A Further Evaluation of the Coupling Relationship between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags: Influence of Slag Chemical Composition
Abstract
:1. Introduction
2. Influence of Slag Chemical Composition on Proposed Coupling Relationships between Dephosphorization and Desulfurization Abilities and Potentials for CaO–based Slags
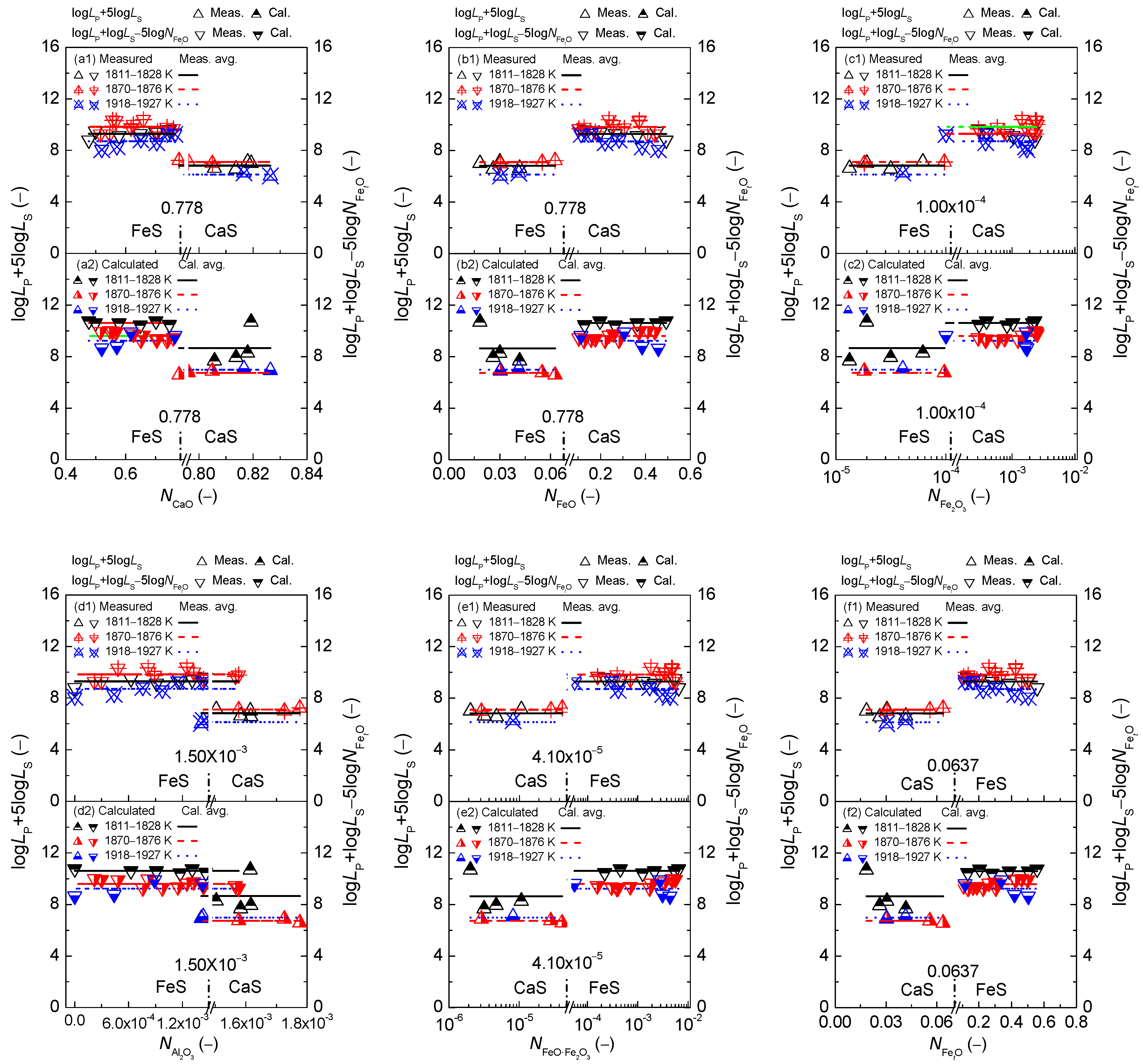
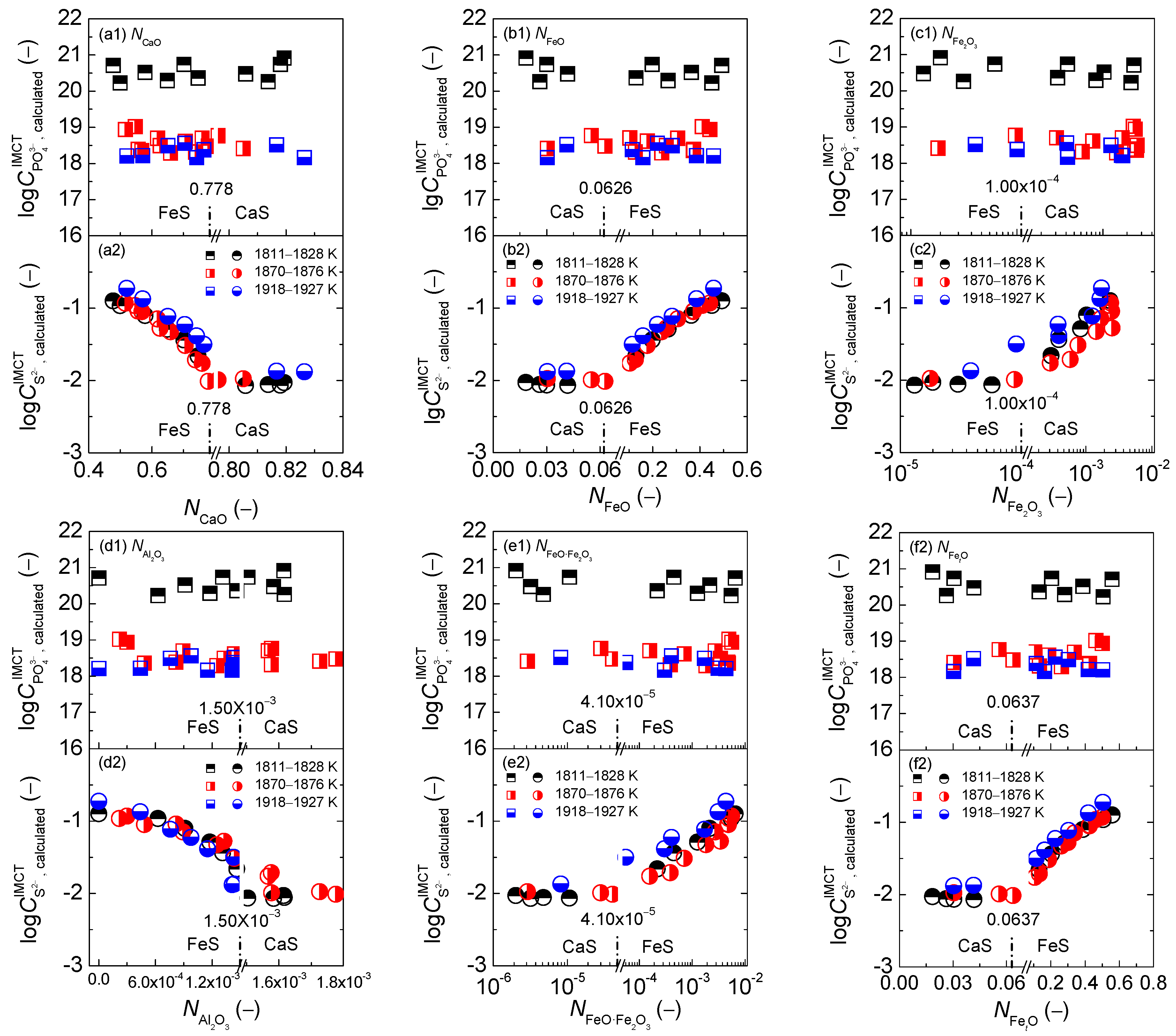
2.1. Influence of Reaction Abilities of Components on Coupling Relationships between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags
2.1.1. Influences of Reaction Abilities of Components on Coupling Relationships between Dephosphorization and Desulfurization Abilities for CaO-based Slags
