Hydrogen Interaction with Deep Surface Modified Zr-1Nb Alloy by High Intensity Ti Ion Implantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Implantation Procedure
2.2. Hydrogenation
2.3. Characterization
3. Results and Discussion
3.1. Structure and Composition of Ti-Implanted Zr-1Nb Alloy
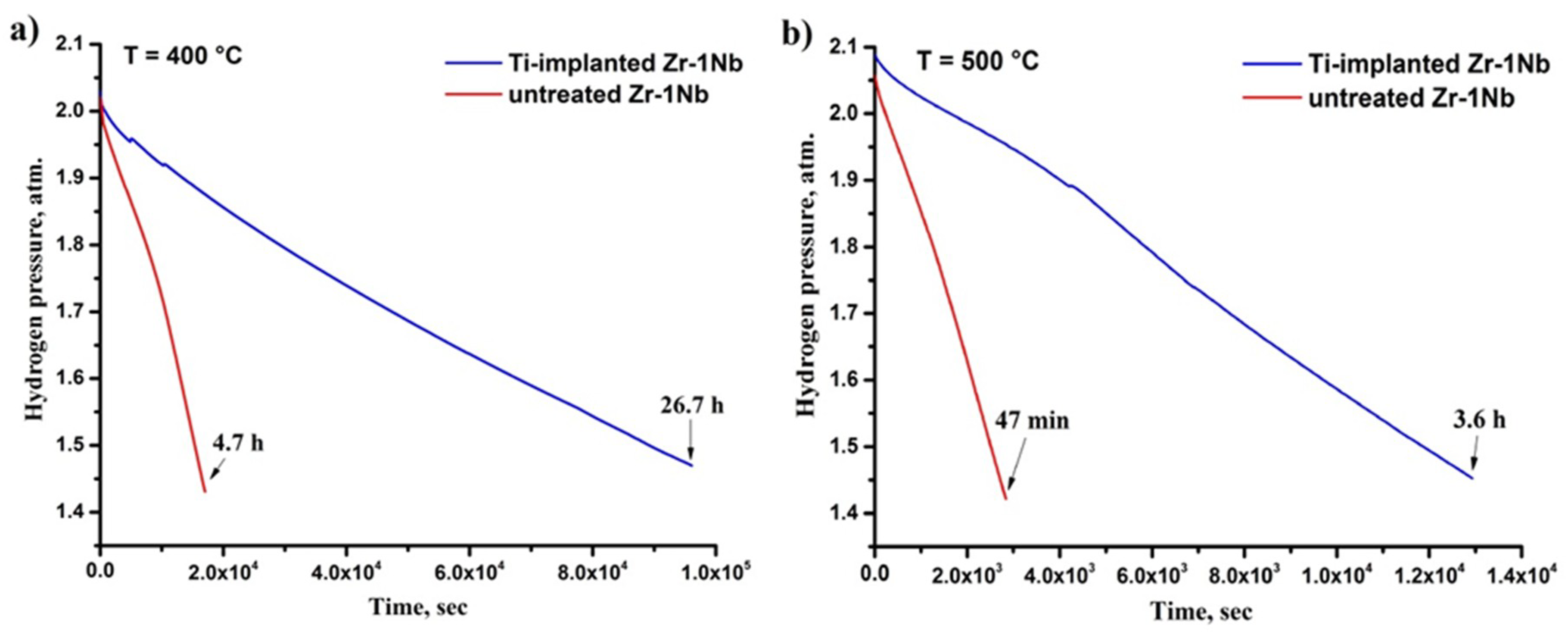
3.2. Hydrogen Sorption Behavior
3.3. Structure and Elemental Distribution in Hydrogenated Samples
3.4. Thermal Desorption Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarkar, A.; Chandanshive, S.A.; Thota, M.K.; Kapoor, R. High temperature deformation behavior of Zr-1Nb alloy. J. Alloys Compd. 2017, 703, 56–66. [Google Scholar] [CrossRef]
- Lee, C.M.; Mok, Y.-K.; Sohn, D.-S. High-temperature steam oxidation and oxide crack effects of Zr-1Nb-1Sn-0.1Fe fuel cladding. J. Nucl. Mater. 2017, 496, 343–352. [Google Scholar] [CrossRef]
- Ensor, B.; Lucente, A.M.; Frederick, M.J.; Sutliff, J.; Motta, A.T. The role of hydrogen in zirconium alloy corrosion. J. Nucl. Mater. 2017, 496, 301–312. [Google Scholar] [CrossRef]
- Kishore, R. Effect of hydrogen on the creep behavior of Zr-2.5%Nb alloy at 723 K. J. Nucl. Mater. 2009, 385, 591–594. [Google Scholar] [CrossRef]
- Wei, T.; Long, C.; Chen, H. Hydrogen uptake behaviors of Zr-1.0Cr-0.4Fe-(0.2Mo) and N18 alloys during corrosion in 500 °C steam. J. Alloys Compd. 2018, 731, 126–134. [Google Scholar] [CrossRef]
- Couet, A.; Motta, A.T.; Comstock, R.J. Hydrogen pickup measurements in zirconium alloys: Relation to oxidation kinetics. J. Nucl. Mater. 2014, 451, 1–13. [Google Scholar] [CrossRef]
- Tagtstrom, P.; Limback, M.; Dahlback, M.; Andersson, T.; Pettersson, H. Effects of Hydrogen Pickup and Second- Phase Particle Dissolution on the In-Reactor Corrosion Performance of BWR Claddings. In Zirconium in the Nuclear Industry: 13th International Symposium, ASTM STP 1423; ASTM International: West Conshohocken, PA, USA, 2002; pp. 96–118. [Google Scholar]
- Krishna, K.M.; Srivastava, D.; Dey, G.K.; Hiwarkar, V.; Samajdar, I.; Banerjee, S. Role of grain/phase boundary nature on the formation of hydrides in Zr–2.5% Nb alloy. J. Nucl. Mater. 2011, 414, 270–275. [Google Scholar] [CrossRef]
- Bair, J.; Asle Zaeem, M.; Tonks, M. A review on hydride precipitation in zirconium alloys. J. Nucl. Mater. 2015, 466, 12–20. [Google Scholar] [CrossRef]
- McRae, G.A.; Coleman, C.E.; Leitch, B.W. The first step for delayed hydride cracking in zirconium alloys. J. Nucl. Mater. 2010, 396, 130–143. [Google Scholar] [CrossRef]
