Enhanced Corrosion Resistance of Ultrafine-Grained Fe-Cr Alloys with Subcritical Cr Contents for Passivity
Abstract
:1. Introduction
2. Materials and Methods
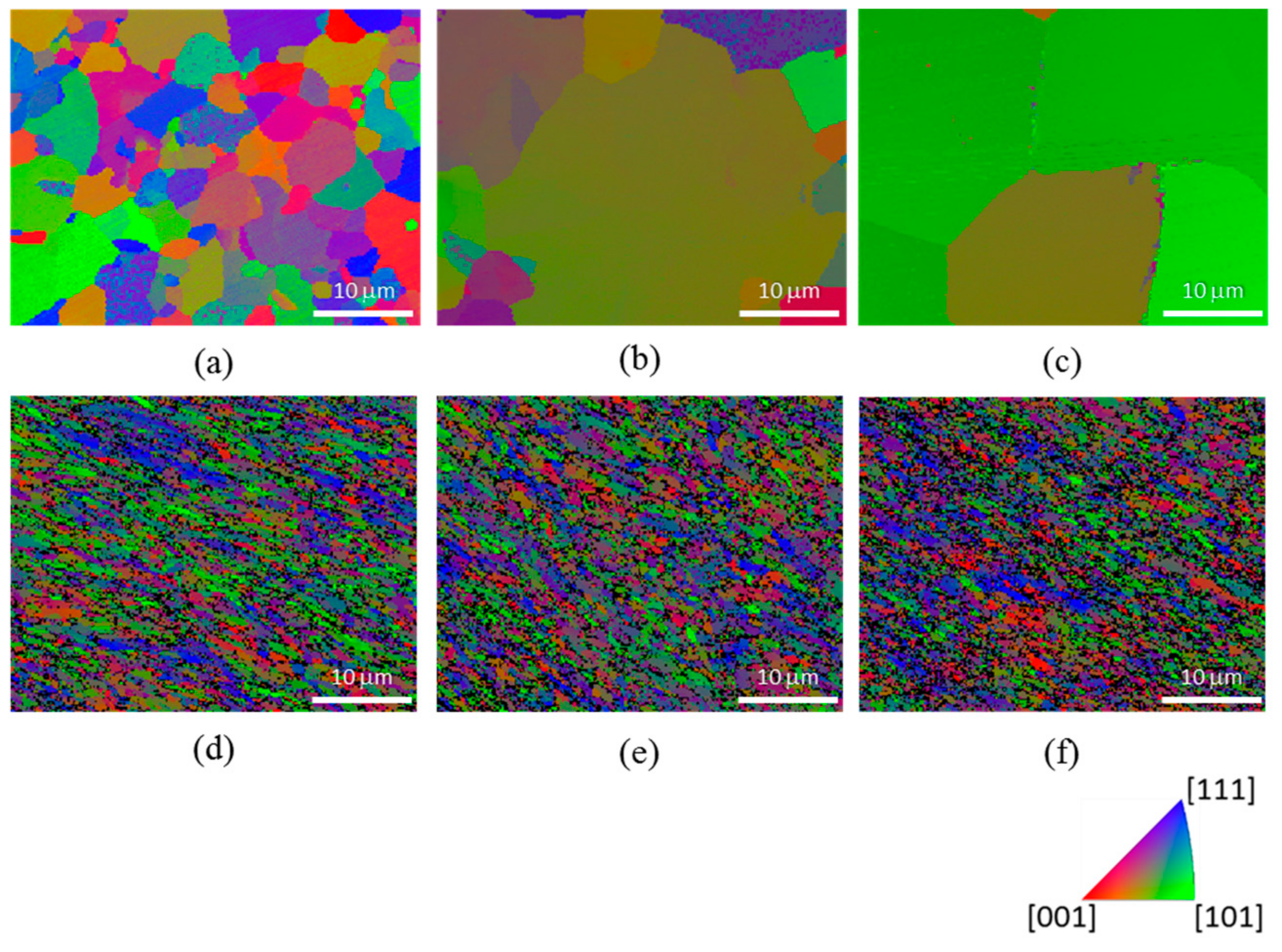
