Thermal Behavior of Hydrated Iron Sulfate in Various Atmospheres
Abstract
:1. Introduction
2. Materials and Methods
3. Results
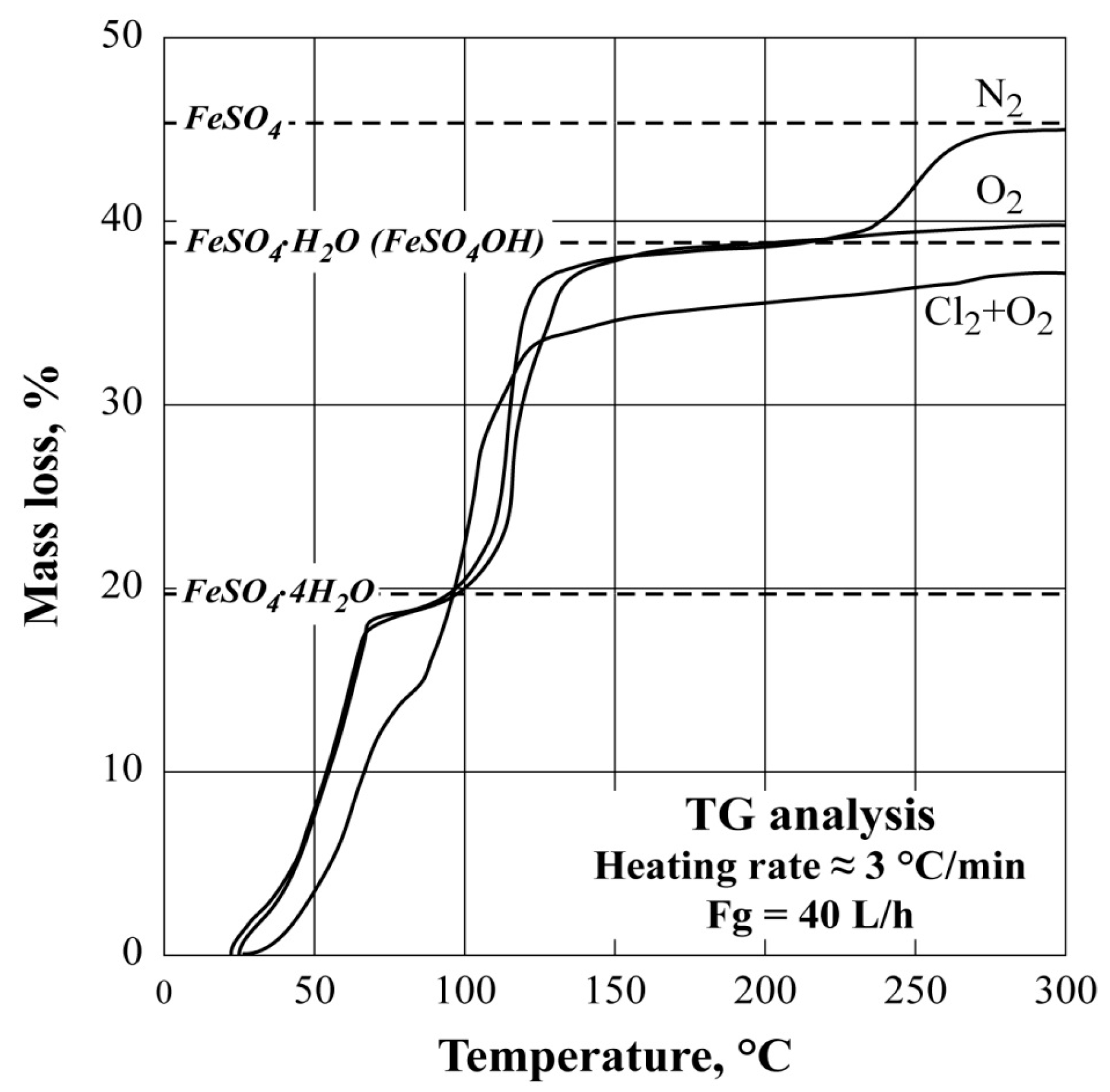