- Große, M.; Lehmann, E.; Steinbrück, M.; Kühne, G.; Stuckert, J. Influence of oxide layer morphology on hydrogen concentration in tin and niobium containing zirconium alloys after high temperature steam oxidation. J. Nucl. Mater. 2009, 385, 339–345. [Google Scholar] [CrossRef]
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Cox, B. Some thoughts on the mechanisms of in-reactor corrosion of zirconium alloys. J. Nucl. Mater. 2005, 336, 331–368. [Google Scholar] [CrossRef]
- Duan, Z.; Yang, H.; Satoh, Y.; Murakami, K.; Kano, S.; Zhao, Z.; Shen, J.; Abe, H. Current status of materials development of nuclear fuel cladding tubes for light water reactors. Nucl. Eng. Des. 2017, 316, 131–150. [Google Scholar] [CrossRef]
- Kuprin, A.S.; Belous, V.A.; Voyevodin, V.N.; Vasilenko, R.L.; Ovcharenko, V.D.; Tolstolutskaya, G.D.; Kopanets, I.E.; Kolodiy, I.V. Irradiation resistance of vacuum arc chromium coatings for zirconium alloy fuel claddings. J. Nucl. Mater. 2018, 510, 163–167. [Google Scholar] [CrossRef]
- Bischoff, J.; Delafoy, C.; Vauglin, C.; Barberis, P.; Roubeyrie, C.; Perche, D.; Duthoo, D.; Schuster, F.; Brachet, J.-C.; Schweitzer, E.W.; et al. AREVA NP’s enhanced accident-tolerant fuel developments: Focus on Cr-coated M5 cladding. Nucl. Eng. Technol. 2018, 50, 223–228. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Khatkhatay, F.; Jiao, L.; Swadener, G.; Cole, J.I.; Gan, J.; Wang, H. TiN-based coatings on fuel cladding tubes for advanced nuclear reactors. J. Nucl. Mater. 2012, 429, 143–148. [Google Scholar] [CrossRef]
- Khatkhatay, F.; Jiao, L.; Jian, J.; Zhang, W.; Jiao, Z.; Gan, J.; Zhang, H.; Zhang, X.; Wang, H. Superior corrosion resistance properties of TiN-based coatings on Zircaloy tubes in supercritical water. J. Nucl. Mater. 2014, 451, 346–351. [Google Scholar] [CrossRef]
- Zou, Z.; Xue, W.; Jia, X.; Du, J.; Wang, R.; Weng, L. Effect of voltage on properties of microarc oxidation films prepared in phosphate electrolyte on Zr-1Nb alloy. Surf. Coat. Technol. 2013, 222, 62–67. [Google Scholar] [CrossRef]
- Van Nieuwenhove, R.; Andersson, V.; Balak, J.; Oberländer, B. In-Pile Testing of CrN, TiAlN, and AlCrN Coatings on Zircaloy Cladding in the Halden Reactor. In Zirconium in the Nuclear Industry: 18th International Symposium; ASTM International: West Conshohocken, PA, USA, 2018; pp. 965–982. [Google Scholar]
- Pushilina, N.S.; Kudiiarov, V.N.; Lider, A.M.; Teresov, A.D. Influence of surface structure on hydrogen interaction with Zr–1Nb alloy. J. Alloys Compd. 2015, 645, S476–S479. [Google Scholar] [CrossRef] [Green Version]
