Bottom-up Synthesis of Porous NiMo Alloy for Hydrogen Evolution Reaction
Abstract
:1. Introduction
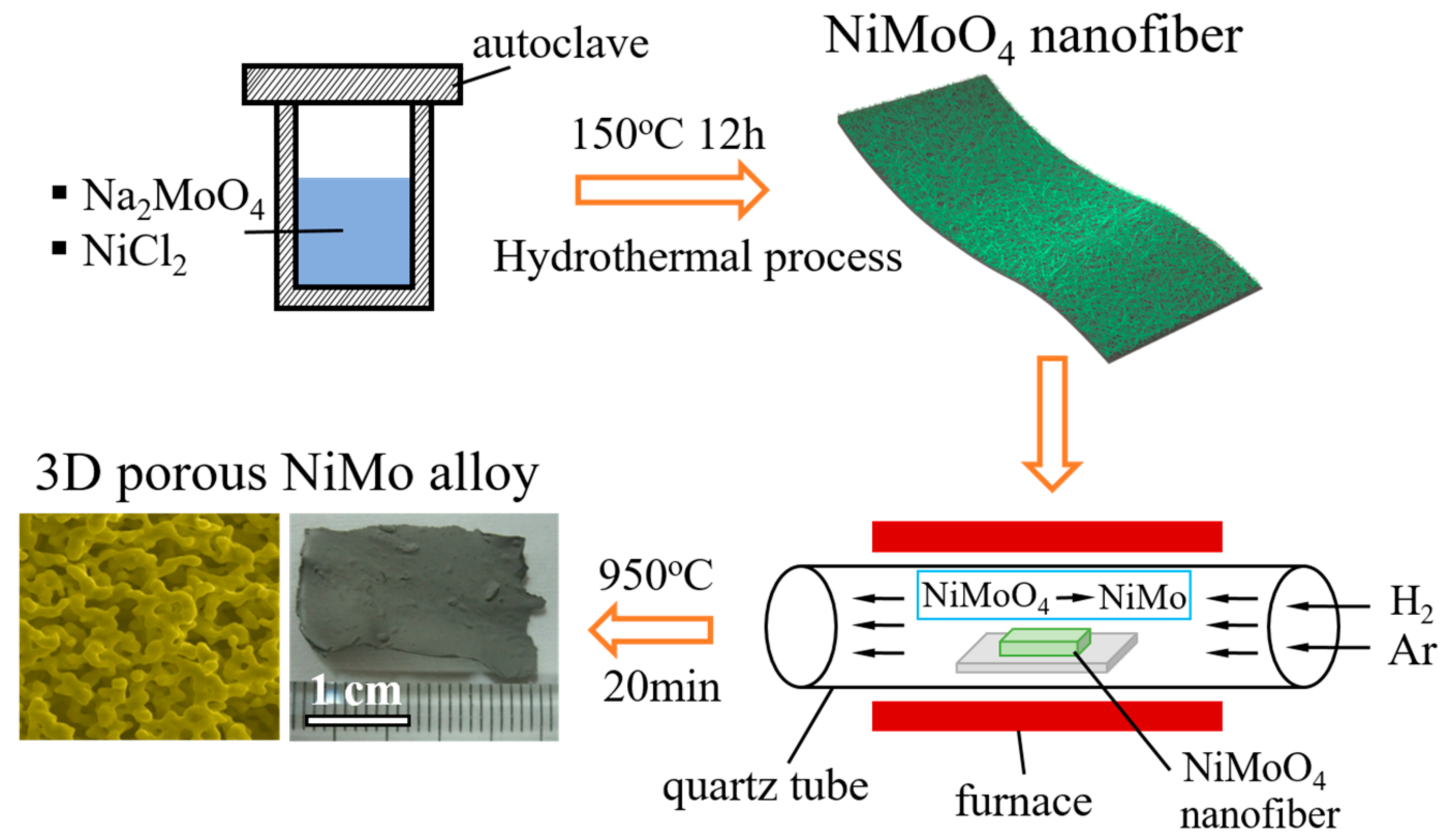
2. Materials and Methods
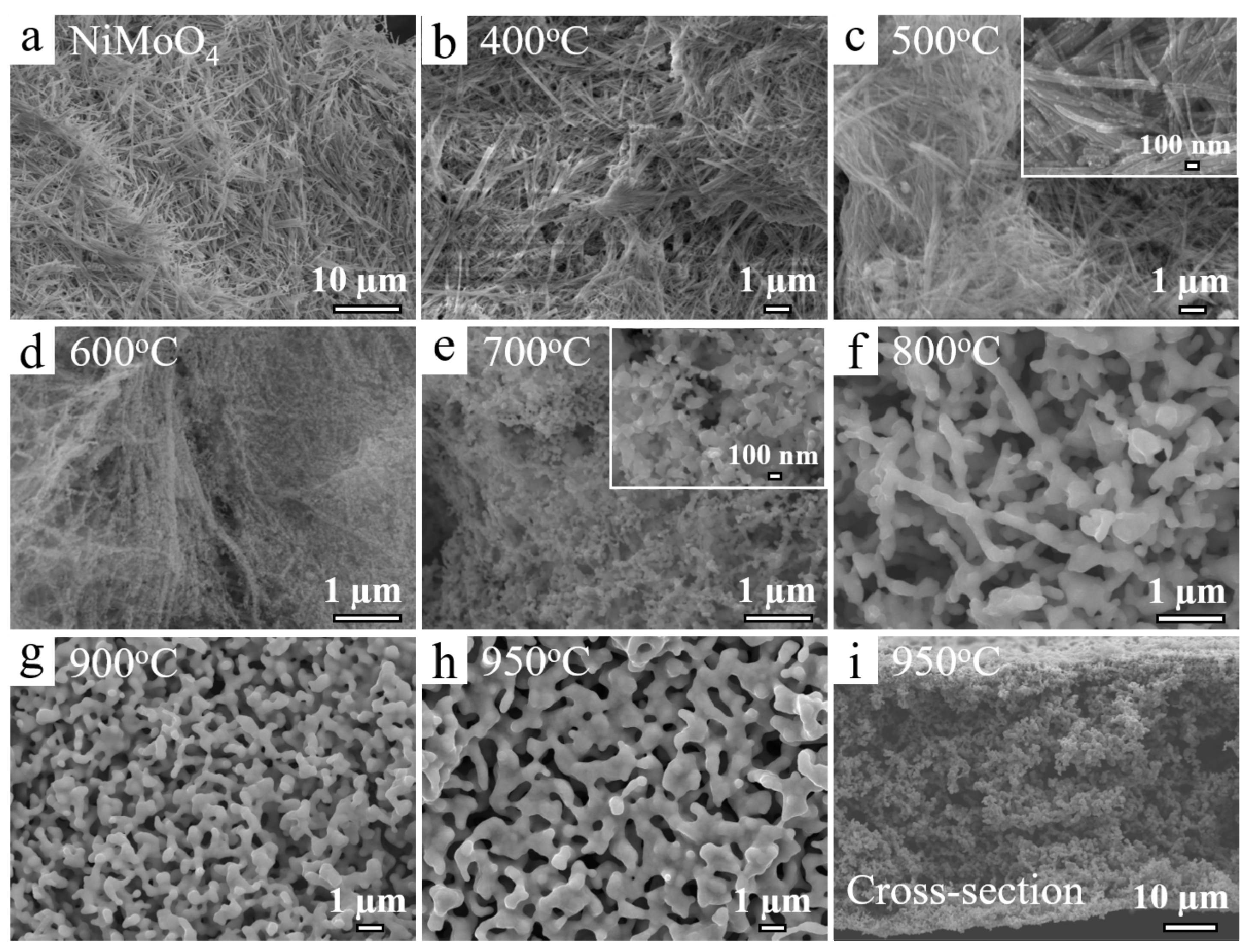
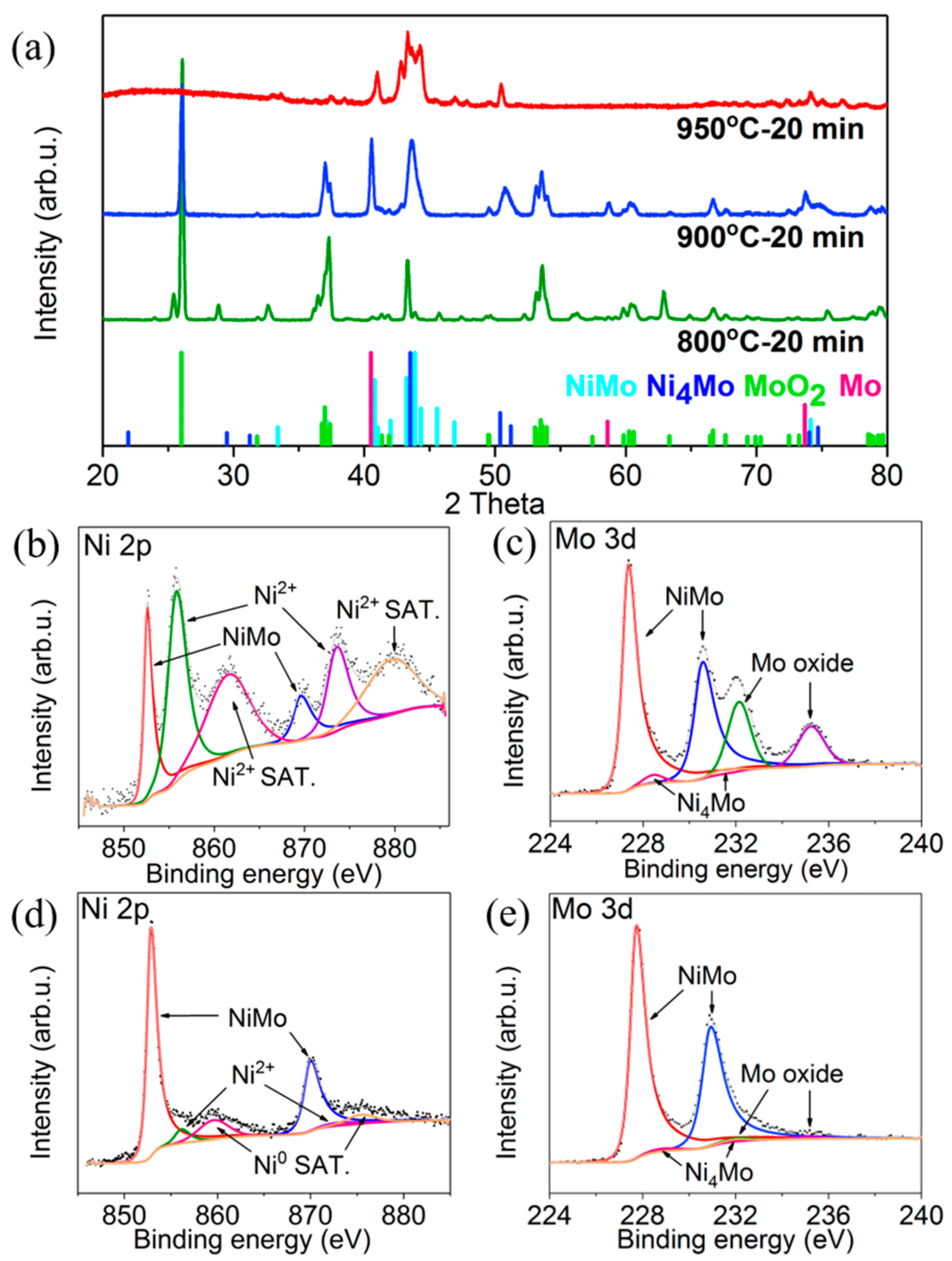
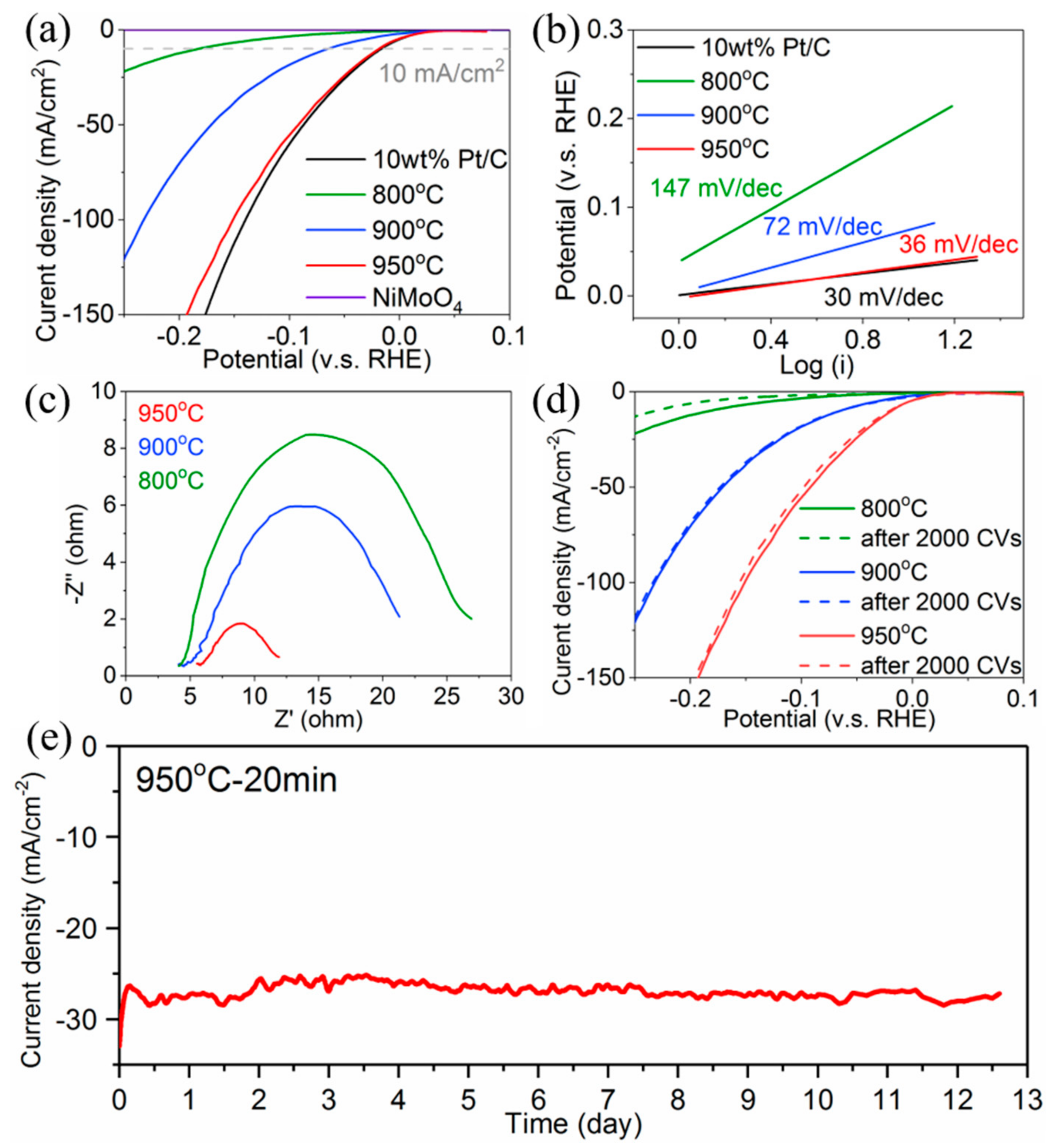
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ding, Y.; Chen, M.W. Nanoporous metals for catalytic and optical applications. MRS Bull. 2009, 34, 569–576. [Google Scholar] [CrossRef]
- Weissmüller, J.; Newman, R.C.; Jin, H.J.; Hodge, A.M.; Kysar, J.W. Nanoporous metals by alloy corrosion: Formation and mechanical properties. MRS Bull. 2009, 34, 577–586. [Google Scholar] [CrossRef]
- Qiu, H.J.; Ito, Y.; Chen, M.W. Hierarchical nanoporous nickel alloy as three-dimensional electrodes for high-efficiency energy storage. Scr. Mater. 2014, 89, 69–72. [Google Scholar] [CrossRef]
- Qiu, H.J.; Kang, J.L.; Liu, P.; Hirata, A.; Fujita, T.; Chen, M.W. Fabrication of large-scale nanoporous nickel with a tunable pore size for energy storage. J. Power Sources 2014, 247, 896–905. [Google Scholar] [CrossRef]
- Guo, X.W.; Han, J.H.; Liu, P.; Ito, Y.; Hirata, A.; Chen, M.W. Graphene@Nanoporous nickel cathode for Li-O2 batteries. ChemNanoMat 2016, 2, 176–181. [Google Scholar] [CrossRef]
