Alkaline Metal Reagent-Assisted Synthesis of Monodisperse Iron Oxide Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
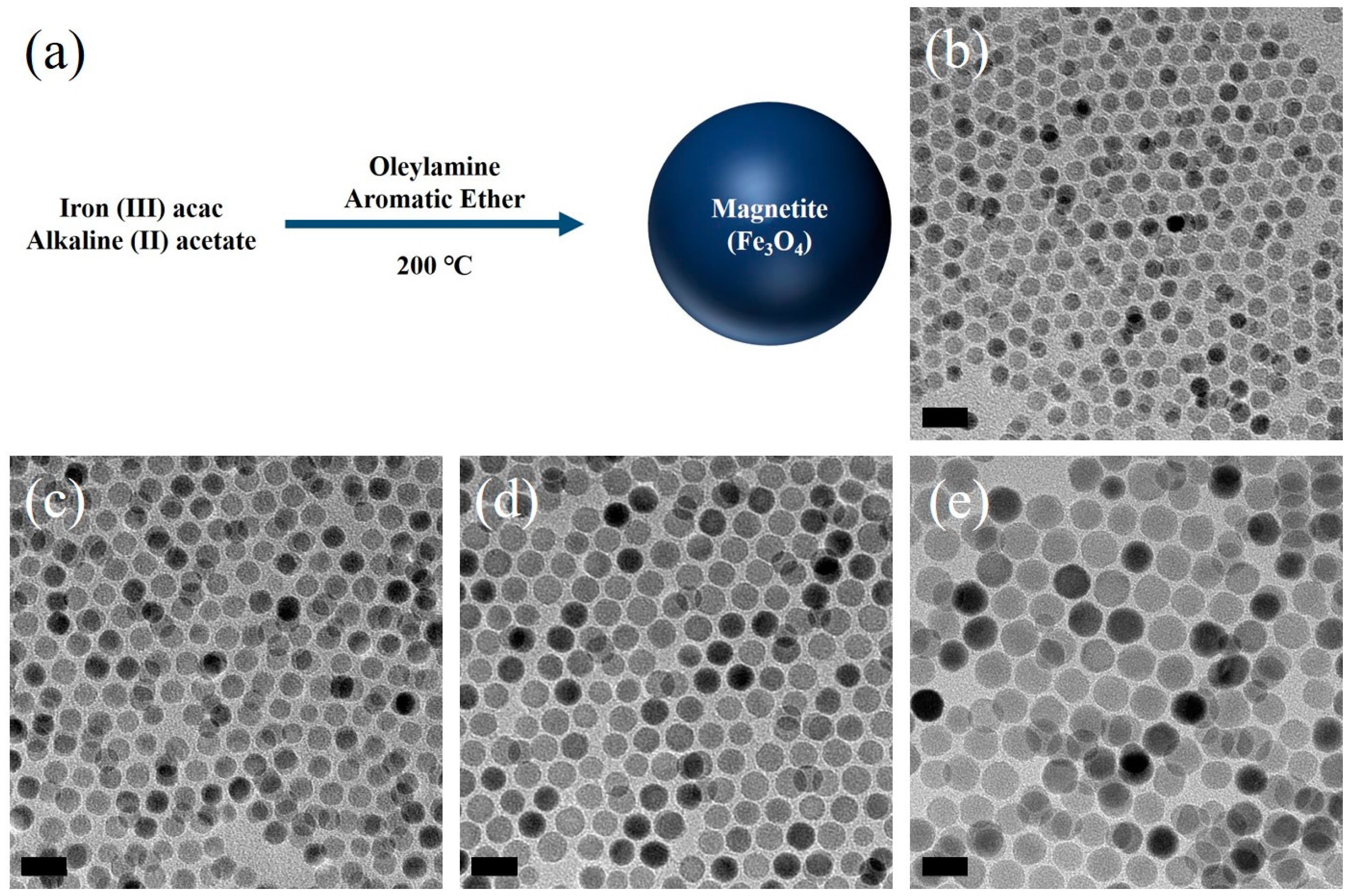
2.1. Iron Oxide Nanoparticles Synthesis
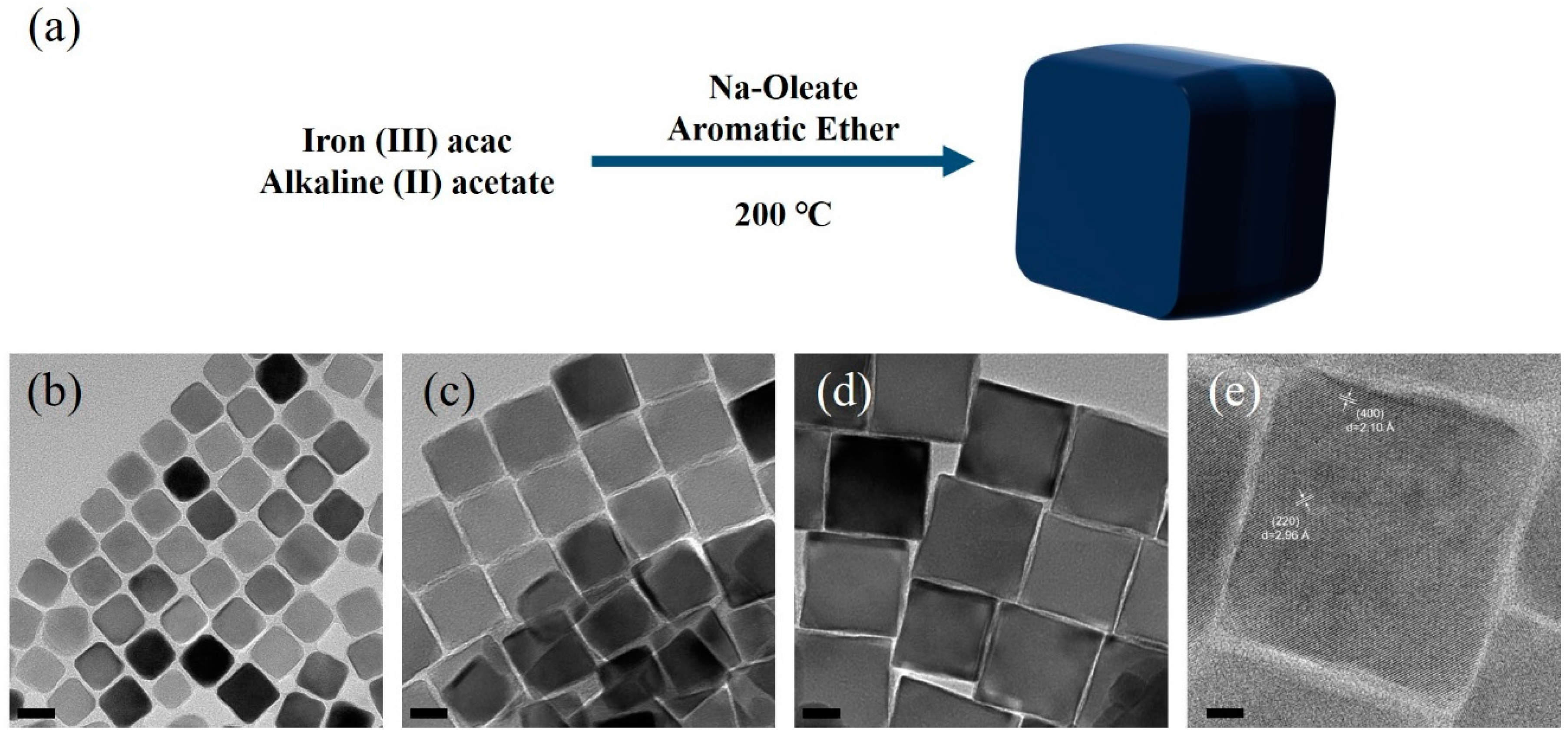
2.2. Iron Oxide Nanocubes Synthesis
2.3. Structural and Compositional Characterization
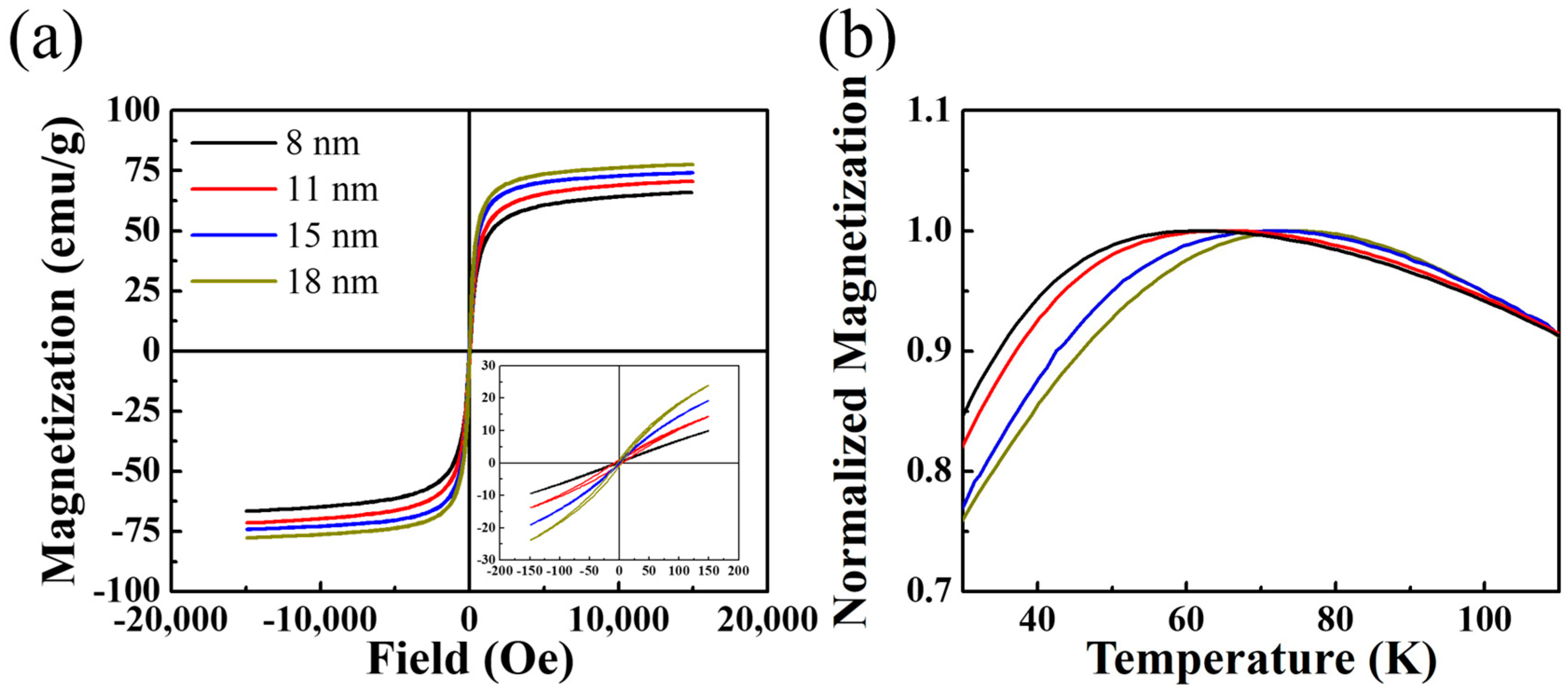
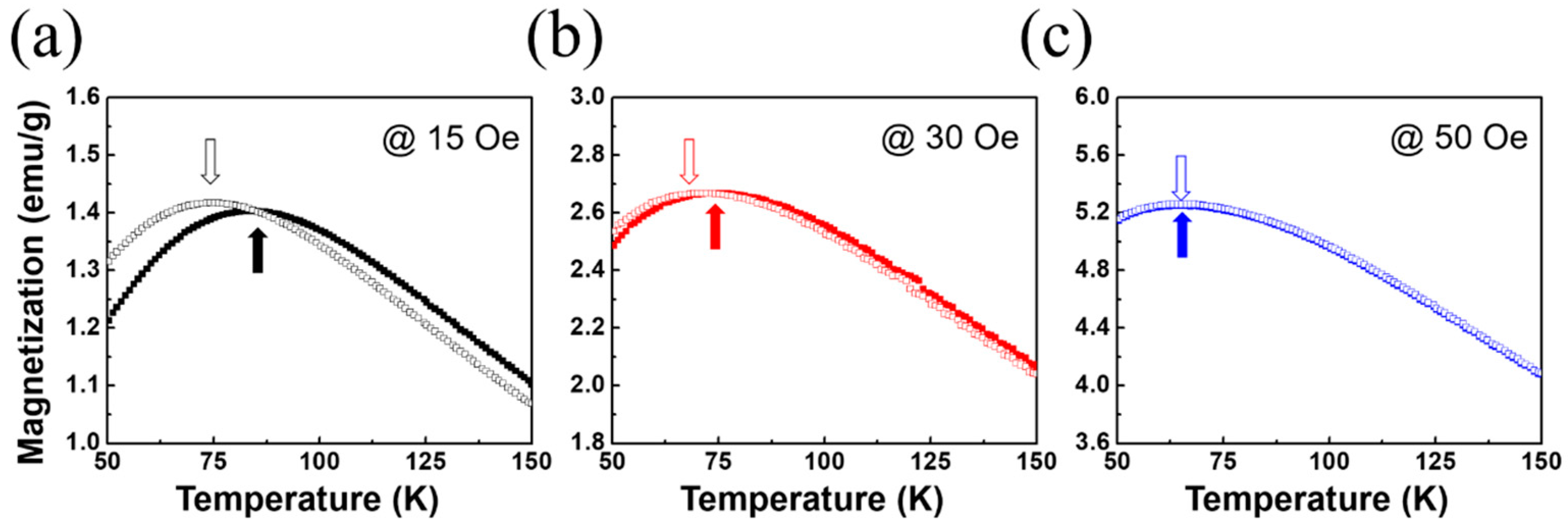
2.4. Magnetic Properties
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt Nanoparticles and Ferromagnetic FePt Nanocrystal Superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Sun, X.; Sun, S. Monodisperse Magnetic Nanoparticles for Theranostic Applications. Acc. Chem. Res. 2011, 44, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Joo, J.; Kwon, S.G.; Jang, Y.; Hyeon, T. Synthesis of Monodisperse Spherical Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 4630–4660. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Jubert, P.-O.; Berman, D.; Imaino, W.; Nelson, A.; Zhu, H.; Zhang, S.; Sun, S. Monolayer Assembly of Ferrimagnetic CoxFe3−xO4 Nanocubes for Magnetic Recording. Nano Lett. 2014, 14, 3395–3399. [Google Scholar] [CrossRef] [PubMed]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Bae, S.; Jeoung, J.W.; Jeun, M.; Jang, J.; Park, J.H.; Kim, Y.J.; Lee, K.; Kim, M.; Lee, J.; Hwang, H.M.; et al. Magnetically Softened Iron Oxide (MSIO) Nanofluid and Its Application to Thermally-Induced Heat Shock Proteins for Ocular Neuroprotection. Biomaterials 2016, 101, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Wang, J.; Wu, C.; Meng, F.; Shi, Z.; Lian, J.; Feng, J.; Meng, J. Novel Complex-Coprecipitation Route to Form High Quality Triethanolamine-Coated Fe3O4 Nanocrystals: Their High Saturation Magnetizations and Excellent Water Treatment Properties. CrystEngComm 2012, 14, 5741–5744. [Google Scholar] [CrossRef]
- Pereira, C.; Pereira, A.M.; Rocha, M.; Freire, C.; Geraldes, C.F.G.C. Architectured Design of Superparamagnetic Fe3O4 Nanoparticles for Application as MRI Contrast Agents: Mastering Size and Magnetism for Enhanced Relaxivity. J. Mater. Chem. B 2015, 3, 6261–6273. [Google Scholar] [CrossRef]
- Mishra, S.; Jeanneau, E.; Rolland, M.; Daniele, S. Structural Isomers of Iron(III) N-Methyl Diethanolaminate as Sol–gel Precursors for Iron-Based Oxide Nanomaterials. RSC Adv. 2016, 6, 1738–1743. [Google Scholar] [CrossRef]
- Liang, X.; Wang, X.; Zhuang, J.; Chen, Y.; Wang, D.; Li, Y. Synthesis of Nearly Monodisperse Iron Oxide and Oxyhydroxide Nanocrystals. Adv. Funct. Mater. 2006, 16, 1805–1813. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Song, S.; Wang, X.; Ge, X.; Zhang, H. Rhombic Dodecahedral Fe3O4: Ionic Liquid-Modulated and Microwave-Assisted Synthesis and Their Magnetic Properties. CrystEngComm 2011, 13, 6017–6020. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shen, C.; Hou, Y.; Gao, H.; Sun, S. Oleylamine as Both Reducing Agent and Stabilizer in a Facile Synthesis of Magnetite Nanoparticles. Chem. Mater. 2009, 21, 1778–1780. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Christiansen, M.G.; Sourakov, A.; Mohr, A.; Matsumoto, Y.; Okada, S.; Jasanoff, A.; Anikeeva, P. High-Performance Ferrite Nanoparticles through Nonaqueous Redox Phase Tuning. Nano Lett. 2016, 16, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Baer, D.R.; Amonette, J.E.; Engelhard, M.H.; Antony, J.; Qiang, Y. Morphology and Electronic Structure of the Oxide Shell on the Surface of Iron Nanoparticles. J. Am. Chem. Soc. 2009, 131, 8824–8832. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.J.; Ferguson, R.M.; Khandhar, A.P.; Krishnan, K.M. Monodisperse Magnetite Nanoparticles with Nearly Ideal Saturation Magnetization. RSC Adv. 2016, 6, 77452–77464. [Google Scholar] [CrossRef]
- Crouse, C.A.; Barron, A.R. Reagent Control over the Size, Uniformity, and Composition of Co–Fe–O Nanoparticles. J. Mater. Chem. 2008, 18, 4146–4153. [Google Scholar] [CrossRef]
- Li, D.; Arachchige, M.P.; Kulikowski, B.; Lawes, G.; Seda, T.; Brock, S.L. Control of Composition and Size in Discrete CoxFe2−xP Nanoparticles: Consequences for Magnetic Properties. Chem. Mater. 2016, 28, 3920–3927. [Google Scholar] [CrossRef]
- Turek, T.; Trimm, D.L.; Cant, N.W. The Catalytic Hydrogenolysis of Esters to Alcohols. Catal. Rev. 1994, 36, 645–683. [Google Scholar] [CrossRef]
- Seo, W.S.; Shim, J.H.; Oh, S.J.; Lee, E.K.; Hur, N.H.; Park, J.T. Phase- and Size-Controlled Synthesis of Hexagonal and Cubic CoO Nanocrystals. J. Am. Chem. Soc. 2005, 127, 6188–6189. [Google Scholar] [CrossRef] [PubMed]
