5 | 0.024 | 0.019 | 0.023 |
| Ti | N. A. | 0.013 | 0.4 | 0.212 | 0.164 | 0.198 |
| Cr | N. A. | 0.032 | 20–23 | 20.1 | 13.5 | 16.8 |
| Mo | N. A. | 0.012 | 8–10 | 9.111 | 7.43 | 9.09 |
| Fe | Balance | 89.671 | 5 | 2.619 | 23.7 | 8.56 |
| Pb | N. A. | 0.004 | 0.5 | 0.041 | 0.032 | 0.038 |
| Al | N. A. | 0.025 | 0.4 | 0.097 | 0.080 | 0.092 |
| Nb + Ta | N. A. | 0.004 | 3.15–4.14 | 3.375 | 2.13 | 2.75 |
| Characteristics | Substrate | Clad I | Clad II |
|---|---|---|---|
| External diameter (mm) | 219.1 | N. A. | N. A. |
| Thickness (mm) | 31.7 | 1.64 | 4.57 |
| Type of pipe | Seamless pipe | N. A. | N. A. |
| Classification | ASTM A333 Gr. 8 | AWS ER NiCrMo-3 | |
| Commercial name | 9%Ni steel | Ni superalloy 625 | |
| Microstructure | Tempered martensite | Columnar grains | |
| Heat treatment | Quench and temper | N. A. | N. A. |
| Dilution (%) | N. A. | 24.2 | 6.3 |
| Procedure before welding | ISO 8501-1 | ||
| Post welding inspection | Approved according to ASME code section IX | ||
| Parameter | Clad | ||
|---|---|---|---|
| I | II * | ||
| Arc welding | - | - | - |
| Current type/polarity | - | CC/direct | CC/direct |
| Current intensity (A) | background | 178 | 178 |
| peak | 260 | 260 | |
| Current time (s) | background | 0.3 | 0.3 |
| peak | 0.3 | 0.3 | |
| Current pulse frequency (Hz) | - | 3.3 | 3.3 |
| Voltage (V) | - | 13 | 13 |
| Length (mm) | - | 1.5 | 1.5 |
| Hot wire | - | - | - |
| Diameter (mm) | - | 1.113 | 1.113 |
| Current type/polarity | - | CC/direct | CC/direct |
| Current intensity (A) | - | 40 | 40 |
| Voltage (V) | - | 12.5 | 12.5 |
| Feed speed (m/min) | - | 1.5 | 2.5 |
| Stick out (mm) | - | 1.5 | 1.5 |
| Electrode | type | EW-La2 | EW-La2 |
| diameter (mm) | 3.2 | 3.2 | |
| Shielding gas | type | argon (99.98%) | argon (99.98%) |
| flow rate (m3/s) | 3.2 × 10−4 | 3.2 × 10−4 | |
| Temperature (°C) | preheat | 25 | 25 |
| interlayer | N.A. | 100 | |
| Weld pass | step (mm) | 3.5 | 3.5 |
| overlap (%) | 50 | 50 | |
| Welding speed (mm/min) ** | - | 300 | 300 |
| Heat input (kJ/mm) | - | 0.75 | 0.71 |
| Welding position | - | IG | IG |
| Etching | Method | Solution | Condition | Aim |
|---|---|---|---|---|
| First | Electrolytic | 10% by weight of oxalic acid dihydrate (HO2CCO2H·2H2O) with distilled water | 6 V, 15 s, stainless steel cathode, and substrate painted with enamel | To reveal the clad microstructure |
| Second | Wet chemical | 2% nitric acid (HNO3) with 98% ethanol (CH3CH2OH) | 25 s | To reveal the HAZ microstructure |
| Samples | Vickers Microhardness, HV0.01 | Shear Strength, τ (MPa) | Bend Test | |||
|---|---|---|---|---|---|---|
| CMZ | TZ-S/C | CGHAZ | Specified (min) | Analyzed | ||
| Clad I | 257 ± 28 | 291 ± 29 | 326 ± 19 | 140 | 465 | Approved |
| Clad II | 237 ± 38 | 247 ± 14 | 307 ± 25 | 140 | 485 | Approved |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipriano Farias, F.W.; Payão Filho, J.d.C.; Barreto de Azevedo, L.M. Microstructural and Mechanical Characterization of the Transition Zone of 9%Ni Steel Cladded with Ni-Based Superalloy 625 by GTAW-HW. Metals 2018, 8, 1007. https://doi.org/10.3390/met8121007
Cipriano Farias FW, Payão Filho JdC, Barreto de Azevedo LM. Microstructural and Mechanical Characterization of the Transition Zone of 9%Ni Steel Cladded with Ni-Based Superalloy 625 by GTAW-HW. Metals. 2018; 8(12):1007. https://doi.org/10.3390/met8121007
Chicago/Turabian StyleCipriano Farias, Francisco Werley, João da Cruz Payão Filho, and Luiz Maurício Barreto de Azevedo. 2018. "Microstructural and Mechanical Characterization of the Transition Zone of 9%Ni Steel Cladded with Ni-Based Superalloy 625 by GTAW-HW" Metals 8, no. 12: 1007. https://doi.org/10.3390/met8121007