- Guo, X.W.; Liu, P.; Han, J.H.; Ito, Y.; Hirata, A.; Fujita, T.; Chen, M.W. 3D nanoporous nitrogen-doped graphene with encapsulated RuO2 nanoparticles for Li-O2 batteries. Adv. Mater. 2015, 27, 6137–6143. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Artero, V.; Kerlidou, M.C.; Fontecave, M. Splitting water with cobalt. Angew. Chem. Int. Ed. Engl. 2011, 50, 7238–7266. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Izumi, M.; Hojo, D.; Wakisaka, M.; Aida, T.; Adschiri, T. One-step nanoporous structure formation using NiO nanoparticles: Pore size control and pore size dependence of hydrogen evolution reaction. Chem. Lett. 2017, 46, 267–270. [Google Scholar] [CrossRef]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of catalytic nanoparticles: Particle migration or ostwald ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, T.; Liu, P.; Liao, Z.Q.; Liu, S.H.; Zhuang, X.D.; Chen, M.W.; Zschech, E.; Feng, X.L. Efficient hydrogen production on MoNi4 electrocatalysts with fast water dissociation kinetics. Nat. Commun. 2017, 8, 15437. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Zhang, Y.; Zhang, X.; Tang, T.; Luo, H.; Niu, S.; Dai, Z.H.; Wan, L.J.; Hu, J.S. Self-templated fabrication of MoNi4/MoO3−X nanorod arrays with dual active components for highly efficient hydrogen evolution. Adv. Mater. 