2.1.2. Influences of Reaction Abilities of Components on Coupling Relationships between Dephosphorization and Desulfurization Potentials for CaO–based Slags
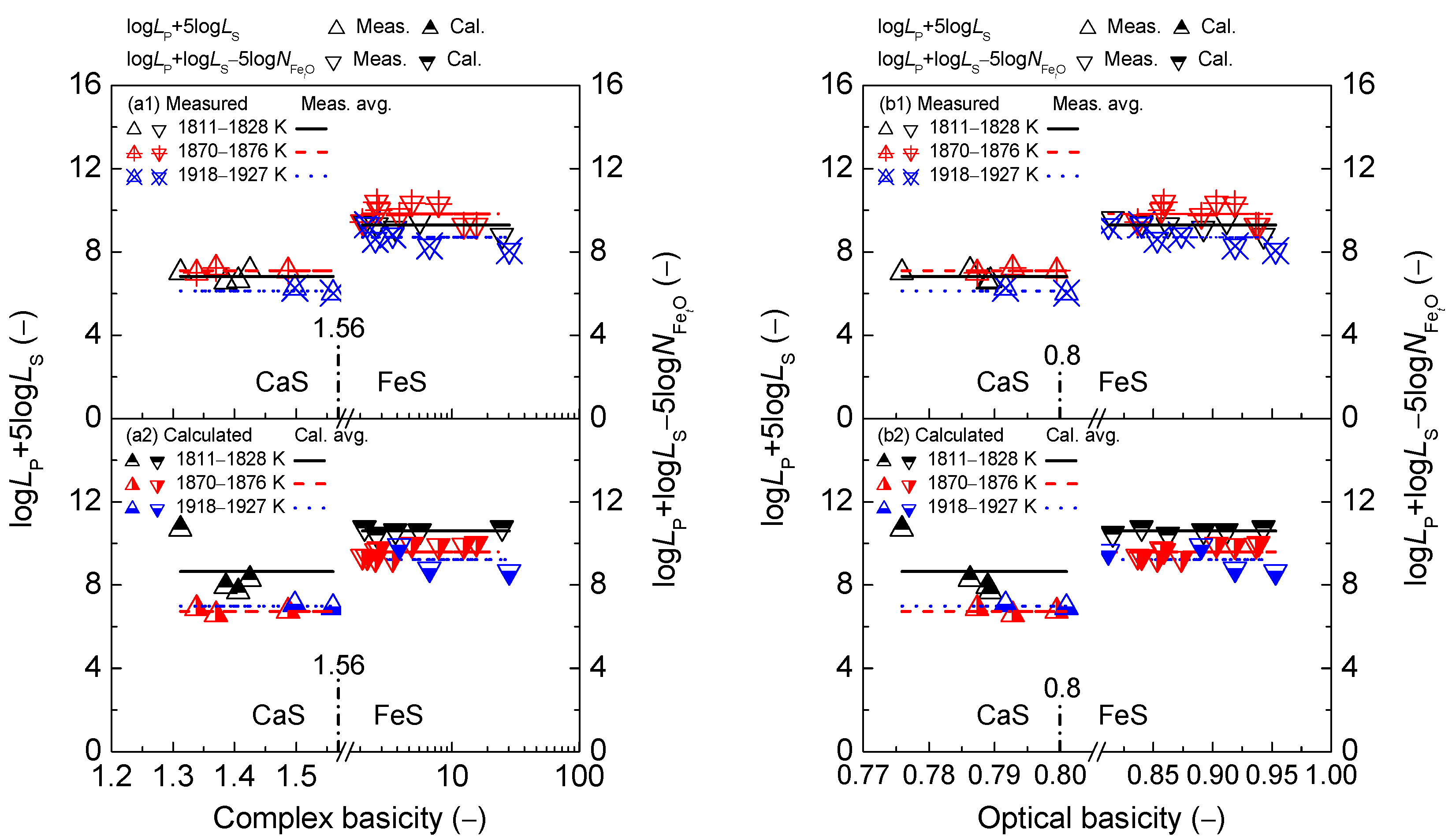
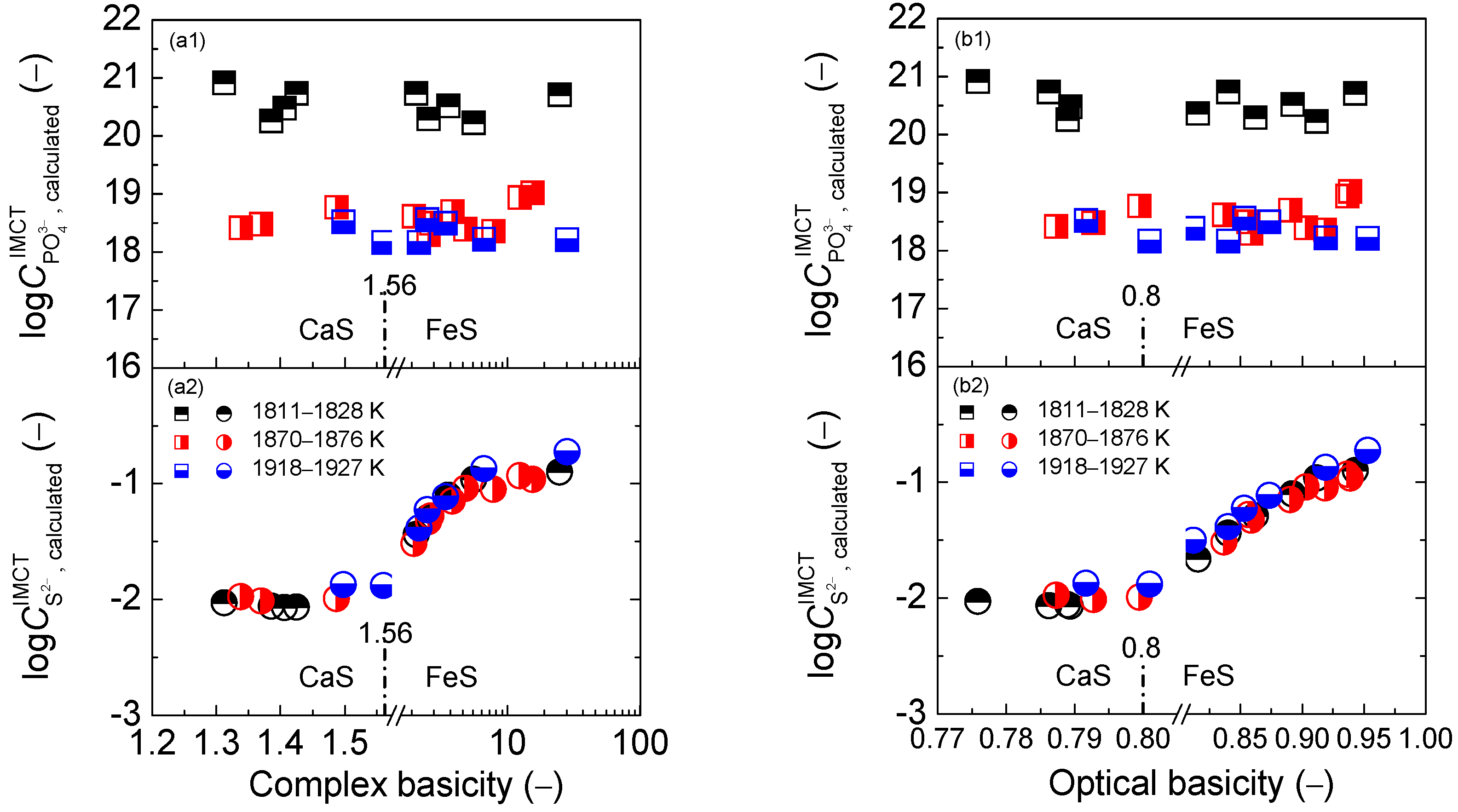
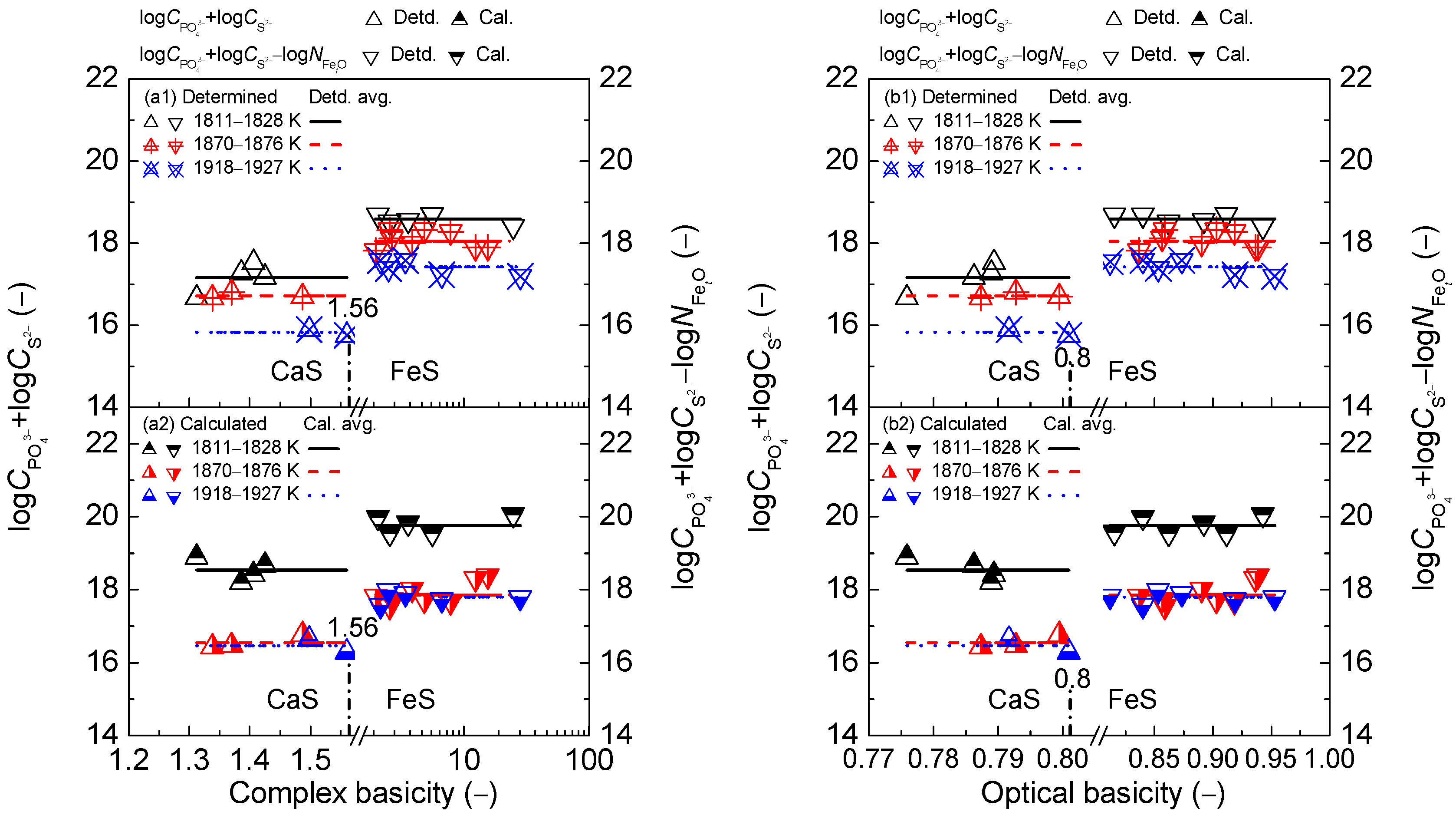
2.2. Influence of Slag Basicity on Coupling Relationships between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags
2.2.1. Influence of Slag Basicity on Coupling Relationships between Dephosphorization and Desulfurization Abilities for CaO-based Slags
2.2.2. Influence of Slag Basicity on Coupling Relationship between Dephosphorization and Desulfurization Potentials for CaO-based Slags
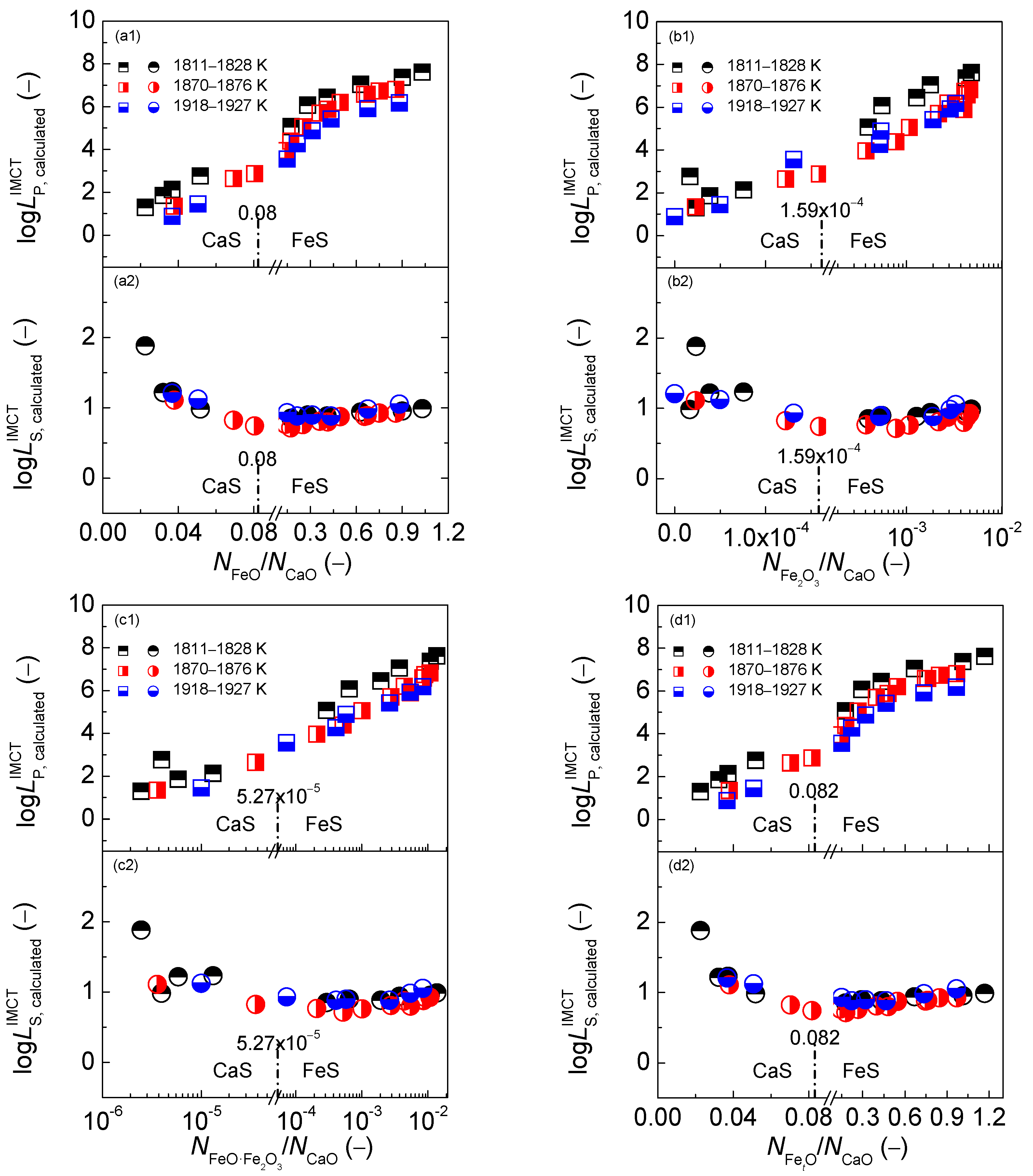
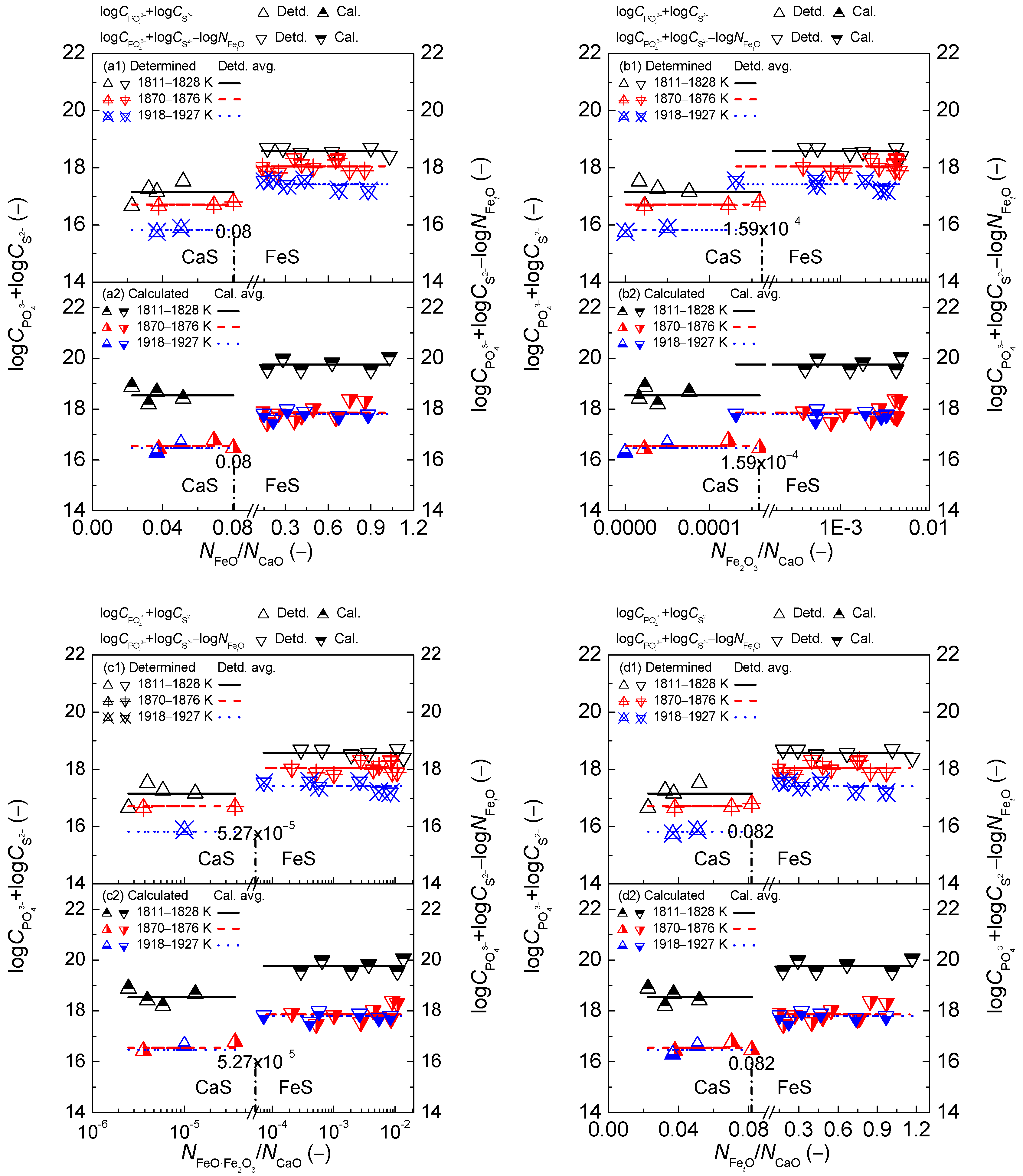
2.3. Comprehensive Effect of FetO and CaO on Coupling Relationships between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags
2.3.1. Comprehensive Effect of FetO and CaO on Coupling Relationships between Dephosphorization and Desulfurization Abilities for CaO-based Slags
2.3.2. Comprehensive Effect of FetO and CaO on Coupling Relationships between Dephosphorization and Desulfurization Potentials for CaO-based Slags
3. Discussion on Proposed Coupling Relationships between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags
3.1. Magnitude of Proposed Coupling Relationships between Dephosphorization and Desulfurization Abilities for CaO-based Slags
3.2. Magnitude of Proposed Coupling Relationships between Dephosphorization and Desulfurization Potentials for CaO-based Slags
3.3. Prospect and application for Proposed Coupling Relationship between Dephosphorization and Desulfurization Abilities or Potentials for CaO–based Slags
4. Conclusions
- (1)
- The proposed coupling relationships for the CaO-based slags in both the reducing and oxidizing zones are not only independent of slag oxidization ability described by the comprehensive mass action concentration of iron oxides but are also irrelevant to the reaction abilities of components expressed by the mass action concentrations over a narrow temperature range in comparison with significant influences of slag oxidization ability as well as reaction abilities of components on dephosphorization and desulfurization abilities or potentials.