3. Results
4. Discussion
5. Conclusions
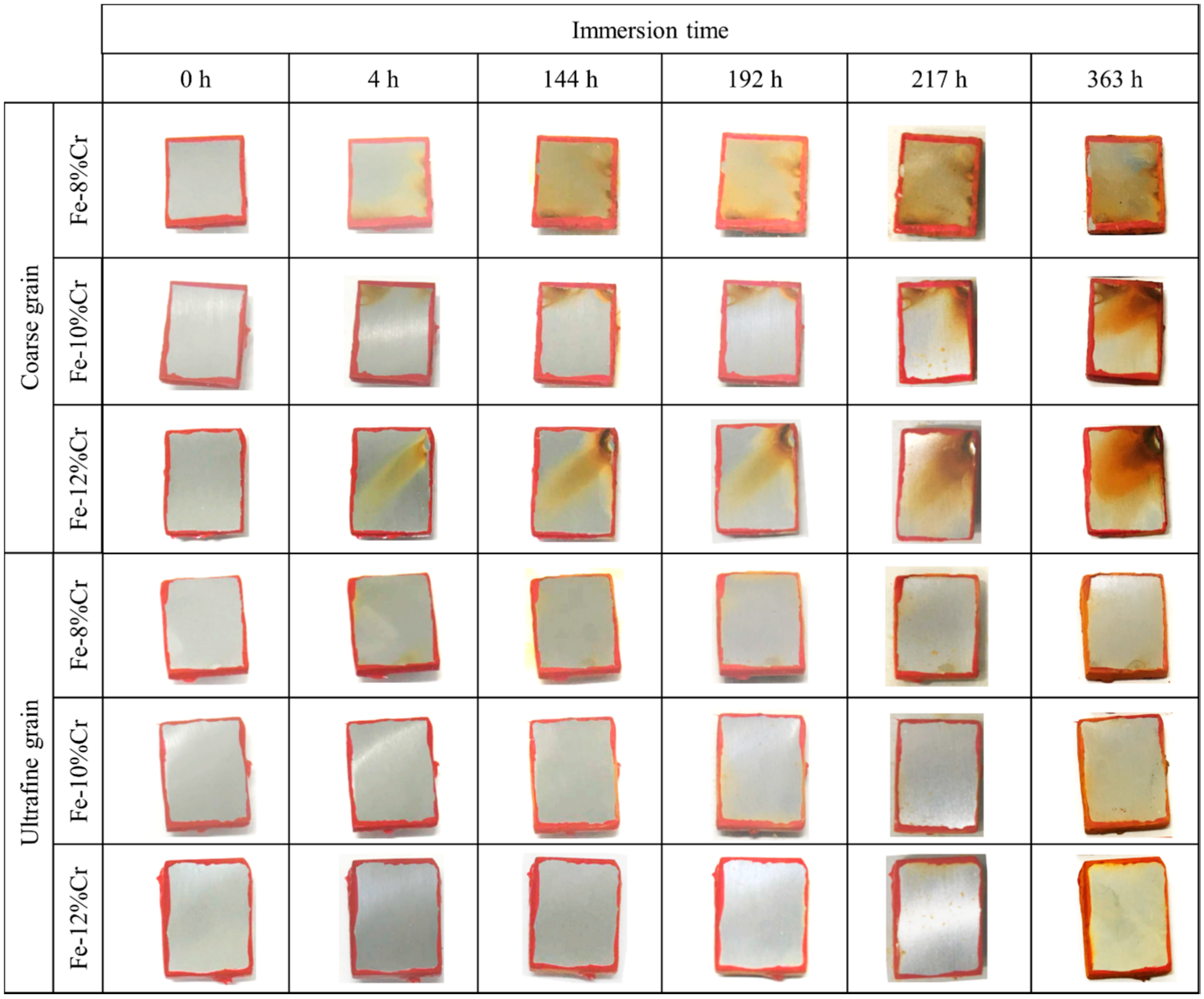
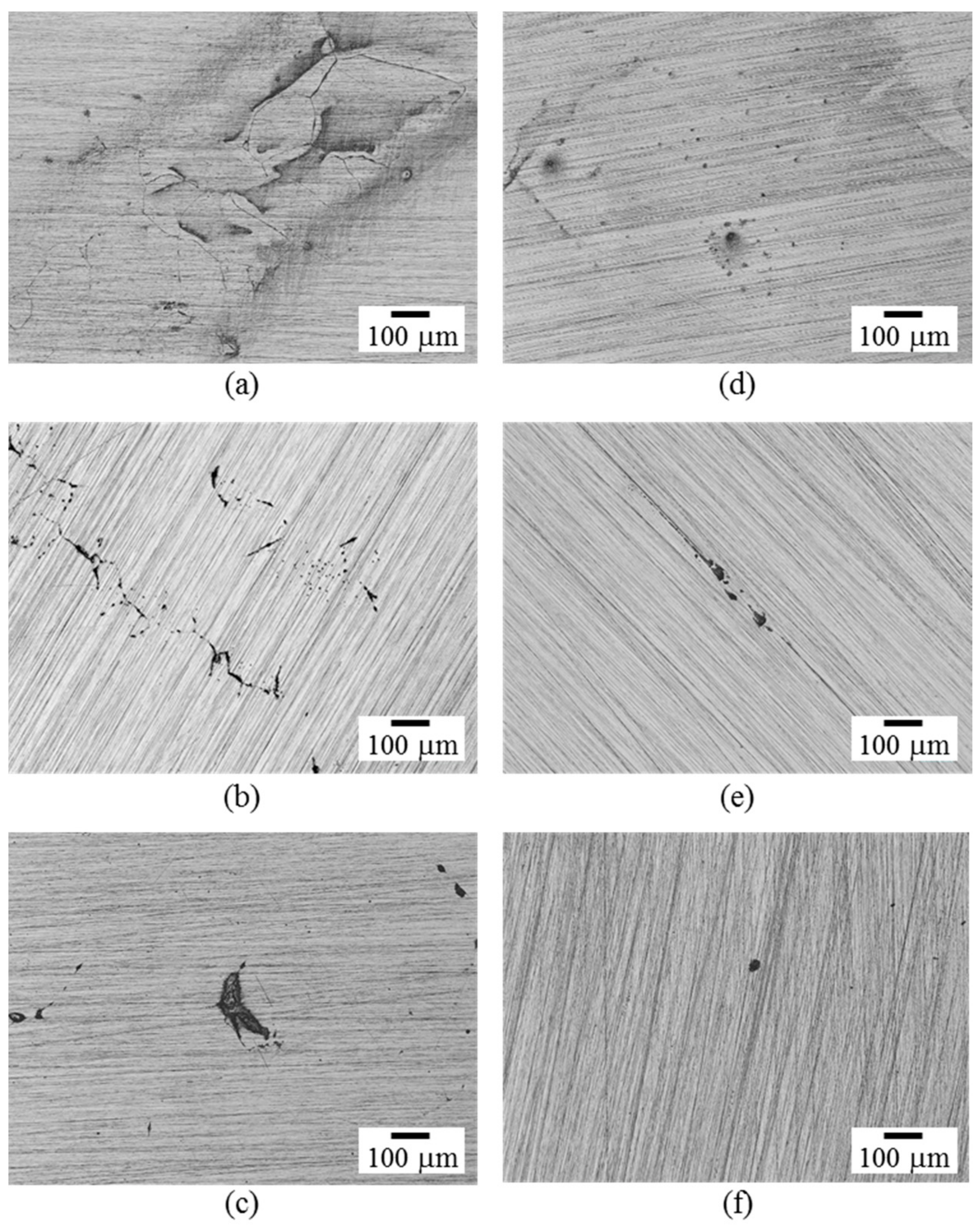
- In immersion test in 0.6 mol/L NaCl solution, UFG alloys exhibited higher corrosion resistance than CG alloys regardless of Cr contents. Importantly, UFG Fe-8 and 10% Cr alloys with subcritical Cr contents exhibited higher corrosion resistance than CG Fe-12% Cr alloys.