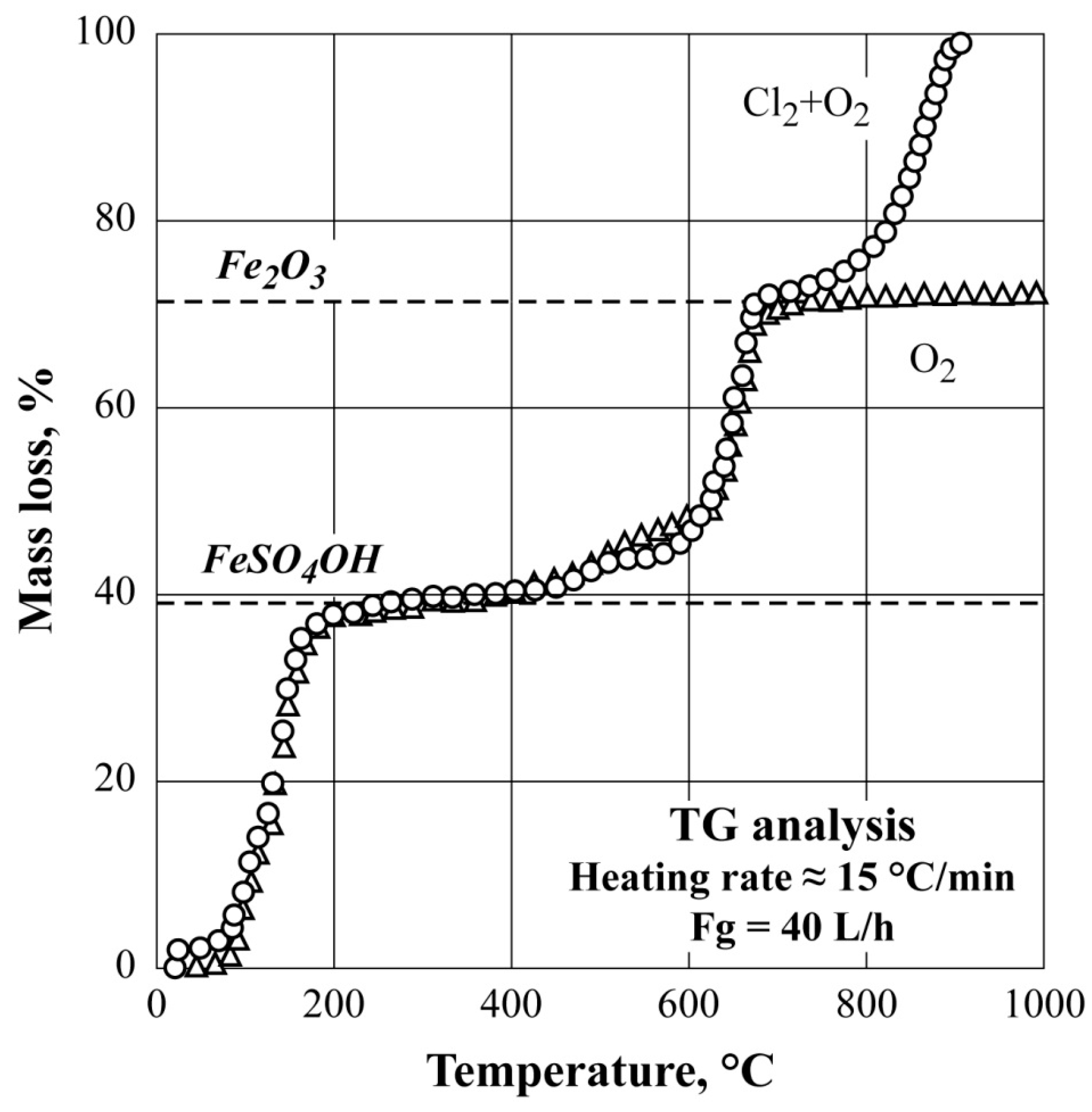
3.1. Nonisothermal TG Analysis of FeSO4·7H2O under Different Atmospheres
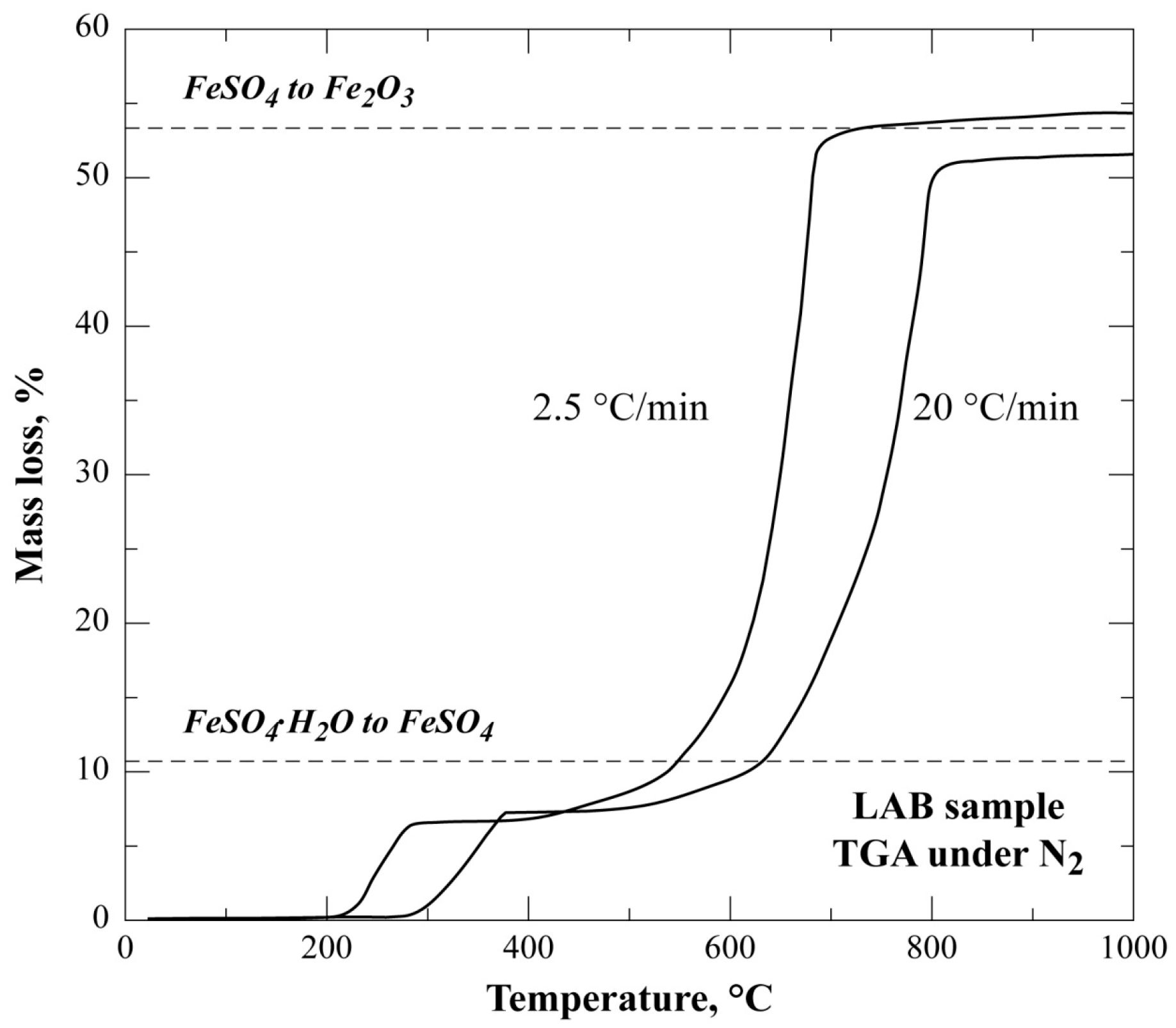