2017, 29, 1703311. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.C.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.S.; Yue, X.; Shu, C.; Huang, S.L.; Shen, P.K. Three-dimensional porous MoNi4 networks constructed by nanosheets as bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2017, 5, 2508–2513. [Google Scholar] [CrossRef]
- McKone, J.R.; Sadtler, B.F.; Werlang, C.L.; Lewis, N.S.; Gray, H.B. Ni–Mo nanopowders for efficient electrochemical hydrogen evolution. ACS Catal. 2013, 3, 166–169. [Google Scholar] [CrossRef]
- Fang, M.; Guo, W.; Dong, G.F.; Xia, Z.M.; Yip, S.P.; Qin, Y.B.; Qu, Y.Q.; Ho, J.C. Hierarchical NiMo-based 3D electrocatalysts for highly-efficient hydrogen evolution in alkaline conditions. Nano Energy 2016, 27, 247–254. [Google Scholar] [CrossRef]
- Jothi, P.R.; Kannan, S.; Velayutham, G. Enhanced methanol electro-oxidation over in-situ carbon and graphene supported one dimensional NiMoO4 nanorods. J. Power Sources 2015, 277, 350–359. [Google Scholar] [CrossRef]
- Kuang, P.Y.; Tong, T.; Fan, K.; Yu, J.G. In situ fabrication of Ni–Mo bimetal sulfide hybrid as an efficient electrocatalyst for hydrogen evolution over a wide pH range. ACS Catal. 2017, 7, 6179–6187. [Google Scholar] [CrossRef]
