The Macroscopic Flow direction and Microscopic Distribution of Mg in Sintered Products and Its Influence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Properties of Raw Materials
2.2. Experimental Methods
3. Results and Discussions
3.1. Mineral Phases of Sintered Products
3.2. Macroscopic Flow Direction of Mg
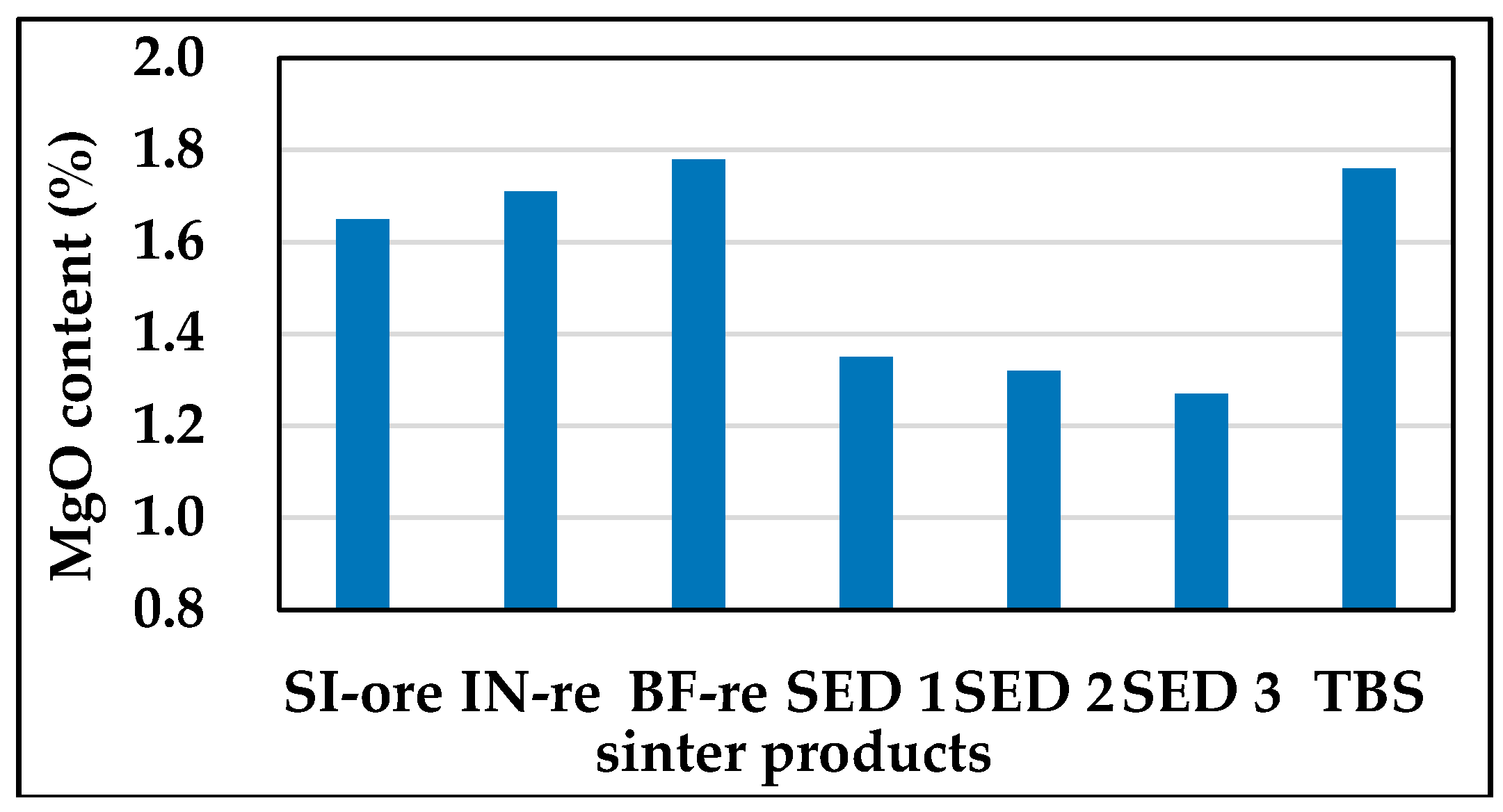
3.2.1. Mg Flow Direction in All Sintered Products
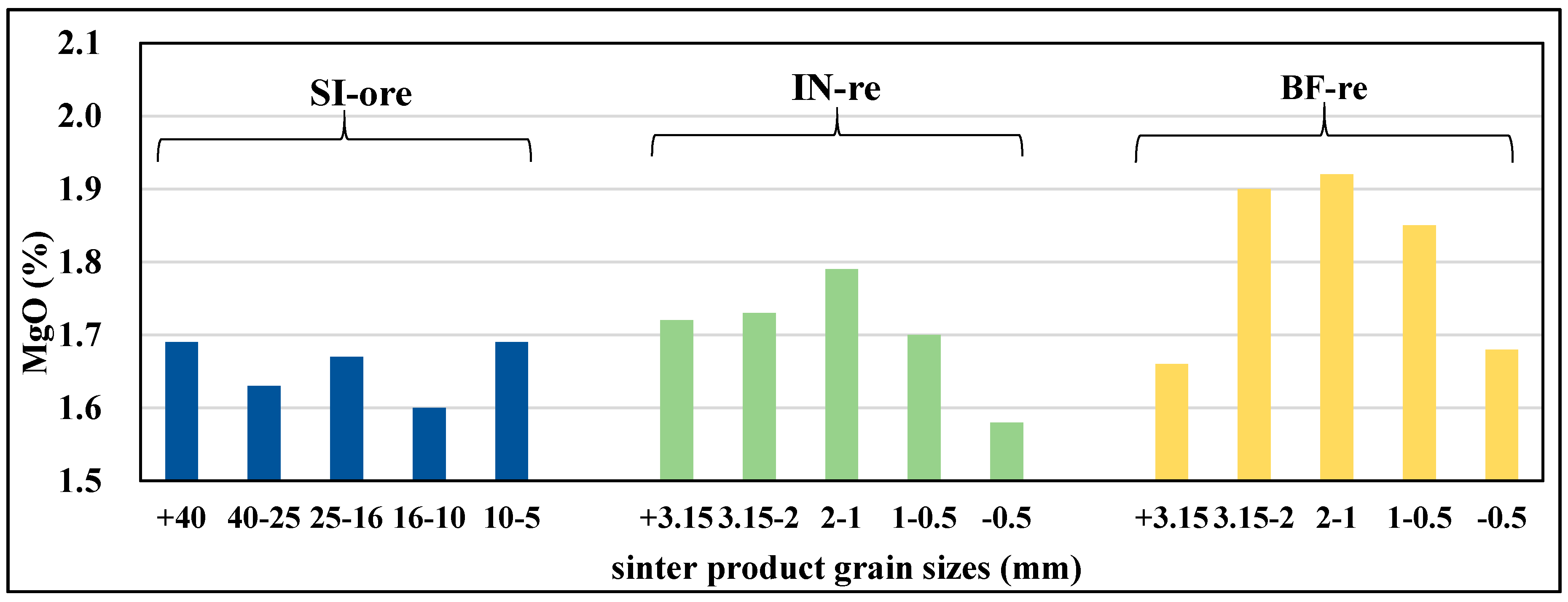
3.2.2. Mg Flow Direction in Various Grain Sizes of Main Sintered Products
3.3. Microscopic Distribution of Mg
3.3.1. Mg Distribution in Different Minerals
3.3.2. Mg Distribution in Different SFCA
3.3.3. Mg Distribution around Dolomite
3.3.4. Mg Distribution characteristics in BF-re
3.3.5. Mg Distribution Characteristics in IN-re
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Masaru, M.; Masahiko, H.; Takazo, K. Improvement of sinter softening property and reducibility by controlling chemical compositions. ISIJ Int. 2005, 45, 594–602. [Google Scholar] [CrossRef]
- Kohei, S.; Kaoru, N.; Masahiko, H.; Takanobu, I.; Shusaku, K.; Takaiku, Y. Effect of High Al2O3 Slag on the Blast Furnace Operations. ISIJ Int. 2008, 48, 420–429. [Google Scholar] [CrossRef]
- Kenichi, H.; Masaaki, N.; Masanori, N.; Yasushi, T. Optimization of chemical composition and microstructure of iron ore sinter for low-temperature drip of molten iron with high permeability. ISIJ Int. 2004, 44, 2057–2066. [Google Scholar] [CrossRef]
- Wang, X.L. Iron and Steel Metallurgy (Iron Making), 3rd ed.; Metallurgy Industry Press: Beijing, China, 2008; pp. 107–156. ISBN 9787502461300. [Google Scholar]
- He, H.Y.; Wang, Q.X.; Zeng, X.N. Effect of MgO content on BF slag viscosity. J. Iron Steel Res. Int. 2006, 18, 11–13. [Google Scholar] [CrossRef]
- Shen, F.M.; Jiang, X.; Wu, G.S. Proper MgO addition in blast furnace operation. ISIJ Int. 2006, 46, 65–69. [Google Scholar] [CrossRef]