3.2. Nonisothermal TG Analysis of FeSO4·H2O under Nitrogen
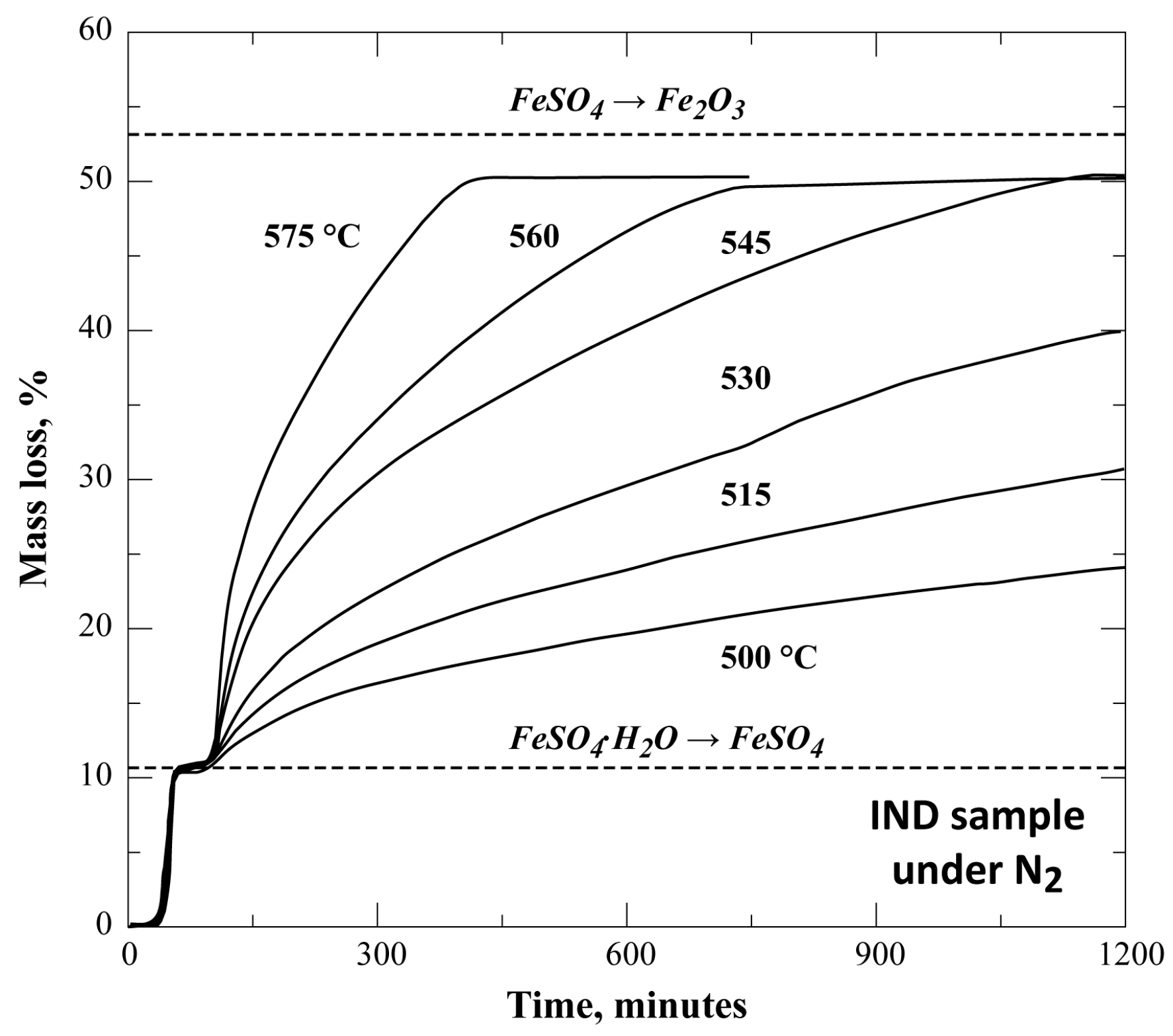
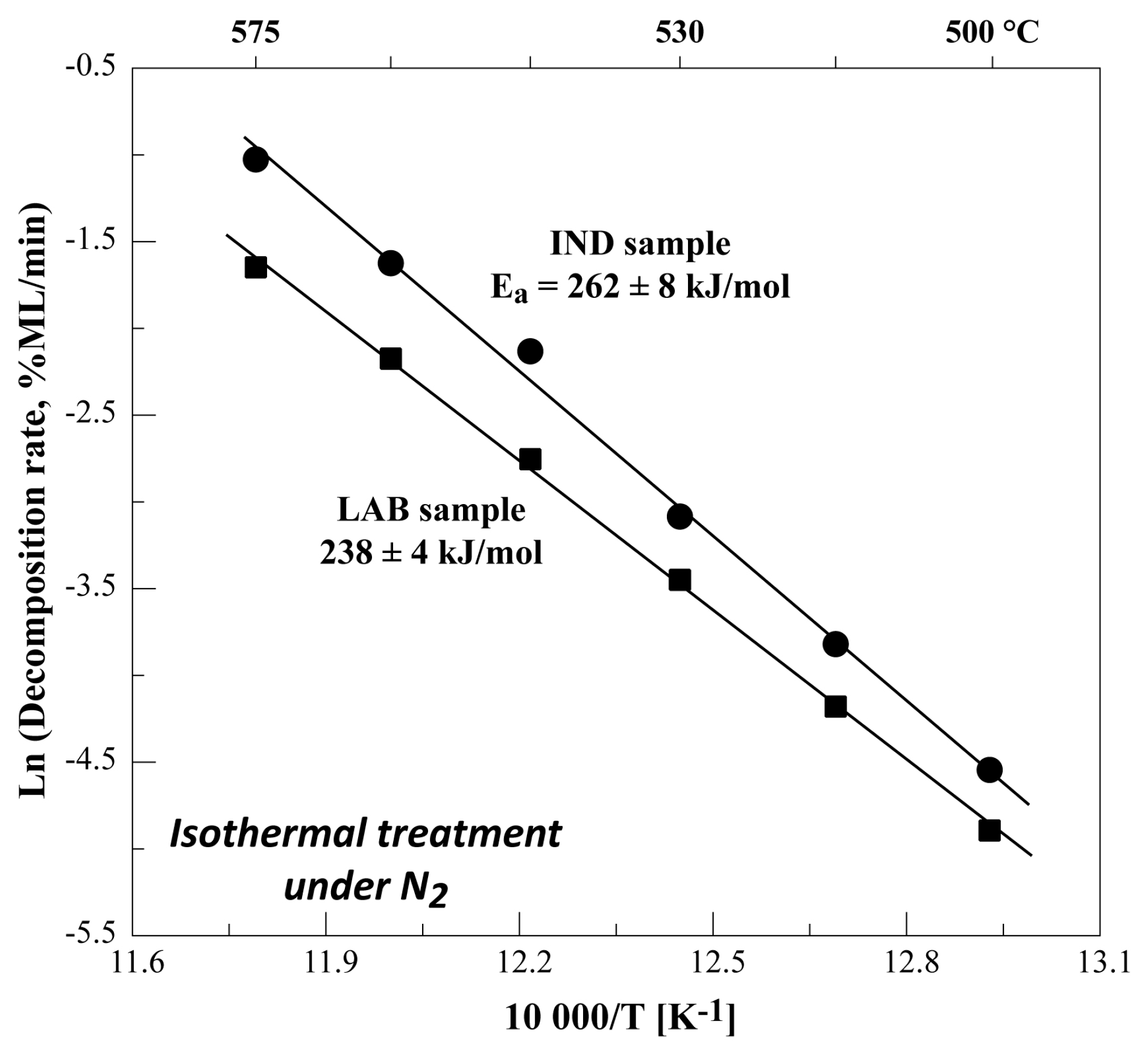
3.3. Isothermal Decomposition of FeSO4·H2O Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kanari, N. Contribution to Chlorine Chemistry and its Applications: Synthesis of Alkali Ferrates (VI). A Study of the Kinetics of Chlorine-Solid Reactions; Defense of the Habilitation Diploma (HDR), Institut National Polytechnique de Lorraine: Nancy, France, 2000.