- (2)
- The proposed coupling relationships for the CaO-based slags in both the reducing and oxidizing zones keep almost constant with the variation of two kinds of slag basicity as the simplified complex basicity and optical basicity over a narrow temperature range compared with the strong effects of two kinds of slags basicity on dephosphorization and desulfurization abilities or potentials.
- (3)
- The proposed coupling relationships for the CaO-based slags in both reducing and oxidizing zones are independent of the comprehensive effect of iron oxides FetO and basic oxide CaO described by the mass action concentration ratio or or or , or the mass percentage ratio (% FeO)/(% CaO) or (% Fe2O3)/(% CaO) or (% FetO)/(% CaO) in comparison with the large influences of the aforementioned comprehensive effect of iron oxides FetO and basic oxide CaO on dephosphorization and desulfurization abilities or potentials.
- (4)
- Increasing the temperature from 1811 to 1927 K (1538 to 1654 °C) can result in a slightly decreasing tendency of the proposed coupling relationships for the CaO-based slags in reducing and oxidizing zones.
- (5)
- Chemical composition of slags or fluxes with the assigned dephosphorization ability or potential can be theoretically designed or optimized by its desulfurization ability or potential, and vice versa, in terms of the obtained maximum values of dephosphorization and desulfurization abilities or potentials for the CaO-based slags in both reducing and oxidizing zones.
- (6)
- The proposed coupling relationships between and for the CaO-based slags as and in reducing and oxidizing zones are recommended to design or optimize the chemical composition of slags or fluxes due to a large difference of magnitude between phosphate capacity and sulfide capacity .
Author Contributions
Funding
Conflicts of Interest
Nomenclatures
| Activity of components i in slags or element i in liquid iron relative to pure solid or liquid component i or element i as standard state with mole fraction as concentration unit and following Raoult’s law under the condition of taking ideal solution as reference state, i.e., , (–); | |
| Phosphate capacity of slags based on gas–slag equilibrium, (–); | |
| Sulfide capacity of slags based on gas–slag equilibrium, (–); | |
| Activity coefficient of element i in liquid iron related with activity , (–); | |
| Standard equilibrium constant of chemical reaction for forming component i or structural unit i, (–); | |
| Phosphorus distribution ratio between slags and liquid iron, defined as , (–); | |
| Sulphur distribution ratio between slags and liquid iron, defined as , (–); | |
| Mi | Relative atomic mass of element i or relative molecular mass of component i, (–); |
| Total mole number of all components in 100 g slags, (mol). | |
| Greek symbols | |
| Activity coefficient of component i in slags related with activity , (–); | |
| Optical basicity of slags, (–). | |
References
- Ban–ya, S.; Hino, M.; Sato, A.; Terayama, A.O. O, P and S Distribution Equilibria between Liquid Iron and CaO–Al2O3–FetO Slag Saturated with CaO. Tetsu–to–Hagané 1991, 77, 361–368. [Google Scholar]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Chai, G.M.; Duan, D.P.; Zhang, J. Coupling Relationship between Dephosphorization and Desulfurization Abilities or Potentials for CaO–FeO–Fe2O3–Al2O3–P2O5 Slags over a Large Variation Range of Slag Oxidization Ability Based on the Ion and Molecule Coexistence Theory. Ironmak. Steelmak. 2018, 45, 25–43. [Google Scholar] [CrossRef]
- Yang, X.M.; Chai, G.M.; Zhang, M.; Li, J.Y.; Liang, Q.; Zhang, J. Thermodynamic Models for Predicting Dephosphorization Ability and Potential of CaO–FeO–Fe2O3–Al2O3–P2O5 Slags during Secondary Refining Process of Molten Steel Based on the Ion and Molecule Coexistence Theory. Ironmak. Steelmak. 2016, 43, 663–687. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Zhang, M.; Zhang, J. Prediction Model of Sulfur Distribution Ratio between CaO–FeO–Fe2O3–Al2O3–P2O5 Slags and Liquid Iron in a Large Variation Range of Oxygen Potential during Secondary Refining Process of Molten Steel Based on the Ion and Molecule Coexistence Theory. Ironmak. Steelmak. 2016, 43, 39–55. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Chai, G.M.; Zhang, M.; Zhang, J. Prediction Model of Sulfide Capacity for CaO–FeO–Fe2O3–Al2O3–P2O5 Slags in a Large Variation Range of Oxygen Potential Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2014, 45, 2118–2137. [Google Scholar] [CrossRef]
- Yang, X.M.; Jiao, J.S.; Ding, R.C.; Shi, C.B.; Guo, H.J. A Thermodynamic Model for Calculating Sulphur Distribution Ratio between CaO–SiO2–MgO–Al2O3 Ironmaking Slags and Carbon Saturated Hot Metal Based on the Ion and Molecule Coexistence Theory. ISIJ Int. 2009, 49, 1828–1837. [Google Scholar] [CrossRef]