- Guan, X.F. The way of heightening MgO of sinter and the effects of metallurgy in BF. Sint and Pellet. 2001, 26, 32–34. (In Chinese) [Google Scholar] [CrossRef]
- Umadevi, T.; Roy, A.K.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of Magnesia on Iron Ore Sinter Properties and Productivity–Use of Dolomite and Dunite. Steel Res. Int. 2009, 80, 800–807. [Google Scholar] [CrossRef]
- Yao, J.H.; Yang, J. Effect of additives on formation of calcium ferrites in magnesia-riched sinters. Iron Steel. 2015, 50, 12–16. (In Chinese) [Google Scholar] [CrossRef]
- Hayashi, M.; Tanaka, H.; Watanabe, T.; Susa, M. Role of MgO in Sinter from Perspective of MgO Distribution between Liquid and Magnetite Phases in FeOx–CaO–SiO2–MgO System. ISIJ Int. 2017, 59, 2124–2130. [Google Scholar] [CrossRef]
- Shigaki, I.; Sawada, M.; Maekawa, M.; Narita, K. Melting property of MgO containing sinter. Tetsu-to-Hagane 1981, 21, 862–869. [Google Scholar] [CrossRef]
- Li, T.L.; Sun, C.Y.; Liu, X.Y. The effects of MgO and Al2O3 behaviours on softening–melting properties of high basicity sinter. Ironmak. Steelmak. 2018, 45, 755–763. [Google Scholar] [CrossRef]
- Yadav, U.S.; Pandey, B.D.; Das, B.K.; Jena, D.N. Influence of magnesia on sintering characteristics of iron ore. Ironmak. Steelmak. 2002, 29, 91–95. [Google Scholar] [CrossRef]
- Eiki, K.; Yorito, S.; Takazo, K.; Takshi, N. Influence of properties of fluxing materials on the flow of melt formed in the sintering process. ISIJ Int. 2000, 40, 857–859. [Google Scholar] [CrossRef]
- Umadevi, T.; Nelson, K.; Mahapatra, P.C.; Prabhu, M.; Ranjan, M. Influence of magnesia on iron ore sinter properties and productivity. Ironmak. Steelmak. 2009, 36, 515–520. [Google Scholar] [CrossRef]
- Wu, S.L.; Han, H.L.; Jiang, W.Z.; Zhu, J.M.; Feng, G.S.; Zhang, Z.C. MgO interaction mechanism in sinter. Chin. J. Eng. 2009, 31, 428–432. (In Chinese) [Google Scholar] [CrossRef]