- Kashkarov, E.; Nikitenkov, N.; Sutygina, A.; Laptev, R.; Bordulev, Y.; Obrosov, A.; Liedke, M.O.; Wagner, A.; Zak, A.; Weiβ, S. Microstructure, defect structure and hydrogen trapping in zirconium alloy Zr-1Nb treated by plasma immersion Ti ion implantation and deposition. J. Alloys Compd. 2018, 732, 80–87. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Nikitenkov, N.N.; Sutygina, A.N.; Obrosov, A.; Manakhov, A.; Polčák, J.; Weiß, S. Hydrogen absorption by Ti-implanted Zr-1Nb alloy. Int. J. Hydrog. Energy 2018, 43, 2484–2491. [Google Scholar] [CrossRef]
- Peng, D.Q.; Bai, X.D.; Pan, F.; Sun, H.; Chen, B.S. Influence of titanium ions implantation on corrosion behavior of zirconium in 1 M H2SO4. Appl. Surf. Sci. 2006, 252, 2196–2203. [Google Scholar] [CrossRef]
- Obrosov, A.; Sutygina, A.; Manakhov, A.; Bolz, S.; Weiß, S.; Kashkarov, E. Oxidation Behavior of Zr–1Nb Corroded in Air at 400 °C after Plasma Immersion Titanium Implantation. Metals 2018, 8, 27. [Google Scholar] [CrossRef]
- Ryabchikov, A.I.; Kashkarov, E.B.; Pushilina, N.S.; Syrtanov, M.S.; Shevelev, A.E.; Korneva, O.S.; Sutygina, A.N.; Lider, A.M. High-intensity low energy titanium ion implantation into zirconium alloy. Appl. Surf. Sci. 2018, 439, 106–112. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Brägger, U.; Meijer, H.J.A.; Naert, I.; Persson, R.; Perucchi, A.; Quirynen, M.; Raghoebar, G.M.; Reichert, T.E.; Romeo, E.; et al. A Double-Blind Randomized Controlled Trial (RCT) of Titanium-13Zirconium versus Titanium Grade IV Small-Diameter Bone Level Implants in Edentulous Mandibles—Results from a 1-Year Observation Period. Clin. Implant Dent. Relat. Res. 2012, 14, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.A.; Rocha-Filho, R.C.; Biaggio, S.R.; Bocchi, N. Corrosion resistance of the Ti–50Zr at.% alloy after anodization in different acidic electrolytes. Corros. Sci. 2010, 52, 4058–4063. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Zhang, F.; Xu, H. Shape memory behavior in Ti-Zr alloys. Scr. Mater. 2011, 64, 584–587. [Google Scholar] [CrossRef]
- Frank, M.J.; Walter, M.S.; Lyngstadaas, S.P.; Wintermantel, E.; Haugen, H.J. Hydrogen content in titanium and a titanium–zirconium alloy after acid etching. Mater. Sci. Eng. C 2013, 33, 1282–1288. [Google Scholar] [CrossRef]
- Ryabchikov, A.I.; Ananin, P.S.; Dektyarev, S.V.; Sivin, D.O.; Shevelev, A.E. High intensity metal ion beam generation. Vacuum 2017, 143, 447–453. [Google Scholar] [CrossRef]