- Shi, C.B.; Yang, X.M.; Jiao, J.S.; Li, C.; Guo, H.J. A Sulphide Capacity Prediction Model of CaO–SiO2–MgO–Al2O3 Ironmaking Slags Based on the Ion and Molecule Coexistence Theory. ISIJ Int. 2010, 50, 1362–1372. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Chai, G.M.; Wang, F. A Thermodynamic Model of Sulfur Distribution Ratio between CaO–SiO2–MgO–FeO–MnO–Al2O3 Slags and Molten Steel during LF Refining Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2011, 42, 1150–1180. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Chai, G.M.; Zhang, J. A Sulfide Capacity Prediction Model of CaO–SiO2–MgO–FeO–MnO–Al2O3 Slags during LF Refining Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2012, 43, 241–266. [Google Scholar] [CrossRef]
- Yang, X.M.; Duan, J.P.; Shi, C.B.; Zhang, M.; Zhang, Y.L.; Wang, J.C. A Thermodynamic Model of Phosphorus Distribution Ratio between CaO–SiO2–MgO–FeO–Fe2O3–MnO–Al2O3–P2O5 Slags and Molten Steel during Top–Bottom Combined Blown Converter Steelmaking Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2011, 42, 738–770. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Duan, J.P.; Zhang, J. A Thermodynamic Model of Phosphate Capacity for CaO–SiO2–MgO–FeO–Fe2O3–MnO–Al2O3–P2O5 Slags Equilibrated with Molten Steel during a Top–Bottom Combined Blown Converter Steelmaking Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2011, 42, 951–976. [Google Scholar] [CrossRef]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Zhang, J. A Thermodynamic Model for Prediction of Iron Oxide Activity in Some FeO–Containing Slag Systems. Steel Res. Int. 2012, 83, 244–258. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhang, M.; Zhang, J.L.; Li, P.C.; Li, J.Y.; Zhang, J. Representation of Oxidation Ability for Metallurgical Slags Based on the Ion and Molecule Coexistence Theory. Steel Res. Int. 2014, 85, 347–375. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, M.; Guo, M.; Yang, X.M. Enrichment Mechanism of Phosphate in CaO–SiO2–FeO–Fe2O3–P2O5 Steelmaking Slags. Metall. Mater. Trans. B 2014, 45, 1666–1682. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Chai, G.M.; Duan, D.P.; Zhang, J. A Thermodynamic Model for Predicting Phosphorus Partition between CaO–based Slags and Hot Metal during Hot Metal Dephosphorization Pretreatment Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2016, 47, 2279–2301. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Chai, G.M.; Duan, D.P.; Zhang, J. Critical Evaluation of Prediction Models for Phosphorus Partition between CaO–based Slags and Iron–based Melts during Dephosphorization Processes. Metall. Mater. Trans. B 2016, 47, 2302–2329. [Google Scholar] [CrossRef]
- Yang, X.M.; Li, J.Y.; Chai, G.M.; Duan, D.P.; Zhang, J. A Thermodynamic Model for Predicting Phosphate Capacity of CaO−based Slags during Hot Metal Dephosphorization Pretreatment Process. Ironmak. Steelmak. 2017, 44, 437–454. [Google Scholar] [CrossRef]
- Zhang, J. Computational Thermodynamics of Metallurgical Melts and Solutions; Metallurgical Industry Press: Beijing, China, 2007. [Google Scholar]
- Tsukihashi, F.; Nakamura, M.; Orimoto, T.; Sano, N. Thermodynamics of Phosphorus for the CaO–BaO–CaF2–SiO2 and CaO–Al2O3 Systems. Tetsu–to–Hagané 1990, 76, 1664–1671. [Google Scholar] [CrossRef]
- Ban-ya, S.; Hobo, M.; Kaji, T.; Itoh, T.; Hino, M. Sulphide Capacity and Sulphur Solubility in CaO-Al2O3 and CaO-Al2O3-CaF2 Slags. ISIJ Int. 2004, 44, 1810–1816. [Google Scholar] [CrossRef]
- Ban-ya, S.; Hino, M. Comments on “Evaluation of Thermodynamic Activity of Metallic Oxide in a Ternary Slag from the Sulphide Capacity of the Slag”. ISIJ Int. 2005, 45, 1754–1756. [Google Scholar] [CrossRef]
- Wei, S.K. Thermodynamics of Metallurgical Processes; Science Press: Beijing, China, 2010. [Google Scholar]
- Zhang, J.Y. Metallurgical Physicochemistry; Metallurgical Industry Press: Beijing, China, 2004. [Google Scholar]
- Chen, J.X. Handbook of Common Figures, Tables and Data for Steelmaking, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2010. [Google Scholar]
- Sosinsky, D.J.; Sommerville, I.D. The Composition and Temperature Dependence of the Sulfide Capacity of Metallurgical Slags. Metall. Trans. B 1986, 17, 331–337. [Google Scholar] [CrossRef]
- Nakamura, T.; Ueda, Y.; Toguri, J.M. A Critical Review of Optical Basicity on Metallurgical application. In Proceedings of the Third International Conference on Metallurgical Slags and Fluxes, University of Strathclyde, Glasgow, Scotland, 27–29 June 1988; The Institute of Metals: London, UK; pp. 146–149. [Google Scholar]
- Mills, K.C.; Sridhar, S. Viscosities of Ironmaking and Steelmaking Slags. Ironmak. Steelmak. 1999, 26, 262–268. [Google Scholar] [CrossRef]
- Young, R.W.; Duffy, J.A.; Hassall, G.J.; Xu, Z. Use of Optical Basicity Concept for Determining Phosphorus and Sulphur Slag–Metal Partitions. Ironmak. Steelmak. 1992, 19, 201–219. [Google Scholar]
- Wagner, C. The Concept of the Basicity of Slags. Metall. Trans. B 1975, 6, 405–409. [Google Scholar] [CrossRef]