- Wertheim, G.K.; Wernick, J.H.; Crecelius, G. Surface effects on valence in rare-earth intermetallic compounds. Phys. Rev. B 1978, 18, 875. [Google Scholar] [CrossRef]
- Roustila, A.; Severac, C.; Chêne, J.; Percheron-Guégan, A. Hydrogen effects on the electronic and microstructural properties of Ce, Ni, and CeNi2 intermetallic compound. Surf. Sci. 1994, 311, 33–44. [Google Scholar] [CrossRef]
- Lebugle, A.; Axelsson, U.; Nyholm, R.; Mårtensson, N. Experimental L and M core level binding energies for the metals 22Ti to 30Zn. Phys. Scr. 1981, 23, 825–827. [Google Scholar] [CrossRef]
- Brainard, W.A.; Wheeler, D.R. An XPS study of the adherence of refractory carbide silicide and boride rf-sputtered wear-resistant coatings. J. Vac. Sci. Technol. 1978, 15, 1800–1805. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Cattania, M.G.; Villa, P. XPS characterization of Ni and Mo oxides before and after “in situ” treatments. Appl. Surf. Sci. 1993, 70, 211–216. [Google Scholar] [CrossRef]
- Takano, I.; Isobe, S.; Sasaki, T.A.; Baba, Y. Nitrogenation of various transition metals by N+2-ion implantation. Appl. Surf. Sci. 1989, 37, 25–32. [Google Scholar] [CrossRef]