- Lak, A.; Niculaes, D.; Anyfantis, G.C.; Bertoni, G.; Barthel, M.J.; Marras, S.; Cassani, M.; Nitti, S.; Athanassiou, A.; Giannini, C.; et al. Facile Transformation of FeO/Fe3O4 Core-Shell Nanocubes to Fe3O4 via Magnetic Stimulation. Sci. Rep. 2016, 6, 33295. [Google Scholar] [CrossRef] [PubMed]
- Hai, H.T.; Kura, H.; Takahashi, M.; Ogawa, T. Facile Synthesis of Fe3O4 Nanoparticles by Reduction Phase Transformation from γ-Fe2O3 Nanoparticles in Organic Solvent. J. Colloid Interface Sci. 2010, 341, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Koga, N.; Suzuki, Y.; Tatsuoka, T. Thermal Dehydration of Magnesium Acetate Tetrahydrate: Formation and in Situ Crystallization of Anhydrous Glass. J. Phys. Chem. B 2012, 116, 14477–14486. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Hou, Y.; Xu, Z.; Sun, S. Controlled Synthesis and Chemical Conversions of FeO Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 6329–6332. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, J.; Mitra, A.; Bahadur, D.; Aslam, M. Surface Controlled Synthesis of MFe2O4 (M = Mn, Fe, Co, Ni and Zn) Nanoparticles and Their Magnetic Characteristics. CrystEngComm 2012, 15, 524–532. [Google Scholar] [CrossRef]
- Lee, K.; Jang, J.; Nakano, H.; Nakagawa, S.; Paek, S.H.; Bae, S. External Magnetic Field Dependent Shift of Superparamagnetic Blocking Temperature Due to Core/Surface Disordered Spin Interactions. Nanotechnology 2017, 28, 075710. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Na, W.; Jang, J.