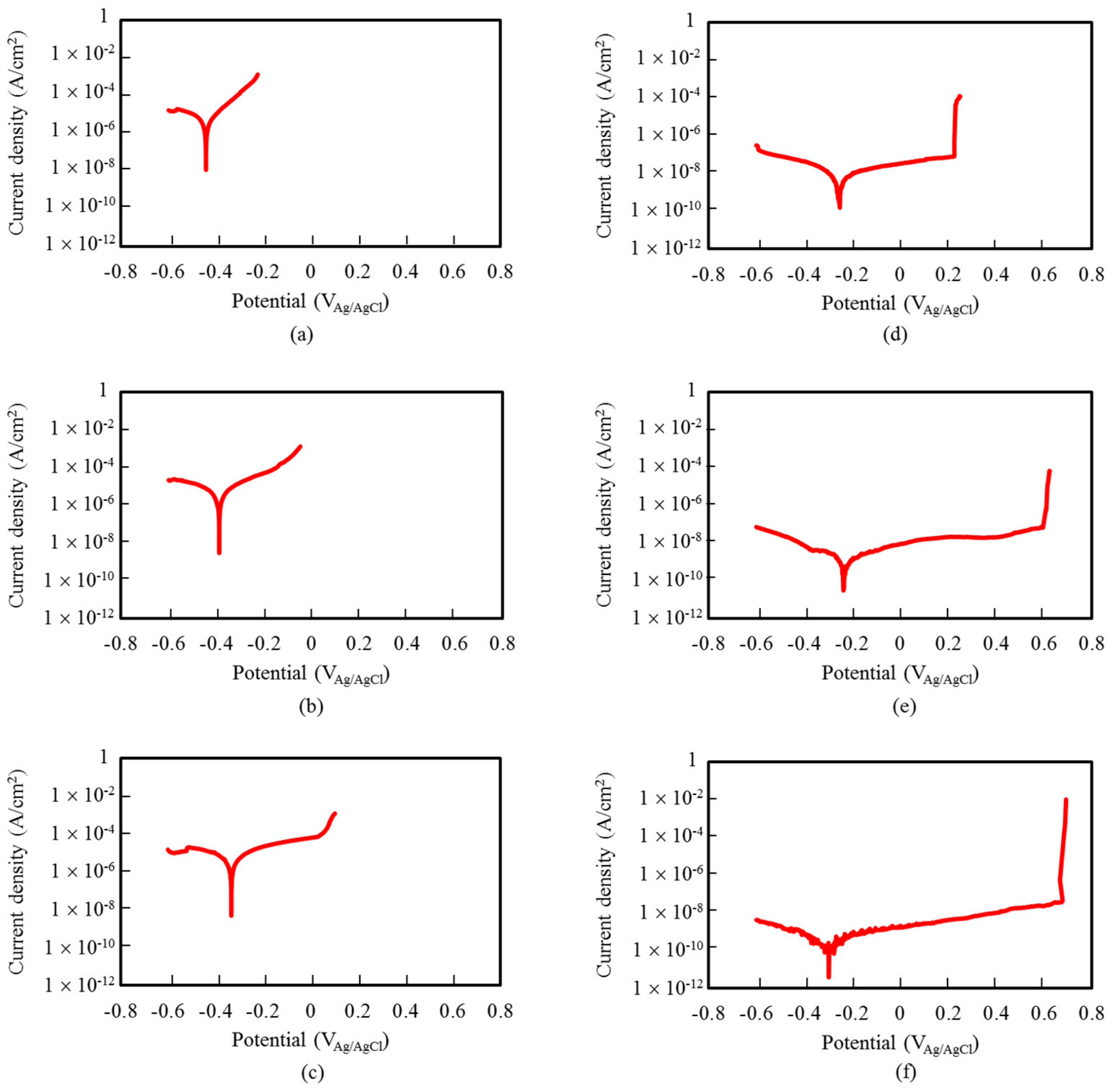
- UFG alloys including 8% and 10% Cr exhibited passivity in anodic polarization tests whereas CG alloys with the same Cr exhibited polarization curve of the active type. Higher corrosion resistance of UFG alloys can be attributed to the formation of the more protective passive film.