| New Test No. [2] | Old Test No. [1] | Chemical Composition of Slags [1] (mass %) | Chemical Composition of Liquid Iron [1] (mass %) | T [K (°C)] | Slag Oxidization Ability | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (CaO) | (FeO) | (Fe2O3) | (Al2O3) | (P2O5) | (S) | [P] | [S] | [O] | (FetO) (mass %) | (–) | (Pa) | |||
| 1 | 18 | 55.19 | 1.61 | 0.23 | 39.65 | 2.5048 | 1.079 | 1.8766 | 0.04 | 0.0039 | 1822 (1549) | 1.88 | 0.019 | 8.18 × 10−8 |
| 2 | 19 | 56.69 | 2.36 | 0.39 | 40.34 | 0.5629 | 0.862 | 0.1973 | 0.072 | 0.0051 | 1818 (1545) | 2.72 | 0.026 | 1.54 × 10–7 |
| 3 | 17 | 56.14 | 2.67 | 0.76 | 37.35 | 2.1138 | 1.364 | 0.5017 | 0.079 | 0.0077 | 1821 (1548) | 3.46 | 0.031 | 2.18 × 10–7 |
| 4 | 20 | 55.64 | 3.74 | 0.16 | 38.25 | 1.348 | 0.944 | 0.1296 | 0.107 | 0.0069 | 1820 (1547) | 3.97 | 0.042 | 4.02 × 10–7 |
| 5 | 10 | 53.69 | 2.63 | 0.19 | 39.89 | 0.2418 | 0.181 | 0.0580 | 0.017 | 0.0087 | 1876 (1603) | 2.91 | 0.031 | 5.38 × 10–7 |
| 6 | 27 | 58.31 | 2.78 | 0.00 | 36.81 | 0.5744 | 0.802 | 0.2766 | 0.075 | 0.0127 | 1918 (1645) | 2.84 | 0.030 | 1.03 × 10–6 |
| 7 | 11 | 53.58 | 4.79 | 1.01 | 35.63 | 0.4165 | 0.216 | 0.0275 | 0.029 | 0.0165 | 1873 (1600) | 6.00 | 0.056 | 1.72 × 10–6 |
| 8 | 14 | 56.84 | 3.71 | 0.42 | 35.71 | 2.3081 | 0.920 | 0.4648 | 0.083 | 0.0150 | 1927 (1654) | 4.23 | 0.042 | 2.20 × 10–6 |
| 9 * | 1 | 52.67 | 5.47 | 1.24 | 38.13 | 0.3284 | 0.154 | 0.0120 | 0.026 | 0.0179 | 1874 (1601) | 6.75* | 0.064* | 2.27 × 10–6* |
| 10 | 21 | 54.47 | 12.01 | 3.25 | 30.36 | 1.5259 | 0.986 | 0.0093 | 0.158 | 0.0242 | 1822 (1549) | 14.92 | 0.133 | 4.18 × 10–6 |
| 11 | 2 | 53.13 | 9.28 | 2.83 | 32.44 | 0.5394 | 0.214 | 0.0060 | 0.035 | 0.0275 | 1874 (1601) | 12.11 | 0.107 | 6.38 × 10–6 |
| 12 | 22 | 51.69 | 18.59 | 3.92 | 23.61 | 1.4744 | 0.899 | 0.0047 | 0.145 | 0.0315 | 1811 (1538) | 22.61 | 0.205 | 8.27 × 10–6 |
| 13 | 3 | 51.28 | 11.09 | 5.26 | 29.78 | 0.3168 | 0.134 | 0.0016 | 0.037 | 0.0356 | 1874 (1601) | 16.24 | 0.133 | 9.93 × 10–6 |
| 14 | 28 | 53.35 | 10.19 | 0.93 | 30.35 | 1.7682 | 1.010 | 0.0283 | 0.168 | 0.0267 | 1922 (1649) | 11.63 | 0.114 | 1.54 × 10–5 |
| 15 | 4 | 50.58 | 15.86 | 6.58 | 25.05 | 0.3575 | 0.167 | 0.0017 | 0.028 | 0.0448 | 1876 (1603) | 22.21 | 0.187 | 2.03 × 10–5 |
| 16 | 23 | 49.27 | 25.15 | 7.22 | 17.78 | 1.3705 | 0.952 | 0.0026 | 0.125 | 0.0448 | 1828 (1555) | 31.83 | 0.281 | 2.08 × 10–5 |
| 17 | 29 | 55.46 | 15.10 | 3.60 | 24.06 | 1.472 | 0.983 | 0.0085 | 0.154 | 0.0407 | 1924 (1651) | 18.67 | 0.164 | 3.27 × 10–5 |
| 18 | 24 | 45.27 | 35.25 | 7.72 | 10.96 | 1.6423 | 0.958 | 0.0022 | 0.096 | 0.0530 | 1824 (1551) | 42.54 | 0.387 | 3.68 × 10–5 |
| 19 | 5 | 47.42 | 20.83 | 10.83 | 18.09 | 0.3611 | 0.181 | 0.0004 | 0.024 | 0.0521 | 1874 (1601) | 31.47 | 0.261 | 3.82 × 10–5 |
| 20 | 9 | 45.40 | 21.92 | 16.63 | 16.48 | 0.4327 | 0.211 | 0.0006 | 0.029 | 0.0541 | 1875 (1602) | 36.73 | 0.301 | 5.15 × 10–5 |
| 21 | 6 | 46.35 | 27.70 | 11.89 | 11.59 | 0.352 | 0.175 | 0.0006 | 0.021 | 0.0584 | 1873 (1600) | 39.37 | 0.338 | 6.29 × 10–5 |
| 22 | 15 | 51.99 | 20.69 | 3.17 | 17.92 | 2.9425 | 0.896 | 0.0126 | 0.129 | 0.0555 | 1927 (1654) | 25.11 | 0.226 | 6.51 × 10–5 |
| 23 | 25 | 37.11 | 40.14 | 11.61 | 5.10 | 1.5949 | 1.027 | 0.0011 | 0.084 | 0.0612 | 1827 (1554) | 53.84 | 0.506 | 6.64 × 10–5 |
| 24 | 26 | 38.61 | 47.49 | 13.02 | 0.00 | 1.5678 | 1.029 | 0.0021 | 0.083 | 0.0624 | 1821 (1548) | 59.73 | 0.558 | 7.28 × 10–5 |
| 25 | 7 | 42.12 | 33.57 | 13.99 | 8.40 | 0.3142 | 0.172 | 0.0002 | 0.018 | 0.0666 | 1870 (1597) | 47.06 | 0.425 | 9.49 × 10–5 |
| 26 | 8 | 43.63 | 33.64 | 15.20 | 5.25 | 0.3065 | 0.168 | 0.0002 | 0.017 | 0.0694 | 1873 (1600) | 48.42 | 0.426 | 9.97 × 10–5 |
| 27 | 16 | 49.13 | 26.21 | 8.98 | 11.50 | 2.9552 | 1.067 | 0.0062 | 0.111 | 0.0690 | 1925 (1652) | 35.79 | 0.304 | 1.14 × 10–4 |
| 28 | 12 | 43.52 | 38.77 | 13.86 | 2.30 | 0.4959 | 0.237 | 0.0008 | 0.021 | 0.0713 | 1873 (1600) | 52.05 | 0.463 | 1.18 × 10–4 |
| 29 | 13 | 39.23 | 40.00 | 12.78 | 2.67 | 0.5014 | 0.241 | 0.0007 | 0.020 | 0.0768 | 1874 (1601) | 54.40 | 0.500 | 1.40 × 10–4 |
| 30 | 30 | 45.80 | 37.65 | 10.16 | 5.34 | 1.5302 | 1.083 | 0.0025 | 0.108 | n/a† | 1927 (1654) | 47.29 | 0.420 | 2.25 × 10–4 |
| 31 | 31 | 42.61 | 45.79 | 9.49 | 0.00 | 1.5405 | 1.139 | 0.0021 | 0.092 | n/a† | 1927 (1654) | 55.50 | 0.503 | 3.23 × 10–4 |
| New Test No. [2] | Old Test No. [1] | De-P and De-S Abilities (–) | De-P and De-S Potentials (–) | Coupling Relationship Term between De-P and De-S Abilities (–) | Coupling Relationship Term between De-P and De-S Potentials (–) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meas.† [1] | Cal.† [3] | Meas.† [1] | Cal.† [4] | Detd.† [3] | Cal.† [3] | Detd.† [5] | Cal.† [5] | Detd.† [2] | Cal.† [2] | Detd.† [2] | Cal.† [2] | Detd.† [2] | Cal.† [2] | Detd.† [2] | Cal.† [2] | ||