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.-S.; Cheon, J. Nanoscale Magnetism Control via Surface and Exchange Anisotropy for Optimized Ferrimagnetic Hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, W.E.M.; Al-Hazmi, F.S.; Memesh, L.S.; Bronstein, L.M. A Novel Approach for Rapid Green Synthesis of Nearly Mono-Disperse Iron Oxide Magnetic Nanocubes with Remarkable Surface Magnetic Anisotropy Density for Enhancing Hyperthermia Performance. Colloids Surf. Physicochem. Eng. Asp. 2017, 529, 239–245. [Google Scholar] [CrossRef]
- Singh, G.; Chan, H.; Baskin, A.; Gelman, E.; Repnin, N.; Král, P.; Klajn, R. Self-Assembly of Magnetite Nanocubes into Helical Superstructures. Science 2014, 345, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, N.; Park, M.; Kim, B.H.; An, K.; Hyeon, T. Synthesis of Uniform Ferrimagnetic Magnetite Nanocubes. J. Am. Chem. Soc. 2009, 131, 454–455. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Lee, S.; Oh, M.C.; Ahn, B. Alkaline Metal Reagent-Assisted Synthesis of Monodisperse Iron Oxide Nanostructures. Metals 2018, 8, 107. https://doi.org/10.3390/met8020107
Lee K, Lee S, Oh MC, Ahn B. Alkaline Metal Reagent-Assisted Synthesis of Monodisperse Iron Oxide Nanostructures. Metals. 2018; 8(2):107. https://doi.org/10.3390/met8020107
Chicago/Turabian StyleLee, Kwan, Sangyeob Lee, Min Chul Oh, and Byungmin Ahn. 2018. "Alkaline Metal Reagent-Assisted Synthesis of Monodisperse Iron Oxide Nanostructures" Metals 8, no. 2: 107. https://doi.org/10.3390/met8020107