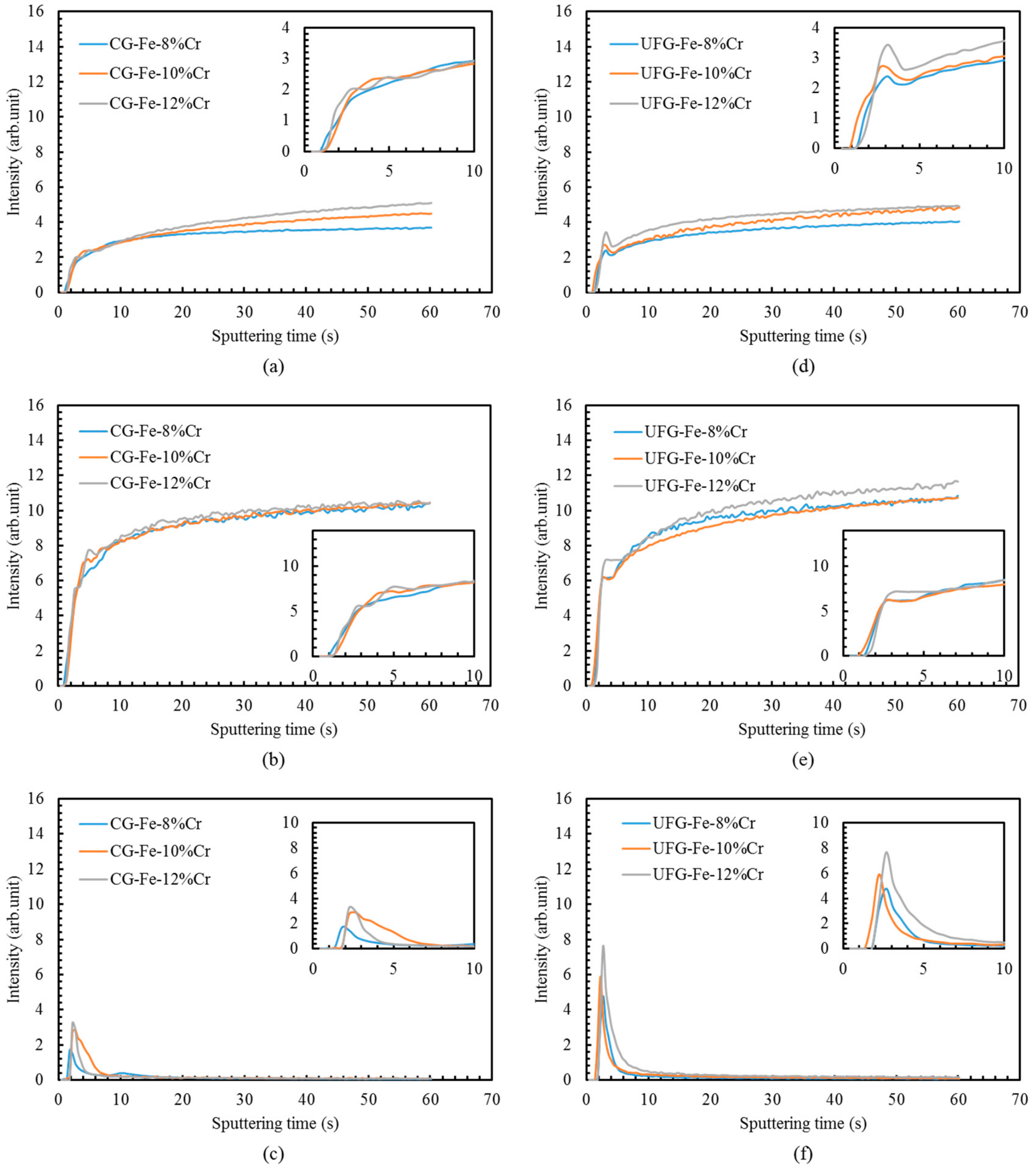
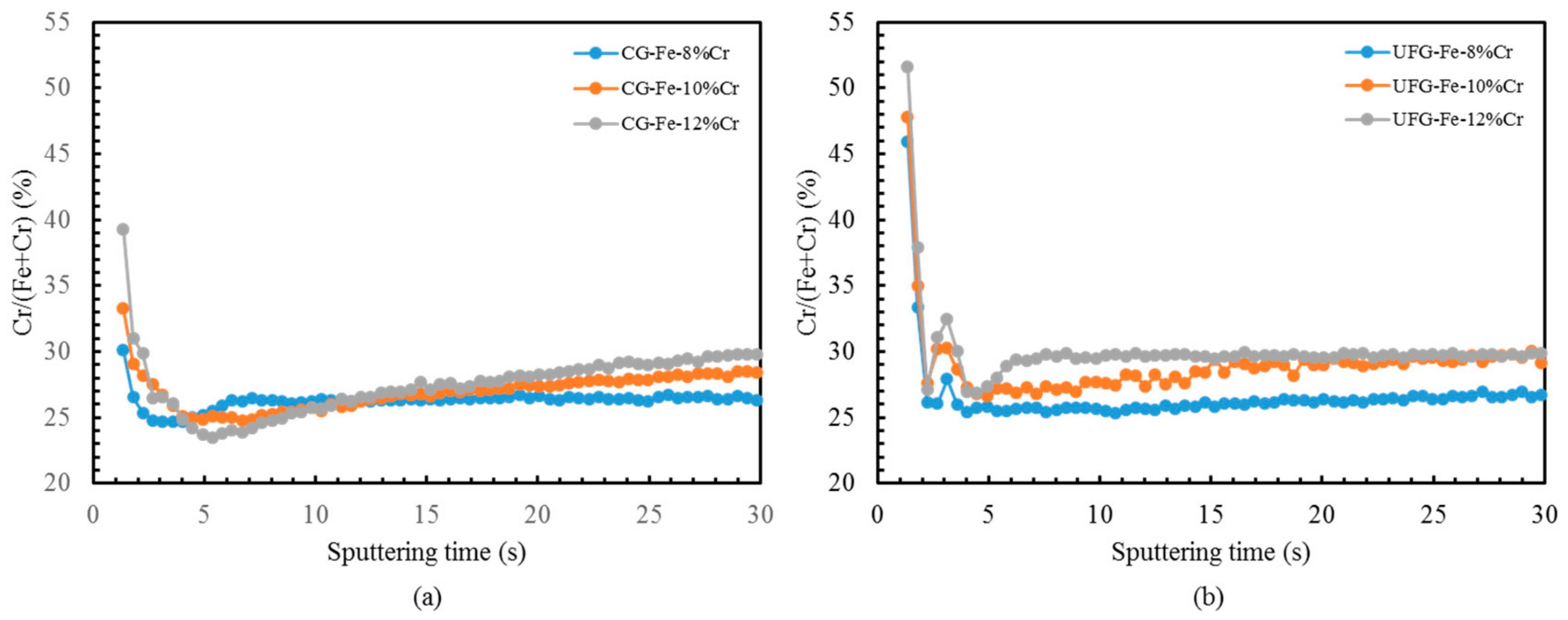
- Greater Cr enrichment in the passive films was manifested in UFG alloys than CG alloys by GD-OES. The UFG structure appears to promote very rapid diffusion of chromium, thus assisting the formation of the protective passive films that in turn leads to enhanced corrosion resistance.
Author Contributions
Conflicts of Interest
References
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 075005. [Google Scholar] [CrossRef]
- Miyamoto, H. Corrosion of ultrafine grained materials by severe plastic deformation, an overview. Mater. Trans. 2016, 57, 559–572. [Google Scholar] [CrossRef]
- Raman, R.K.S.; Gupta, R.K. Oxidation resistance of nanocrystalline vis-à-vis microcrystalline Fe-Cr alloys. Corros. Sci. 2009, 51, 316–321. [Google Scholar] [CrossRef]
- Raman, R.K.S.; Gupta, R.K.; Koch, C.C. Resistance of nanocrystalline vis-à-vis microcrytalline Fe-Cr alloys to environmental degradation and challenge to their synthesis. Philos. Mag. 2010, 90, 3233–3260. [Google Scholar] [CrossRef]
- Gupta, R.K.; Birbilis, N. The influence of nanocrystalline structure and processing route on corrosion of stainless steel: A review. Corros. Sci. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Chen, G.; Gao, Y.; Wu, S.; Hu, J. Corrosion behavior of AISI304 austentic stainless steel fabricated by equal-channel angular pressing. Adv. Mater. Res. 2011, 194–196, 411–415. [Google Scholar] [CrossRef]
- Zheng, Z.J.; Gao, Y.; Gui, Y.; Zhu, M. Corrosion behaviour of nanocrystalline 304 stainless steel prepared by equal channel angular pressing. Corros. Sci. 2012, 54, 60–67. [Google Scholar] [CrossRef]
- Rifai, M.; Miyamoto, H.; Fujiwara, H. Effects of Strain Energy and Grain Size on Corrosion Resistance of Ultrafine Grained Fe-20%Cr Steels with Extremely low C and N Fabricated by ECAP. Int. J. Corros. 2015, 2015, 386865. [Google Scholar] [CrossRef]
- Gupta, R.K.; Singh Raman, R.K.; Koch, C.C. Fabrication and oxidation resistance of nanocrystalline Fe10Cr alloy. J. Mater. Sci. 2010, 45, 4884–4888. [Google Scholar] [CrossRef]
- Gupta, R.; Singh Raman, R.K.; Koch, C.C. Electrochemical characteristics of nano and microcrystalline Fe–Cr alloys. J. Mater. Sci. 2012, 47, 6118–6124. [Google Scholar] [CrossRef]