- Conway, B.E.; Tilak, B.V. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.H.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.J.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Z.X.; Ahn, H.S.; Bard, A.J. A study of the mechanism of the hydrogen evolution reaction on nickel by surface interrogation scanning electrochemical microscopy. J. Am. Chem. Soc. 2017, 139, 4854–4858. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, K.; Jeong, S.; Wakisaka, M.; Fujita, J.-i.; Ito, Y. Bottom-up Synthesis of Porous NiMo Alloy for Hydrogen Evolution Reaction. Metals 2018, 8, 83. https://doi.org/10.3390/met8020083
Hu K, Jeong S, Wakisaka M, Fujita J-i, Ito Y. Bottom-up Synthesis of Porous NiMo Alloy for Hydrogen Evolution Reaction. Metals. 2018; 8(2):83. https://doi.org/10.3390/met8020083
Chicago/Turabian StyleHu, Kailong, Samuel Jeong, Mitsuru Wakisaka, Jun-ichi Fujita, and Yoshikazu Ito. 2018. "Bottom-up Synthesis of Porous NiMo Alloy for Hydrogen Evolution Reaction" Metals 8, no. 2: 83. https://doi.org/10.3390/met8020083
APA StyleHu, K., Jeong, S., Wakisaka, M., Fujita, J.-i., & Ito, Y. (2018). Bottom-up Synthesis of Porous NiMo Alloy for Hydrogen Evolution Reaction. Metals, 8(2), 83. https://doi.org/10.3390/met8020083