- Stout, V.L.; Gibbons, M.D. Gettering of gas by titanium. J. Appl. Phys. 1955, 26, 1488–1492. [Google Scholar] [CrossRef]
- Anders, A. (Ed.) Handbook of Plasma Immersion Ion Implantation and Deposition; Wiley: New York, NY, USA, 2000; Volume 17, p. 46. [Google Scholar]
- Yushkov, G.Y.; Anders, A.; Oks, E.M.; Brown, I.G. Ion velocities in vacuum arc plasmas. J. Appl. Phys. 2000, 88, 5618–5622. [Google Scholar] [CrossRef]
- Ryabchikov, A.I.; Sivin, D.O.; Korneva, O.S.; Lopatin, I.V.; Ananin, P.S.; Prokopenko, N.A.; Akhmadeev, Y.K. High-current-density gas ion ribbon beam formation. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2018, 906, 56–60. [Google Scholar] [CrossRef]
- Richmond, C.J. Ion Implantation; Ryssel, H., Ruge, I., Eds.; Wiley: Chichester, UK, 1986; 478p. [Google Scholar]
- Babikhina, M.N.; Kudiiarov, V.N.; Mostovshchikov, A.V.; Lider, A.M. Quantitative and Qualitative Analysis of Hydrogen Accumulation in Hydrogen-Storage Materials Using Hydrogen Extraction in an Inert Atmosphere. Metals 2018, 8, 672. [Google Scholar] [CrossRef]
- Dobromyslov, A.V.; Taluts, N.I. Structure investigation of quenched and tempered alloys of the Zr–Ti system. Phys. Met. Met. 1987, 63, 114–120. [Google Scholar]
- Zhou, Y.K.; Jing, R.; Ma, M.Z.; Liu, R.P. Tensile strength of Zr-Ti binary alloy. Chin. Phys. Lett. 2013, 30, 116201. [Google Scholar] [CrossRef]
- Liang, S.X.; Yin, L.X.; Zhou, Y.K.; Feng, X.J.; Ma, M.Z.; Liu, R.P.; Tan, C.L. Abnormal martensitic transformation of high Zr-containing Ti alloys. J. Alloys Compd. 2014, 615, 804–808. [Google Scholar] [CrossRef]
- Lin, C.; Yin, G.; Zhao, Y.; Ge, P.; Liu, Z. Analysis of the effect of alloy elements on martensitic transformation in titanium alloy with the use of valence electron structure parameters. Mater. Chem. Phys. 2011, 125, 411–417. [Google Scholar] [CrossRef]
- Ma, M.; Liang, L.; Wang, L.; Wang, Y.; Cheng, Y.; Tang, B.; Xiang, W.; Tan, X. Phase transformations of titanium hydride in thermal desorption process with different heating rates. Int. J. Hydrog. Energy 2015, 40, 8926–8934. [Google Scholar] [CrossRef]
- Suwarno, S.; Yartys, V.A. Kinetics of hydrogen absorption and desorption in titanium. Bull. Chem. React. Eng. Catal. 2017, 12, 312–317. [Google Scholar] [CrossRef]
- Laptev, R.S.; Syrtanov, M.S.; Kudiiarov, V.N.; Shmakov, A.N.; Vinokurov, Z.S.; Mikhaylov, A.A.; Zolotarev, K.V. In Situ Investigation of Thermo-stimulated Decay of Hydrides of Titanium and Zirconium by Means of X-ray Diffraction of Synchrotron Radiation. In Physics Procedia; Elsevier: Amsterdam, The Netherlands, 2016; Volume 84, pp. 337–341. [Google Scholar]