- Gupta, R.K.; Singh Raman, R.K.; Koch, C.C.; Murty, B.S. Effect of nanocrystalline structure on the corrosion of a Fe20Cr alloy. Int. J. Electrochem. Sci. 2013, 8, 6791–6806. [Google Scholar]
- Pisarek, M.; Kedzierzawski, P.; Janik-Czachor, M.; Kurzydlowski, K.J. Effect of hydrostatic extrusion on the corrosion resistance of type316 stainless steel. Corrosion 2008, 64, 131–137. [Google Scholar] [CrossRef]
- Pisarek, M.; Kȩdzierzawski, P.; Janik-Czachor, M.; Kurzydłowski, K.J. Effect of hydrostatic extrusion on passivity breakdown on 303 austenitic stainless steel in chloride solution. J. Solid State Electrochem. 2009, 13, 283–291. [Google Scholar] [CrossRef]
- Krawczynska, A.T.; Gloc, M.; Lublinska, K. Intergranular corrosion resistance of nanostructured austenitic stainless steel. J. Mater. Sci. 2013, 48, 4517–4523. [Google Scholar] [CrossRef]
- Balusamy, T.; Kumar, S.; Sankara Narayanan, T.S.N. Effect of surface nanocrystallization on the corrosion behaviour of AISI 409 stainless steel. Corros. Sci. 2010, 52, 3826–3834. [Google Scholar] [CrossRef]
- Mordyuk, B.N.; Prokopenko, G.I.; Vasylyev, M.A.; Iefimov, M.O. Effect of structure evolution induced by ultrasonic peening on the corrosion behavior of AISI-321 stainless steel. Mater. Sci. Eng. A 2007, 458, 253–261. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Wang, F. The corrosion behavior of Fe–10Cr nanocrystalline coating. Electrochim. Acta 2006, 51, 4277–4284. [Google Scholar] [CrossRef]
- Pan, C.; Liu, L.; Bin, Z.; Wang, F. The electrochemical corrosion behaivor of nanocrystalline 304 stainless steel prepared by magnetron sputtering. J. Electrochem. Soc. 2012, 159, C453–C460. [Google Scholar] [CrossRef]
- Olefjord, I.; Brox, B.; Jelvestam, U. Surface Composition of Stainless Steels during Anodic Dissolution and Passivation Studied by ESCA. J. Electrochem. Soc. 1985, 132, 2854–2861. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1996; ISBN 10-0029464390. [Google Scholar]
- Schneider, M.; Zieiger, W.; Scharnweber, D.; Worch, H. Electrochemical behaivor of nanocrystalline FeAl8 and FeCr10 alloys. Mater. Sci. Forum 1996, 225–227, 819–824. [Google Scholar] [CrossRef]
- Streicher, M.A. Austenitic and Ferritic stainless steels. In Uhlig’s Corrosion Handbook; Revie, R.W., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2011; ISBN 0470080329. [Google Scholar]