- Yajima, K.; Matsuura, H.; Tsukihashi, F. Effect of simultaneous addition of Al2O3 and MgO on the liquidus of the CaO–SiO2–FeOx system with various oxygen partial pressures at 1573 K. ISIJ Int. 2010, 50, 191–194. [Google Scholar] [CrossRef]
- Feng, Q.; Yu, S.J.; Hou, H.Y.; Zhang, D.K.; Geng, J.S.; Xu, P.F. Recycling of the electric dust in sintering machine head. Iron Steel. 2015, 50, 67–71. (In Chinese) [Google Scholar] [CrossRef]
- Fan, X.H.; Li, W.Q.; Gan, M.; Chen, X.L.; Yuan, L.S.; Ji, Z.Y.; Yu, Z.Y.; Huang, X.X.; Su, D. Influence and mechanism of MgO on strength of high basicity sinter. J. Cent. South Univ. 2012, 43, 3325–3330. [Google Scholar]
- Jiang, X.; Wu, G.S.; Wei, G.; Li, X.G.; Sheng, F.M. Effect of MgO on Sintering Process and Metallurgical Properties of Sinter. Iron Steel. 2006, 41, 8–11. (In Chinese) [Google Scholar] [CrossRef]
- Gan, M.; Fan, X.H.; Ji, Z.Y.; Chen, X.L.; Yin, L.; Jiang, T.; Li, G.H.; Yu, Z.Y. High Temperature Mineralization Behavior of Mixtures during Iron Ore Sintering and Optimizing Methods. ISIJ Int. 2015, 55, 742–750. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Lv, X.W.; Xiang, S.L.; Bai, C.G.; Yin, J.Q. Wetting behavior of calcium ferrite melts on sintered MgO. ISIJ Int. 2015, 55, 1558–1564. [Google Scholar] [CrossRef]
- Long, H.M.; Wu, X.J.; Chun, T.J.; Di, Z.X.; Yu, B. Assimilation behavior of calcium ferrite and calcium differrite with sintered Al2O3 and MgO. Metall. Mater. Trans. B 2016, 47, 2830–2836. [Google Scholar] [CrossRef]
- Sugiyama, K.; Monkawa, A.; Sugiyama, T. Crystal structure of the SFCAM phase Ca2(Ca, Fe, Mg, Al)6(Fe, Al, Si)6O2. ISIJ Int. 2005, 45, 560–568. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Tan, Y.C. Metallurgy and Heat Treatment, 2nd ed.; Mechanical Industry Press: Beijing, China, 2007; pp. 36–54. ISBN 9787111017967. [Google Scholar]