- Jiménez, C.; Garcia-Moreno, F.; Pfretzschner, B.; Klaus, M.; Wollgarten, M.; Zizak, I.; Schumacher, G.; Tovar, M.; Banhart, J. Decomposition of TiH2studied in situ by synchrotron X-ray and neutron diffraction. Acta Mater. 2011, 59, 6318–6330. [Google Scholar] [CrossRef]
- Ma, M.; Liang, L.; Tang, B.; Xiang, W.; Wang, Y.; Cheng, Y.; Tan, X. Decomposition kinetics study of zirconium hydride by interrupted thermal desorption spectroscopy. J. Alloys Compd. 2015, 645, S217–S220. [Google Scholar] [CrossRef]







| Sample | Phase | Phase Content, % | Lattice Parameters, Å | c/a | |
|---|---|---|---|---|---|
| Zr-1Nb | α-Zr (hcp) | 100 | a = 3.2364 c = 5.1451 | 1.590 | 1.5 |
| Ti-implanted Zr-1Nb | α-Zr (hcp) | 4 | a = 3.2325 c = 5.1507 | 1.594 | 2.2 |
| Zr0.5Ti0.5(hcp) | 54 | a = 3.0886 c = 4.8438 | 1.568 | 4.3 | |
| Zr0.7Ti0.3(hcp) | 43 | a = 3.1613 c = 5.0229 | 1.589 | 4.8 |
| Temperature, °C | Hydrogen Absorption Rate Q, ×10−6 cm3Н2/(s∙cm2) | Reduction Ratio | |
|---|---|---|---|
| Zr-1Nb Alloy | Ti-implanted Zr-1Nb | ||
| 400 | 4.4 | 0.7 | 6.3 |
| 500 | 28.7 | 6.1 | 4.7 |
| Sample | Phase | Content, vol.% | Lattice Parameter, Å | c/a |
|---|---|---|---|---|
| Zr-1Nb | α-Zr (hcp) | 42 | a = 3.2347; c = 5.1624 | 1.596 |
| δ-ZrH1.66 (fcc) | 53 | a = 4.7654 | - | |
| γ-ZrH (fct) | 5 | a = 4.5811; c = 4.9612 | 1.083 | |
| Ti-implanted Zr-1Nb | α-Zr (hcp) | 4 | a = 3.2567; c = 5.1508 | 1.582 |
| Zr0.5Ti0.5 (hcp) | 50 | a = 3.0901; c = 4.8931 | 1.583 | |
| Zr0.7Ti0.3 (hcp) | 46 | a = 3.1899; c = 4.9612 | 1.555 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashkarov, E.B.; Ryabchikov, A.I.; Kurochkin, A.V.; Syrtanov, M.S.; Shevelev, A.E.; Obrosov, A.; Weiß, S. Hydrogen Interaction with Deep Surface Modified Zr-1Nb Alloy by High Intensity Ti Ion Implantation. Metals 2018, 8, 1081. https://doi.org/10.3390/met8121081
Kashkarov EB, Ryabchikov AI, Kurochkin AV, Syrtanov MS, Shevelev AE, Obrosov A, Weiß S. Hydrogen Interaction with Deep Surface Modified Zr-1Nb Alloy by High Intensity Ti Ion Implantation. Metals. 2018; 8(12):1081. https://doi.org/10.3390/met8121081
Chicago/Turabian StyleKashkarov, Egor B., Alexander I. Ryabchikov, Alexander V. Kurochkin, Maxim S. Syrtanov, Alexey E. Shevelev, Aleksei Obrosov, and Sabine Weiß. 2018. "Hydrogen Interaction with Deep Surface Modified Zr-1Nb Alloy by High Intensity Ti Ion Implantation" Metals 8, no. 12: 1081. https://doi.org/10.3390/met8121081