- Valiev, R.Z.; Gertsman, V.Y.; Kaibyshev, O.A. Non-equillibrium state and recovery of grain boundary structure. Phys. Status Solidi 1983, 78, 177–187. [Google Scholar] [CrossRef]
- Sergueeva, A.V.; Stolyarov, V.V.; Valiev, R.Z.; Mukherjee, A.K. Enhanced superplasticity in a Ti-6Al-4V alloy processed by severe plastic deformation. Scr. Mater. 2000, 43, 819–824. [Google Scholar] [CrossRef]
- Divinski, S.V.; Reglitz, G.; Rösner, H.; Estrin, Y.; Wilde, G. Ultra-fast diffusion channels in pure Ni severely deformed by equal-channel angular pressing. Acta Mater. 2011, 59, 1974–1985. [Google Scholar] [CrossRef]
- Miyamoto, H.; Harada, K.; Mimaki, T.; Vinogradov, A.; Hashimoto, S. Corrosion of ultra-fine grained copper fabricated by equal-channel angular pressing. Corros. Sci. 2008, 50, 1215–1220. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Z.H.; Ma, Q.F.; Li, Z. Influence of cold working and grain size on pitting corrosion resistance of ferritic stainless steel. Adv. Mater. Res. 2011, 217–218, 1180–1184. [Google Scholar] [CrossRef]
- Rifai, M.; Bagherpour, E.; Yamamoto, G.; Yuasa, M.; Miyamoto, H. Transition of Dislocation Structures in Severe Plastic Deformation and Its Effect on Dissolution in Dislocation Etchant. Adv. Mater. Sci. Eng. 2018, 2018, 4254156. [Google Scholar] [CrossRef]
- Fischmeister, H.; Roll, U. Passive layers on stainless steels: A survey of surface analysis: A survey of surface analysis results. Z. Anal. Chem. 1984, 319, 639–645. [Google Scholar] [CrossRef]
- Kirchheim, R.; Heine, B.; Fischmeister, H.; Hofmann, S.; Knote, H.; Stolz, U. The passivity of iron-chromium alloys. Corros. Sci. 1989, 29, 899–917. [Google Scholar] [CrossRef]
- Hamm, D.; Ogle, K.; Olsson, C.O.A.; Weber, S.; Landolt, D. Passivation of Fe-Cr alloys studied with ICP-AES and EQCM. Corros. Sci. 2002, 44, 1443–1456. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive films on stainless steels—Chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Sieradzki, K.; Newman, R.C. A percolation model for passivation in stainless steels. J. Electrochem. Soc. 1986, 133, 1979–1980. [Google Scholar] [CrossRef]
- Newman, R.C.; Foong, T.M.; Sieradzki, K. Validation of a percolation model for passivation of Fe-Cr alloys: Current efficiency in the incompletely passivated state. Corros. Sci. 1988, 28, 523–527. [Google Scholar] [CrossRef]