| 1 | 18 | –0.148 | 1.294 | 1.431 | 1.880 | 18.921 | 20.912 | –2.258 | –2.028 | 7.007 | 10.696 | – | – | 16.663 | 18.884 | – | – |
| 2 | 19 | 1.160 | 1.848 | 1.078 | 1.218 | 19.398 | 20.258 | –2.127 | –2.057 | 6.551 | 7.936 | – | – | 17.271 | 18.201 | – | – |
| 3 | 17 | 0.924 | 2.133 | 1.237 | 1.231 | 19.030 | 20.734 | –1.859 | –2.063 | 7.110 | 8.286 | – | – | 17.172 | 18.671 | – | – |
| 4 | 20 | 1.904 | 2.772 | 0.946 | 0.983 | 19.647 | 20.479 | –2.118 | –2.067 | 6.632 | 7.688 | – | – | 17.528 | 18.412 | – | – |
| 5 | 10 | 1.857 | 1.344 | 1.027 | 1.109 | 18.597 | 18.408 | –1.927 | –1.975 | 6.993 | 6.886 | – | – | 16.671 | 16.433 | – | – |
| 6 | 27 | 0.876 | 0.885 | 1.029 | 1.209 | 17.580 | 18.164 | –1.826 | –1.877 | 6.021 | 6.928 | – | – | 15.753 | 16.287 | – | – |
| 7 | 11 | 2.741 | 2.649 | 0.872 | 0.822 | 18.497 | 18.765 | –1.799 | –1.993 | 7.101 | 6.758 | – | – | 16.698 | 16.772 | – | – |
| 8 | 14 | 1.029 | 1.454 | 1.045 | 1.121 | 17.680 | 18.514 | –1.783 | –1.871 | 6.252 | 7.058 | – | – | 15.896 | 16.643 | – | – |
| 9* | 1 | 3.358 | 2.874 | 0.773 | 0.741 | 18.662 | 18.479 | –1.860 | –2.012 | 7.221 | 6.577 | – | – | 16.802 | 16.467 | – | – |
| 10 | 21 | 4.247 | 5.068 | 0.795 | 0.849 | 19.521 | 20.362 | –1.707 | –1.660 | – | – | 9.442 | 10.317 | – | – | 18.692 | 19.579 |
| 11 | 2 | 4.176 | 3.960 | 0.786 | 0.764 | 18.721 | 18.702 | –1.661 | –1.763 | – | – | 9.831 | 9.593 | – | – | 18.031 | 17.911 |
| 12 | 22 | 4.824 | 6.079 | 0.792 | 0.898 | 19.594 | 20.734 | –1.591 | –1.440 | – | – | 9.075 | 10.435 | – | – | 18.692 | 19.983 |
| 13 | 3 | 5.093 | 4.389 | 0.559 | 0.716 | 18.788 | 18.316 | –1.778 | –1.713 | – | – | 10.050 | 9.503 | – | – | 17.886 | 17.478 |
| 14 | 28 | 3.344 | 3.553 | 0.779 | 0.932 | 18.323 | 18.379 | –1.713 | –-1.498 | – | – | 8.837 | 9.199 | – | – | 17.553 | 17.823 |
| 15 | 4 | 5.092 | 5.051 | 0.776 | 0.762 | 18.551 | 18.611 | –1.463 | –1.516 | – | – | 9.528 | 9.473 | – | – | 17.816 | 17.821 |
| 16 | 23 | 5.307 | 6.438 | 0.882 | 0.882 | 19.315 | 20.288 | –1.354 | –1.291 | – | – | 8.967 | 10.098 | – | – | 18.512 | 19.548 |
| 17 | 29 | 4.309 | 4.273 | 0.805 | 0.882 | 18.301 | 18.163 | –1.501 | –1.383 | – | – | 9.054 | 9.096 | – | – | 17.585 | 17.565 |
| 18 | 24 | 5.531 | 7.041 | 0.999 | 0.941 | 19.304 | 20.518 | –1.161 | –1.100 | – | – | 8.620 | 10.072 | – | – | 18.556 | 19.830 |
| 19 | 5 | 6.354 | 5.698 | 0.877 | 0.808 | 19.035 | 18.289 | –1.295 | –1.328 | – | – | 10.184 | 9.460 | – | – | 18.323 | 17.543 |
| 20 | 9 | 6.080 | 5.892 | 0.862 | 0.797 | 18.892 | 18.494 | –1.296 | –1.277 | – | – | 9.616 | 9.363 | – | – | 18.118 | 17.739 |
| 21 | 6 | 5.990 | 6.197 | 0.921 | 0.876 | 18.732 | 18.695 | –1.203 | –1.151 | – | – | 9.309 | 9.470 | – | – | 18.000 | 18.015 |
| 22 | 15 | 4.268 | 4.882 | 0.842 | 0.898 | 18.070 | 18.560 | –1.332 | –1.225 | – | – | 8.350 | 9.021 | – | – | 17.384 | 17.981 |
| 23 | 25 | 6.120 | 7.378 | 1.087 | 0.955 | 19.414 | 20.225 | –1.011 | –0.964 | – | – | 8.737 | 9.862 | – | – | 18.699 | 19.556 |
| 24 | 26 | 5.551 | 7.618 | 1.093 | 0.986 | 19.147 | 20.711 | –0.995 | –0.901 | – | – | 7.966 | 9.927 | – | – | 18.405 | 20.063 |
| 25 | 7 | 6.895 | 6.587 | 0.980 | 0.889 | 19.039 | 18.390 | –1.087 | –1.042 | – | – | 9.788 | 9.388 | – | – | 18.323 | 17.719 |
| 26 | 8 | 6.884 | 6.584 | 0.995 | 0.878 | 18.964 | 18.353 | –1.056 | –1.048 | – | – | 9.792 | 9.376 | – | – | 18.279 | 17.676 |
| 27 | 16 | 4.886 | 5.429 | 0.983 | 0.883 | 18.156 | 18.496 | –1.095 | –1.113 | – | – | 8.486 | 8.929 | – | – | 17.579 | 17.899 |
| 28 | 12 | 5.889 | 6.735 | 1.053 | 0.926 | 18.544 | 19.014 | –0.987 | –0.964 | – | – | 8.666 | 9.385 | – | – | 17.891 | 18.385 |
| 29 | 13 | 6.010 | 6.809 | 1.081 | 0.924 | 18.521 | 18.935 | –0.928 | –0.931 | – | – | 8.650 | 9.292 | – | – | 17.894 | 18.305 |
| 30 | 30 | 5.389 | 5.914 | 1.001 | 0.980 | 17.697 | 18.221 | –0.851 | –0.872 | – | – | 8.310 | 8.814 | – | – | 17.222 | 17.726 |
| 31 | 31 | 5.543 | 6.180 | 1.093 | 1.051 | 17.572 | 18.208 | –0.685 | –0.726 | – | – | 8.181 | 8.776 | – | – | 17.189 | 17.784 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.-m.; Li, J.-y.; Zhang, M.; Yan, F.-j.; Duan, D.-p.; Zhang, J. A Further Evaluation of the Coupling Relationship between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags: Influence of Slag Chemical Composition. Metals 2018, 8, 1083. https://doi.org/10.3390/met8121083
Yang X-m, Li J-y, Zhang M, Yan F-j, Duan D-p, Zhang J. A Further Evaluation of the Coupling Relationship between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags: Influence of Slag Chemical Composition. Metals. 2018; 8(12):1083. https://doi.org/10.3390/met8121083
Chicago/Turabian StyleYang, Xue-min, Jin-yan Li, Meng Zhang, Fang-jia Yan, Dong-ping Duan, and Jian Zhang. 2018. "A Further Evaluation of the Coupling Relationship between Dephosphorization and Desulfurization Abilities or Potentials for CaO-based Slags: Influence of Slag Chemical Composition" Metals 8, no. 12: 1083. https://doi.org/10.3390/met8121083