- Zhang, C.J. Research on crystallization behavior of mold flux in magnetic field. Master’s Thesis, Chongqing University, Chongqing, China, May 2015. [Google Scholar]
- Jin, M.F.; Li, G.S.; Chu, M.S.; Shen, F.M. Diffusion between Mg and hematite during sintering. Iron Steel 2008, 43, 10–14. (In Chinese) [Google Scholar] [CrossRef]










| Materials | Mixed Mine | IN-re | BF-re | Dolomite | Limestone | Quicklime | Fuel |
|---|---|---|---|---|---|---|---|
| ratio (%) | 55.6 | 25.0 | 8.12 | 2.76 | 2.08 | 3.11 | 3.33 |
| Composition | TFe | FeO | SiO2 | CaO | Al2O3 | MgO | LOI |
|---|---|---|---|---|---|---|---|
| mixed mine (%) | 62.19 | 3.1 | 4.52 | 0.77 | 1.59 | 0.21 | 3.78 |
| dolomite (%) | - | - | 2.46 | 30.95 | 0.26 | 19.85 | 44.97 |
| Granularity (mm) | −0.5 | 0.5–1 | 1–2 | 2–3.15 | +3.15 |
| Proportion (%) | 32.61 | 20.79 | 27.96 | 17.92 | 0.72 |
| Region | Fe | O | Si | Al | Mg | Ca |
|---|---|---|---|---|---|---|
| region 1 | 60.15 | 17.26 | 6.76 | 1.97 | 0.73 | 13.13 |
| region 2 | 55.34 | 15.81 | 6.41 | 1.88 | 4.49 | 16.07 |
| Point | Fe | O | Si | Al | Mg | Ca |
|---|---|---|---|---|---|---|
| point 1 | 67.50 | 29.20 | 0.06 | 0.29 | 2.08 | 0.87 |
| point 2 | 48.71 | 17.41 | 5.61 | 1.58 | 0.63 | 26.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zhang, W.; Kou, M.; Zhou, H. The Macroscopic Flow direction and Microscopic Distribution of Mg in Sintered Products and Its Influence. Metals 2018, 8, 1008. https://doi.org/10.3390/met8121008
Wu S, Zhang W, Kou M, Zhou H. The Macroscopic Flow direction and Microscopic Distribution of Mg in Sintered Products and Its Influence. Metals. 2018; 8(12):1008. https://doi.org/10.3390/met8121008
Chicago/Turabian StyleWu, Shengli, Weili Zhang, Mingyin Kou, and Heng Zhou. 2018. "The Macroscopic Flow direction and Microscopic Distribution of Mg in Sintered Products and Its Influence" Metals 8, no. 12: 1008. https://doi.org/10.3390/met8121008