- Qian, S.; Newman, R.C.; Cottis, R.A. Validation of a percolation model for passivation of Fe-Cr alloys: Two-dimensional computer simulation. J. Electrochem. Soc. 1990, 137, 435–439. [Google Scholar] [CrossRef]
- Williams, D.E.; Newman, R.C.; Song, Q.; Kelly, R.G. Passivity breakdown and pitting corrosion of binary alloys. Nature 1991, 350, 216–219. [Google Scholar] [CrossRef]
- Hamada, E.; Yamada, K.; Nagoshi, M.; Makiishi, N.; Sato, K.; Ishii, T.; Fukuda, K.; Ishikawa, S.; Ujiro, T. Direct imaging of native passive film on stainless steel by aberration corrected STEM. Corros. Sci. 2010, 52, 3851–3854. [Google Scholar] [CrossRef]
- Wang, Z.B.; Tao, N.R.; Tong, W.P.; Lu, J.; Lu, K. Diffusion of chromium in nanocrystalline iron produced by means of surface mechanical attrition treatment. Acta Mater. 2003, 51, 4319–4329. [Google Scholar] [CrossRef]
- Chao, C.Y.; Lin, L.F.; Macdonald, D.D. A point defect model for anodic passive films 2. Chemical breakdown and pit initiation. J. Electrochem. Soc. 1981, 128, 1194–1198. [Google Scholar] [CrossRef]
- Chao, C.Y.; Lin, L.F.; Macdonald, D.D. A point defect model for anodic passive films 1. Film growth kinetics. J. Electrochem. Soc. 1981, 128, 1187–1194. [Google Scholar] [CrossRef]
- Macdonald, D.D. The point defect model for the passive state. J. Electrochem. Soc. 1992, 139, 3434–3449. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rifai, M.; Yuasa, M.; Miyamoto, H. Enhanced Corrosion Resistance of Ultrafine-Grained Fe-Cr Alloys with Subcritical Cr Contents for Passivity. Metals 2018, 8, 149. https://doi.org/10.3390/met8030149
Rifai M, Yuasa M, Miyamoto H. Enhanced Corrosion Resistance of Ultrafine-Grained Fe-Cr Alloys with Subcritical Cr Contents for Passivity. Metals. 2018; 8(3):149. https://doi.org/10.3390/met8030149
Chicago/Turabian StyleRifai, Muhammad, Motohiro Yuasa, and Hiroyuki Miyamoto. 2018. "Enhanced Corrosion Resistance of Ultrafine-Grained Fe-Cr Alloys with Subcritical Cr Contents for Passivity" Metals 8, no. 3: 149. https://doi.org/10.3390/met8030149
APA StyleRifai, M., Yuasa, M., & Miyamoto, H. (2018). Enhanced Corrosion Resistance of Ultrafine-Grained Fe-Cr Alloys with Subcritical Cr Contents for Passivity. Metals, 8(3), 149. https://doi.org/10.3390/met8030149