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar] [CrossRef]
- Kanari, N.; Filippova, I.; Diot, F.; Mochón, J.; Ruiz-Bustinza, I.; Allain, E.; Yvon, J. Utilization of a waste from titanium oxide industry for the synthesis of sodium ferrate by gas-solid reactions. Thermochim. Acta 2014, 575, 219–225. [Google Scholar] [CrossRef]
- Huang, P.; Deng, S.; Zhang, Z.; Wang, X.; Chen, X.; Yang, X.; Yang, L. A sustainable process to utilize ferrous sulfate waste from titanium oxide industry by reductive decomposition reaction with pyrite. Thermochim. Acta 2015, 620, 18–27. [Google Scholar] [CrossRef]
- Kanari, N.; Ostrosi, O.; Ninane, N.; Neveux, N.; Evrard, O. Synthesizing alkali ferrates using a waste as a raw material. JOM 2005, 57, 39–42. [Google Scholar] [CrossRef]
- Kanari, N. Method of Producing Ferrates (VI). French Patent n° 2 905 609, 14 March 2008. [Google Scholar]
- Wei, Y.-L.; Wang, Y.-S.; Liu, C.-H. Preparation of potassium ferrate from spent steel pickling liquid. Metals 2015, 5, 1770–1787. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2: Part I: Low temperature reduction of hematite. Thermochim. Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2: Part II. Low temperature reduction of magnetite. Thermochim. Acta 2007, 456, 75–88. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.S.; Ying, Z.W.; Li, F.; Feng, C.; Liu, Z.G. Non-isothermal gas-based direct reduction behavior of high chromium vanadium-titanium magnetite pellets and the melting separation of metallized pellets. Metals 2017, 7, 153. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Sun, T. Dephosphorization behavior of high-phosphorus oolitic hematite-solid waste containing carbon briquettes during the process of direct reduction-magnetic separation. Metals 2018, 8, 897. [Google Scholar] [CrossRef]
- Oh, J.; Noh, D. The reduction kinetics of hematite particles in H2 and CO atmospheres. Fuel 2017, 196, 144–153. [Google Scholar] [CrossRef]
- Chen, Z.; Dang, J.; Hu, X.; Yan, H. Reduction kinetics of hematite powder in hydrogen atmosphere at moderate temperatures. Metals 2018, 8, 751. [Google Scholar] [CrossRef]
- Tang, H.; Yun, Z.; Fu, X.; Du, S. Modeling and experimental study of ore-carbon briquette reduction under CO–CO2 atmosphere. Metals 2018, 8, 205. [Google Scholar] [CrossRef]
- Fukushima, J.; Takizawa, H. In situ spectroscopic analysis of the carbothermal reduction process of iron oxides during microwave irradiation. Metals 2018, 8, 49. [Google Scholar] [CrossRef]
- Kanari, N.; Mishra, D.; Filippov, L.; Diot, F.; Mochón, J.; Allain, E. Kinetics of hematite chlorination with Cl2 and Cl2+O2: Part I. Chlorination with Cl2. Thermochim. Acta 2010, 497, 52–59. [Google Scholar] [CrossRef]
- Kanari, N.; Mishra, D.; Filippov, L.; Diot, F.; Mochón, J.; Allain, E. Kinetics of hematite chlorination with Cl2 and Cl2+O2. Part II. Chlorination with Cl2+O2. Thermochim. Acta 2010, 506, 34–40. [Google Scholar] [CrossRef]




| Sample | Chemical Analysis (%) | XRD | ||
|---|---|---|---|---|
| Fetotal | Fe(II) | Fe(III) | ||
| IND | 30.9 | 30.9 | trace | FeIISO4·H2O |
| LAB | 32.8 | 14.8 | 18.0 1 | FeIISO4·H2O and FeIIISO4·OH |
| Heating Rate, °C/min | Possible Reaction Steps | ||||
|---|---|---|---|---|---|
| 2.5 | 5.0 | 10.0 | |||
| 70 | 80 | 98 | FeSO4·7H2O | → | FeSO4·4H2O |
| 86 | 133 | 159 | FeSO4·4H2O | → | FeSO4·H2O |
| 227 | 250 | 283 | FeSO4·H2O | → | FeSO4 |
| 653 | 687 | 716 | FeSO4 | → | Fe2O3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanari, N.; Menad, N.-E.; Ostrosi, E.; Shallari, S.; Diot, F.; Allain, E.; Yvon, J. Thermal Behavior of Hydrated Iron Sulfate in Various Atmospheres. Metals 2018, 8, 1084. https://doi.org/10.3390/met8121084
Kanari N, Menad N-E, Ostrosi E, Shallari S, Diot F, Allain E, Yvon J. Thermal Behavior of Hydrated Iron Sulfate in Various Atmospheres. Metals. 2018; 8(12):1084. https://doi.org/10.3390/met8121084
Chicago/Turabian StyleKanari, Ndue, Nour-Eddine Menad, Etleva Ostrosi, Seit Shallari, Frederic Diot, Eric Allain, and Jacques Yvon. 2018. "Thermal Behavior of Hydrated Iron Sulfate in Various Atmospheres" Metals 8, no. 12: 1084. https://doi.org/10.3390/met8121084





